Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2336
Peer-review started: December 21, 2022
First decision: January 20, 2023
Revised: February 1, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: April 6, 2023
Processing time: 99 Days and 9.2 Hours
Neurofibromatosis type 1 (NF1) is characterized by café-au-lait patches on the skin and the presence of neurofibromas. Gastrointestinal stromal tumor (GIST) is the most common non-neurological tumor in NF1 patients. In NF1-associated GIST, KIT and PDGFRA mutations are frequently absent and imatinib is ineffective. Surgical resection is first-line treatment.
A 56-year-old woman with NF1 was hospitalized because of an incidental pelvic mass. Physical examination was notable for multiple café-au-lait patches and numerous subcutaneous soft nodular masses of the skin of the head, face, trunk, and limbs. Her abdomen was soft and nontender. No masses were palpated. Digital rectal examination was unremarkable. Abdominal computed tomography was suspicious for GIST or solitary fibrous tumor. Laparoscopy was performed, which identified eight well-demarcated masses in the jejunum. All were resected and pathologically diagnosed as GISTs. The patient was discharged on day 7 after surgery without complications. No tumor recurrence was evident at the 6-mo follow-up.
Laparoscopy is effective for both diagnosis and treatment of NF1-associated GIST.
Core Tip: A thorough medical history and physical examination are imperative in patients with neurofibromatosis type 1 and gastrointestinal symptoms. Such symptoms may indicate the presence of one or more gastrointestinal stromal tumors. Abdominal computed tomography, capsule endoscopy, or small bowel endoscopy should be performed. Laparoscopy may also be performed for both diagnosis and treatment.
- Citation: Yao MQ, Jiang YP, Yi BH, Yang Y, Sun DZ, Fan JX. Neurofibromatosis type 1 with multiple gastrointestinal stromal tumors: A case report. World J Clin Cases 2023; 11(10): 2336-2342
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2336.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2336
Neurofibromatosis type 1 (NF1), also known as von Recklinghausen's disease, is an autosomal dominant disorder with an incidence of 1/3000 to 1/4000[1]. Clinical diagnosis of NF1 is based on the presence of at least two of the following criteria: (1) Six or more café-au-lait patches over 5 mm in diameter in prepubertal children and over 15 mm in diameter in postpubertal individuals and adults; (2) two or more neurofibromas or one plexiform neurofibroma; (3) freckles in the axilla or inguinal region; (4) optic glioma; (5) two or more Lisch nodes; (6) osseous lesion; and (7) first-degree relative with NF1[2]. Gastrointestinal stromal tumor (GIST) is a tumor of mesenchymal origin that usually arises in the stomach (60%–70%) or small intestine (20%–30%). Multiple GISTs are uncommon[3]. We report an NF1 patient with a pelvic mass who underwent laparoscopic examination of the abdomen and pelvis and was diagnosed with multiple GISTs.
A 56-year-old woman with NF1 was hospitalized because of an incidental pelvic mass.
Ultrasonography of the pelvis performed at another hospital showed a mixed echoic mass approximately 4.5 cm × 4.5 cm × 4.0 cm in size in the left lower abdomen. Boundaries were clear and shape was irregular. No internal fluid echoes were visualized. Color Doppler flow imaging showed abundant blood flow signals within the mass. Clinically, the patient denied any gastrointestinal symptoms.
The patient had multiple café-au-lait patches and cutaneous nodular masses on the head, face, trunk, and limbs which had been increasing in number and size for more than 30 years. She had no symptoms of itchy skin. Biopsy of a nodular upper limb mass 10 years previously was consistent with neurofibroma. She had undergone laparoscopic cholecystectomy for a gallbladder stone 10 years previously and partial ileal resection for a strangulated bowel obstruction associated with adhesions 8 years previously. She had no history of hypertension, diabetes mellitus, or heart disease.
The patient had no history of smoking or drinking. One sister and one brother had NF1.
The patient exhibited soft cutaneous nodular masses of 0.2 to 3 cm in diameter on the head, face, trunk, and limbs as well as multiple café-au-lait patches (Figure 1). Examinations of the eyes, lungs, and heart were normal. Hearing testing was normal. Freckles were seen in the axilla and inguinal region. No lymphadenopathy was detected in the neck, axilla, or inguinal region. The abdomen was soft and without tenderness or palpable masses. Bowel sounds were normal. Digital rectal exam was unremarkable.
White blood cell count was 7.2 × 109/L. Red blood cell count was 4.45 × 1012/L. Hemoglobin concentration was 136 g/L. Platelet count was 260.0 × 109/L. C-reactive protein concentration was 3.65 mg/L. Fecal occult blood testing was negative. Concentrations of carcinoembryonic antigen, cancer antigen 125, and carbohydrate antigen 19-9 were all within normal range.
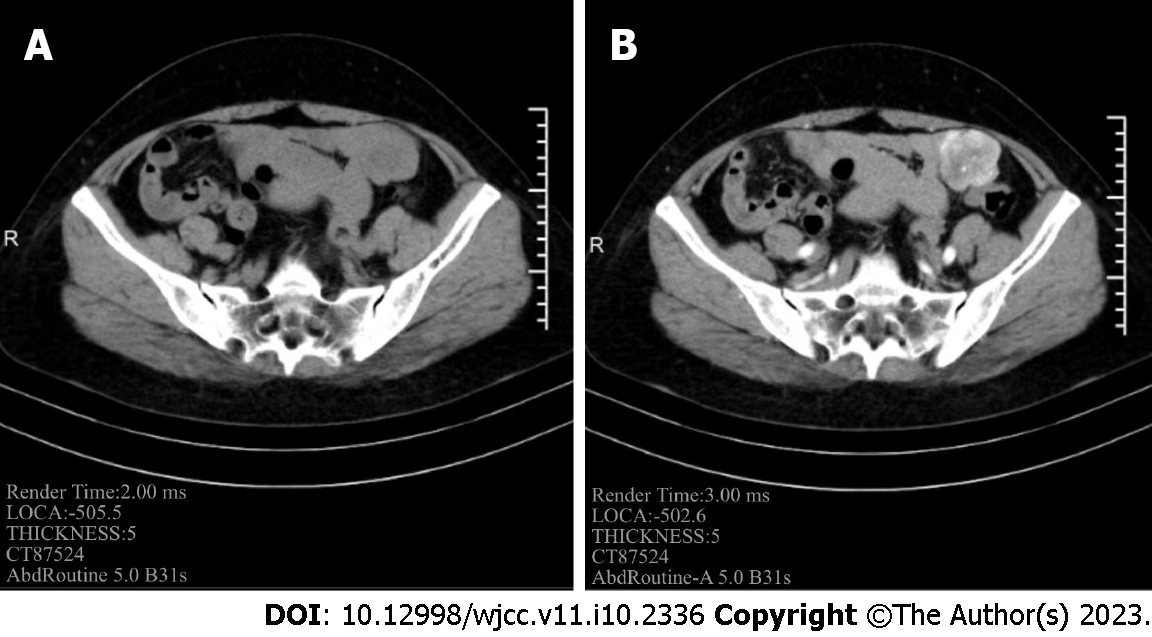
Contrast-enhanced computed tomography (CT) of the abdomen showed an irregular cystic mass with an oblique diameter of approximately 44 mm in the left pelvis. Its solid component exhibited contrast enhancement (Figure 2) and had no clear border with the adjacent intestinal wall. GIST of the small intestine or solitary fibrous tumor was suspected.
NF1 with GISTs.
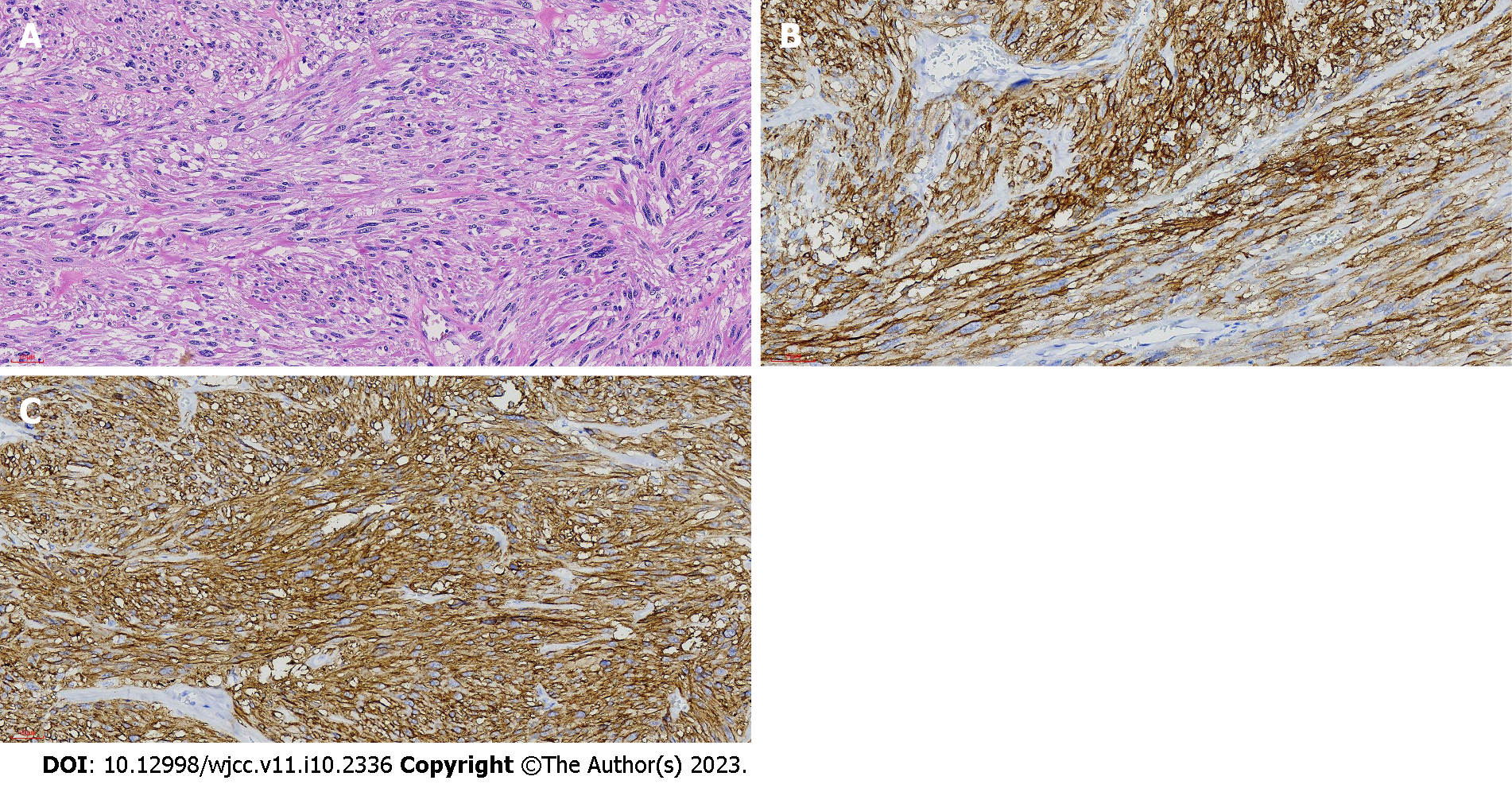
After multidisciplinary team consultation, laparoscopy was performed. Eight well-demarcated encapsulated masses were found in the jejunum between 20 and 120 cm from the Trietz ligament. The remainder of the small intestine was free of masses. The largest mass was approximately 4.5 cm in diameter and located 20 cm from the Trietz ligament. The others were 0.3 to 0.8 cm in diameter. The small intestine was approximately 200 cm in length and the original small intestine anastomosis from her previous surgery was open. Because resecting 100 cm of the intestine could cause short bowel syndrome, we elected to perform resection of the jejunal masses. A 6 cm median upper abdominal incision was made to raise the small intestine into the abdominal cavity. Partial jejunostomy was performed 20 cm from the Trietz ligament and the masses were resected (Figure 3). Histopathology of the 4.5 cm jejunal mass was consistent with GIST with hemorrhagic infarction. Mitosis count was < 5 in 50 high-power fields. Based on the modified National Institutes of Health criteria, the tumor was classified as low risk. The other masses were also diagnosed as GISTs. None exhibited invasive growth. CD34, CD117, and DOG-1 staining was positive (Figure 4). S-100 staining was negative. Ki-67 index was less than 5%. Genetic testing demonstrated wild type KIT and PDGFRA genes. The final diagnosis was multiple NF1-associated GISTs.
The patient was discharged on day 7 after surgery. No complications occurred. No sign of tumor recurrence or metastasis was present at the 6-mo follow-up. Annual follow-up by contrast-enhanced abdominal CT or magnetic resonance imaging is planned. Any small recurrent tumors in the small intestine will be removed enteroscopically.
The gene responsible for NF1 is located at chromosome 17q11.2. Mutations can result in inability to produce the corresponding neurofibromin protein. Deletion leads to abnormal activation of the RAS/RAF/MAP signaling pathway, which results in loss of neurofibromin’s tumor suppressor function and increases susceptibility to development of tumors such as neurofibroma, optic glioma, malignant peripheral nerve sheath tumor, neuroblastoma, pheochromocytoma, and breast cancer[1]. GIST is rare and develops in approximately 7% of all NF1 patients[4]. It originates from mesenchymal tissue of the gastrointestinal tract, usually in the stomach and small intestine, and pathology mainly shows spindle cells and epithelioid cells. KIT mutations are detected in approximately 90% of GISTs, while PDGFRA mutations are found in approximately 5%[5]. Mutations of these two genes are the main cause of GIST, although BRAF mutations are present in some cases. GISTs are multiple in less than 5% of cases. Multiple GISTs are frequently associated with NF1 and familial GIST. GIST is the most common non-neurological tumor in NF1 patients. Incidence of GIST is 45 times higher in NF1 patients than in the general population. Unlike sporadic GIST, NF1-associated GIST develops at a younger age and is more common in women[6]; moreover, most tumors are multiple and located in the small intestine (90%)[7]. Pathologically, NF1-associated GIST consists predominantly of spindle cells and behaves as a low-grade malignancy with slow progression. KIT and PDGFRA mutations are frequently not present, suggesting a different pathogenesis from the sporadic form. Prognosis is better for NF1-associated GIST than the sporadic form (Table 1). GIST may present with abdominal pain, nausea and vomiting, gastrointestinal bleeding, anemia, abdominal mass, intestinal obstruction. However, it may be asymptomatic and found incidentally. Since NF1-associated GIST is usually located in the small intestine, gastroscopy is often negative. Therefore, middle-aged and elderly NF1 patients with gastrointestinal symptoms should undergo abdominal CT or magnetic resonance imaging[8]. Abdominal CT can reveal tumor size, location, border, and relationship with surrounding tissues, as well as metastases. Capsule endoscopy and small bowel endoscopy are also commonly used for diagnosis, which is confirmed via pathologic examination and immunohistochemical and genetic testing of a tumor specimen. CD117 and CD34 are positive in most cases of NF1-associated GIST, which facilitates diagnosis[9]. Tumor location, size, and mitotic activity are GIST prognostic factors. Ki-67 index is associated with recurrence and survival[10]. Imatinib is ineffective in most NF1-associated GISTs because of their lack of KIT and PDGFRA mutations[11]. Surgical resection of tumor or bowel segment is the first choice of treatment, depending on tumor size. Usually a resection margin of 1 cm is sufficient[12]. Complete removal without lymph node dissection is recommended[13]. Even after radical surgical treatment, approximately 50% of patients experience relapse[14]. The recurrence rate in patients with NF1 is similar to that in patients with sporadic GIST. Laparoscopic surgery is as effective as open surgery and associated with a similar rate of recurrence; however, procedure-related pain is less, recovery is faster, and length of hospital stay is shorter.
| NF1 with GIST | Sporadic GIST | |
| Age of onset | Young | Older |
| Most common sites | Small intestine (90%) | Stomach (60%-70%) |
| Number of tumors | Often multiple | Often solitary |
| Nuclear fission rate | Low | High |
| KIT and PDGFRA gene mutation | Rare | Common |
| Imatinib treatment | Ineffective | Effective |
| Disease progression | Slow | Quick |
| Postoperative recurrence rate | Low | High |
| Prognosis | Good | Common |
GIST should be suspected in NF1 patients with gastrointestinal symptoms. NF1 should be considered in patients with multiple GISTs located outside the stomach. A thorough medical history and physical examination are imperative, and abdominal CT, capsule endoscopy, or small bowel endoscopy should be performed. Laparoscopy may also be performed for both diagnosis and treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Almousa M, Syria; Otani K, Japan; Wani I, India S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575-578. [PubMed] |
| 3. | Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 482] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 4. | Nishida T, Tsujimoto M, Takahashi T, Hirota S, Blay JY, Wataya-Kaneda M. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J Gastroenterol. 2016;51:571-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Takazawa Y, Sakurai S, Sakuma Y, Ikeda T, Yamaguchi J, Hashizume Y, Yokoyama S, Motegi A, Fukayama M. Gastrointestinal stromal tumors of neurofibromatosis type I (von Recklinghausen's disease). Am J Surg Pathol. 2005;29:755-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 321] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013;2013:398570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Ylä-Outinen H, Loponen N, Kallionpää RA, Peltonen S, Peltonen J. Intestinal tumors in neurofibromatosis 1 with special reference to fatal gastrointestinal stromal tumors (GIST). Mol Genet Genomic Med. 2019;7:e927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Pan D, Liang P, Xiao H. Neurofibromatosis type 1 associated with pheochromocytoma and gastrointestinal stromal tumors: A case report and literature review. Oncol Lett. 2016;12:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Mandalà S, Lupo M, Guccione M, La Barbera C, Iadicola D, Mirabella A. Small bowel gastrointestinal stromal tumor presenting with gastrointestinal bleeding in patient with type 1 Neurofibromatosis: Management and laparoscopic treatment. Case report and review of the literature. Int J Surg Case Rep. 2021;79:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | von Mehren M, Joensuu H. Gastrointestinal Stromal Tumors. J Clin Oncol. 2018;36:136-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 12. | Tian H, Wu XD, Ma Y, Jin SZ, Song SW. [Multiple gastrointestinal stromal tumors of the small intestine with gastrointestinal bleeding: a case report]. Zhonghua Putongwaike Zazhi. 2019;34:639. [DOI] [Full Text] |
| 13. | Kim JJ, Lim JY, Nguyen SQ. Laparoscopic resection of gastrointestinal stromal tumors: Does laparoscopic surgery provide an adequate oncologic resection? World J Gastrointest Endosc. 2017;9:448-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Xu MM, Angeles A, Kahaleh M. Endoscopic full-thickness resection of gastric stromal tumor: one and done. Endoscopy. 2018;50:E42-E43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |