Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2301
Peer-review started: December 4, 2022
First decision: February 7, 2023
Revised: February 18, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: April 6, 2023
Processing time: 115 Days and 22.7 Hours
Lichenoid amyloidosis (LA) is a subtype of primary cutaneous amyloidosis characterized by persistent multiple groups of hyperkeratotic papules, usually on the lower leg, back, forearm, or thigh. LA may be associated with several skin diseases, including atopic dermatitis (AD). The treatment of LA is considered to be difficult. However, as there is some overlap in the etiopathogenesis of LA and AD, AD treatment may also be effective for LA.
Case 1: A 70-year-old man was diagnosed with severe AD with LA based on large dark erythema and papules on the trunk and buttocks and dense hemispherical millet-shaped papules with pruritus on the extensor side of the lower limbs. He had a long history of the disease (8 years), with repeated and polymorphic skin lesions. Given the poor efficacy of traditional treatments, this patient was recommended to receive dupilumab treatment. At the initial stage, 300 mg was injected subcutaneously every 2 wk. After 28 wk, the drug interval was extended to 1 mo due to the pandemic. Follow-up observations revealed that the patient reached an Eczema Area Severity Index of 90 (skin lesions improved by 90% compared with the baseline) by the end of the study. Moreover, Investigator's Global Assessment score was 1, and scoring atopic dermatitis index and numeric rating scale improved by 97.7% and 87.5% compared with the baseline, respectively, with LA skin lesions having largely subsided. Case 2: A 30-year-old woman was diagnosed with severe AD with LA, due to dense and substantial papules on the dorsal hands similar to changes in cutaneous amyloidosis, and erythema and papules scattered on limbs and trunk with pruritus, present for 25 years. After 16 wk of dupilumab treatment, she stopped, and skin lesions completely subsided, without recurrence since the last follow-up.
Dupilumab shows rational efficacy and safety in the treatment of severe AD with LA, in addition to benefits in the quality of life of the patients.
Core Tip: Although cases of atopic dermatitis (AD) with lichenoid amyloidosis (LA) are not uncommon, there are few reports on the use of AD treatment for LA. In this paper, two adult patients with severe AD and LA are described. Treatment with dupilumab significantly improved the AD and LA skin lesions with no adverse reactions, confirming the efficacy and safety of dupilumab in the treatment of AD with LA, and providing a reference for clinically similar diseases.
- Citation: Zhao XQ, Zhu WJ, Mou Y, Xu M, Xia JX. Dupilumab for treatment of severe atopic dermatitis accompanied by lichenoid amyloidosis in adults: Two case reports. World J Clin Cases 2023; 11(10): 2301-2307
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2301.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2301
Atopic dermatitis (AD), a chronic recurrent disease driven by type-2 inflammation, is clinically characterized as chronic eczematoid manifestations[1]. Lichenoid amyloidosis (LA) is a subtype of primary cutaneous amyloidosis, which is characterized by persistent multiple groups of hyperkeratotic papules, usually in the lower leg, back, forearm, or thigh[2]. It has been demonstrated that primary cutaneous amyloidosis is associated with AD, with an elusive mechanism[3-5]. Dupilumab, a human monoclonal antibody targeting for interleukin (IL)-4/13 receptor α chain, can inhibit the development of type-2 inflammation, and has shown promising efficacy and safety in the treatment of medium and severe AD[6,7]. Currently, there are cases reported that dupilumab may hold promise in the treatment of AD-related LA, but with minor cases, and few studies focused on the changes of skin lesions after drug withdrawal and the efficacy and safety of the long-term application. This paper reports two cases of severe AD with LA in adults. The patients were treated with subcutaneous dupilumab injection after ineffective traditional treatment. In case 1, the skin lesions of AD and LA virtually disappeared after long-term application of dupilumab, with promising efficacy and safety. In case 2, the skin lesions of AD and LA skin lesions disappeared after dupilumab treatment, without recurrence after discontinuation.
Case 1: On August 12, 2021, a 70-year-old man was admitted to our department due to the appearance of large dark erythematous papules on his trunk and buttocks, together with dense hemispherical millet granular miliary papules with itching on the extensor sides of his lower limbs.
Case 2: On December 21, 2020, a 30-year-old woman was admitted to our department due to substantial dense papules on the back of her hands and scattered erythema, papules, and itching on her limbs and torso.
Case 1: A 70-year-old man developed erythema and papules on his lower limbs 8 years ago, and pruritus. He was diagnosed with chronic eczema at a local hospital and treated with a Chinese medicine decoction. He stopped taking this medicine after most skin lesions subsided. The patient subsequently complained of mildly symptomatic recurrent skin lesions following the cessation of treatment. The patient did not seek further treatment at this time. Three years ago, he was diagnosed with AD at the Dermatology Department of the Second Hospital of Jilin University. Treatment with a topical glucocorticoid and oral levocetirizine controlled the skin lesions and prevented recurrence. However, two years ago, the skin lesions became aggravated without obvious inducement and spread to the entire body. Dense, hemispherical, millet-shaped papules appeared on the extensor surfaces of both lower limbs. The patient’s condition did not improve despite self-medication with traditional Chinese medicine and a topical glucocorticoid and the patient experienced expanded skin lesions and sleep severely affected by pruritus. In August 2021, he visited our department for further treatment.
Case 2: A 30-year-old woman developed several coin-sized erythema lesions and papules on her hips and both lower limbs along with pruritus without obvious inducement 25 years ago. She was diagnosed with chronic eczema at a local hospital and was treated with a topical glucocorticoid and oral loratadine. Following treatment, the skin lesions partially subsided, with occasional recurrence. Two years ago, the patient developed dense erythema and papules covered by small scales, keratosis, and chapped skin on the dorsal side of both hands. After self-medication with antihistamines and a topical glucocorticoid, the skin lesions showed no improvement and gradually progressed to the elbows and trunk. Concomitantly, her pruritus severely affected her sleep. In December 2020, she visited our department for further treatment.
Case 1: The patient’s past history was unremarkable.
Case 2: The patient had a history of allergic rhinitis for 5 years, which aggravated in autumn every year. Oral loratadine or levocetirizine was required to relieve the allergic rhinitis.
Case 1: There was no family history of similar disease.
Case 2: The patient’s grandfather and aunt suffered from allergic rhinitis.
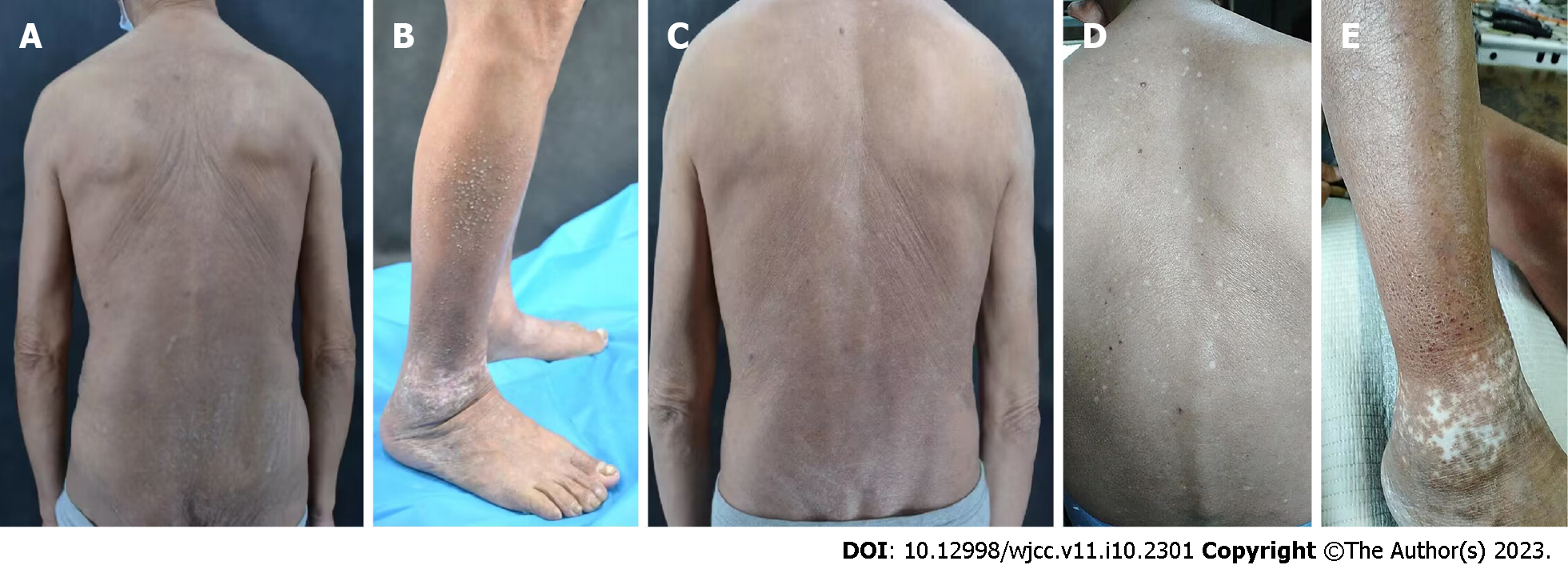
Case 1: Systemic examination revealed no obvious abnormalities. The dermatological conditions included dryness of the skin over the whole body, while the trunk and buttocks showed extensive dark erythema and papules. The skin lesions were thickened, lichenized, and pruritic. Similarly, dense hemispherical millet-shaped papules were observed on the lateral sides of both lower legs, with scattered erythema and papules on both feet (Figure 1A and B). The scoring atopic dermatitis index (SCORAD), Eczema Area Severity Index (EASI), numeric rating scale (NRS), and Investigator's Global Assessment (IGA) scores were 60.8, 13.5, 8, and 4, respectively.
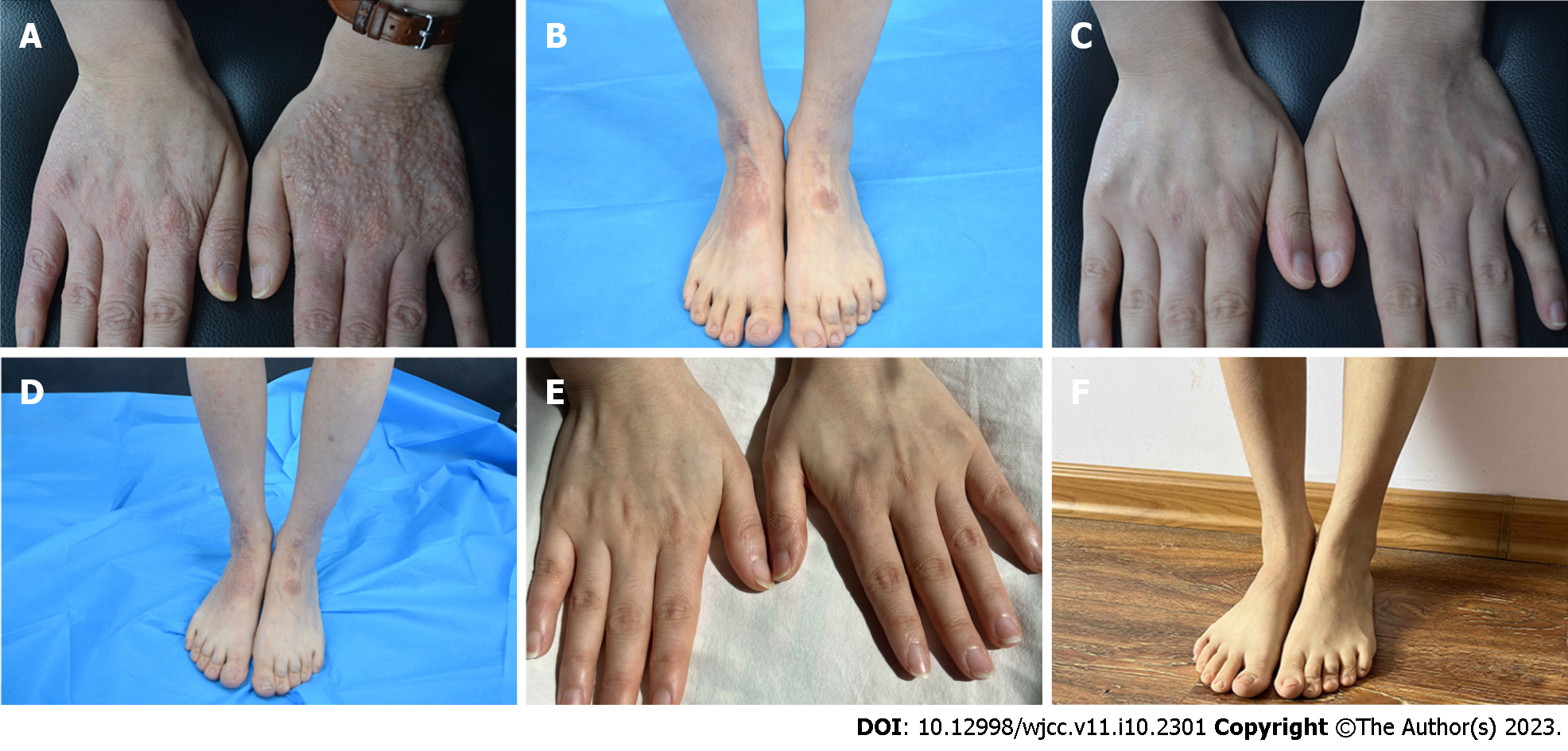
Case 2: Systemic examination revealed no obvious abnormalities. Dermatological conditions included dry skin affecting the entire body, chapping erythema covered by a few scales affecting the palms of both hands, densely filled millet grain-to-sorghum grain-sized papules on the dorsal surface of both hands with keratinized surfaces reminiscent of cutaneous amyloidosis, thick and lichenized lesions on both elbows and feet, erythema and papules scattered on the trunk, and scratches and blood scabs on the buttocks (Figure 2A and B). SCORAD, EASI, NRS, and IGA scores were 53.1, 13.1, 7, and 4, respectively.
Case 1: Routine blood analysis showed an eosinophil count of 1.96 × 109/L.
Case 2: Allergen-specific immunoglobulin E: Artemisia > 100 IU/mL, positive value: 6.0; ragweed: 9.4 IU/mL, positive value: 3.4.
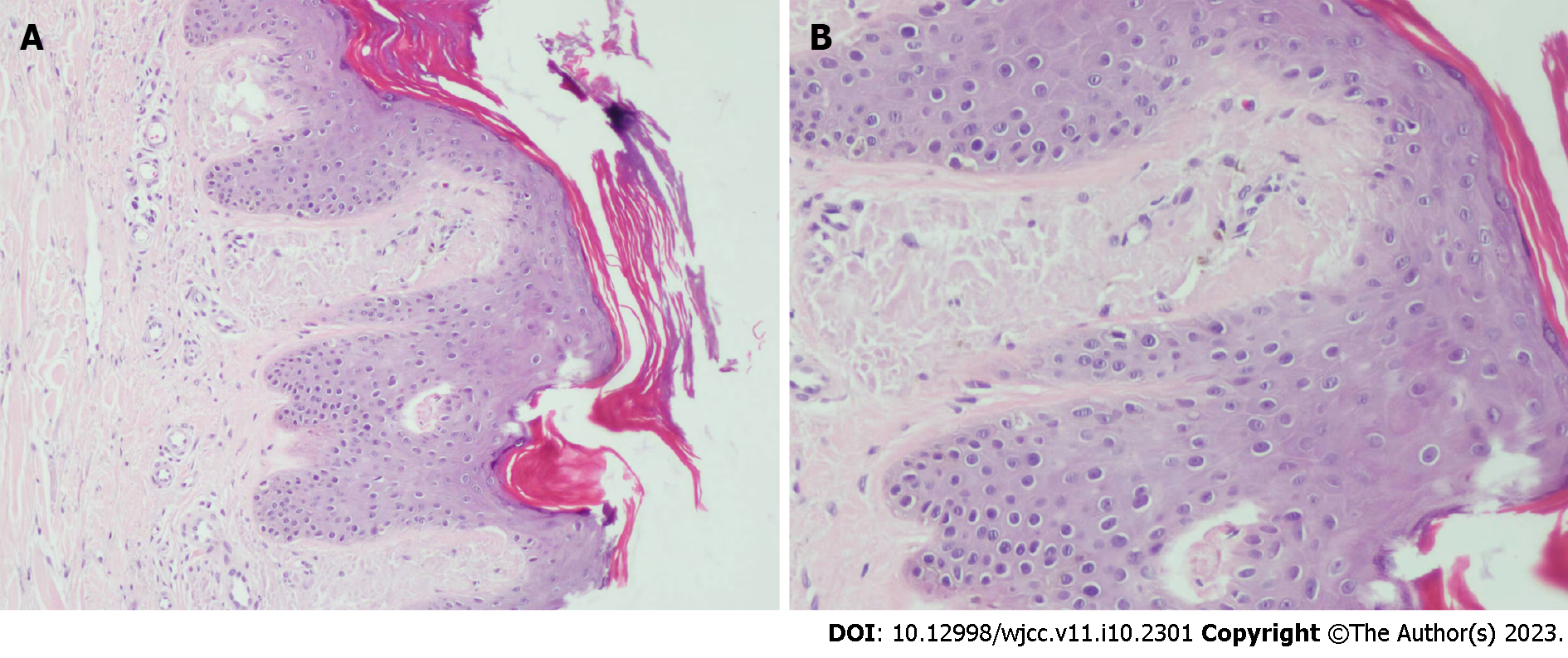
Case 1: Histopathology of dense papules on the extensor surface of the lower leg showed amorphous amyloid lump deposition with mild eosinophilic staining in the dermal papillary layer, which was consistent with amyloidosis (Figure 3).
Case 2: No histological examination was performed because the patient refused invasive examination.
Severe AD accompanied by LA.
A designated staff assigned to cases 1 and 2 independently collected the basic information, clinical features, medical history, drug allergy history, and family history of the patients and established personal AD files. Both patients received an initial subcutaneous injection of 600 mg dupilumab followed by 300 mg every 2 wk. During the treatment period, the patients were followed regularly, during which the skin lesions were photographed, SCORAD, EASI, NRS, and IGA were recorded, and the incidence of any adverse reactions was noted.
Case 1: The patient was first treated with dupilumab on August 21, 2021. The pruritus was relieved after 1 wk and the skin lesions improved after 4 wk. During the treatment, all scores continued to decline, without adverse reactions. After 16 wk of treatment, most skin lesions had improved, and papules on both legs had partially flattened. In the 28th week (February 8, 2022), most of the skin lesions had subsided with some pigmentation remaining on the trunk and buttocks, without recurrence. The skin over the entire body was still dry, and the patient was advised to use external moisturizing emollients regularly. SCORAD, EASI, NRS, and IGA scores were 7.1, 2.3, 2, and 1, respectively (Figure 1C). Due to the access limitations associated with the COVID-19 pandemic, the subcutaneous injection of dupilumab was adjusted to 300 mg every month. The patient was treated with dupilumab for a total of 55 wk, after which the skin lesions of the whole body had largely subsided, without adverse reactions during the treatment (Figure 1D and E). SCORAD, EASI, NRS, and IGA scores were 1.4, 1.2, 1, and 1, respectively, reaching EASI90 (skin lesions improved by 90% from baseline). Notably, SCORAD and NRS scores improved by 97.7% and 87.5% from baseline, respectively.
Case 2: The patient was first treated with dupilumab on December 21, 2020. The pruritus was relieved after 1 wk, and the skin lesions improved after 2 wk of treatment. During the treatment, all scores continued to decline, without adverse reactions. The final injection was administered on April 9, 2021, by which the patient had received a total of eight dupilumab injections. At this point, the skin lesions had completely subsided and with each score reaching 0 (Figure 2C and D). The last follow-up was done 1.5 years since the termination of drug treatment, and no recurrence of skin lesions was observed. The symptoms of allergic rhinitis were also significantly improved, and no further medical treatment was deemed necessary (Figure 2E and F).
AD, a chronic recurrent disease driven by type-2 inflammation, is clinically characterized by chronic eczematoid manifestations with pruritus, which can be diagnosed according to the previously reported clinical criteria[1,8]. In addition to eczematoid manifestations, the skin lesions of the two patients in this study exhibited lichen amyloidosis, a subtype of primary cutaneous amyloidosis which manifested as numerous persistent groups of hyperkeratotic papules, usually on the lower leg, back, forearm or thigh[2]. It was first proposed by Shanon et al[9] in 1970 that cutaneous amyloidosis was related to atopic disease. Currently, studies have found that AD is related to primary cutaneous amyloidosis; however, the exact mechanism remains elusive[3-5]. Primary cutaneous amyloidosis can be caused by the deposition of skin amyloid protein through the "itching-scratching" cycle. Since AD patients generally have pruritus, it is currently believed that the destruction of the skin barrier in AD through chronic scratching and friction can lead to the damage and death of keratinocytes and the subsequent deposition of amyloid protein, resulting in amyloidosis[10,11]. The treatment of AD is typically individualized and different treatment methods are selected according to the severity of the disease. Moderate and severe AD patients can be treated with topical drugs, systemic drugs, and immunosuppressants[12]. In our study, two patients with a long history of repeated dermatological illness were selected and treated with dupilumab based on the inefficacies of the traditional medicines that had been trialed in these patients previously. Dupilumab is a human monoclonal antibody that targets the receptor for Th2 cytokines IL-4/13 (IL-4 receptor α chain), thereby inhibiting type-2 inflammation by blocking the pro-inflammatory effects of IL-4/13[6]. It has been demonstrated that dupilumab effectively inhibits type-2 pathway genes as early as the 4th week of treatment, and significantly improves the clinical efficacy indexes of AD in the 16th week of treatment[13].
Currently, several treatments exist for primary cutaneous amyloidosis associated with AD, including oral antihistamines, CO2 laser, cyclosporine, and acitretin. However, it is proven difficult to achieve complete skin lesion regression through these methods[14-17]. Primary cutaneous amyloidosis is related to pruritus, and IL-4 and IL-13 have been shown to promote pruritus via their role in the type-2 inflammation pathway. Thus, dupilumab may relieve pruritus and inhibit the development of amyloidosis by blocking the effects of IL-4 and IL-13[18]. However, the specific mechanism of dupilumab in the treatment of primary cutaneous amyloidosis has not been clarified. Previous studies have detected increased expression of IL-31 in the skin lesions of patients with familial primary cutaneous amyloidosis. IL-31 is also one of the pro-pruritus factors secreted by Th2 cells. Dupilumab can indirectly inhibit the secretion of IL-31 by Th2 cells by inhibiting type-2 inflammation, and therefore may exert beneficial effects in primary cutaneous amyloidosis through this mechanism[19,20]. To date, there is one case where remission of AD-related lichen amyloidosis skin lesions was achieved after treatment with dupilumab, indicating that dupilumab can have a therapeutic benefit in AD-related amyloidosis[11]. In this study, case 1 experienced reduced pruritus after one week of dupilumab. To date, the skin lesions of lichen amyloidosis have largely subsided in this patient, without adverse reactions, demonstrating that dupilumab has a rational efficacy against AD-related lichen amyloidosis, and long-term treatment can achieve skin lesion control with high safety. In case 2, the skin lesions of lichen amyloidosis completely subsided 16 wk after dupilumab was applied, and have not recurred since the termination of treatment, indicating that dupilumab may inhibit the recurrence of AD-related lichen amyloidosis on the basis of its therapeutic effect.
In this study, the treatment of AD with dupilumab substantially reduced the appearance of skin lesions in two patients with lichen amyloidosis. Long-term treatment of dupilumab for AD and lichen amyloidosis in case 1 was deemed effective and safe. In case 2, there was no recurrence of skin lesions of AD and lichen amyloidosis following termination of treatment. Moreover, compared to the previous traditional treatments, dupilumab was more effective and deemed safe in the treatment of AD and its associated amyloidosis. The above two cases further indicate that the treatments indicated for AD may also have rational efficacy against AD-related lichen amyloidosis.
We thank the patients and their family members for their support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Dermatopathology Group, Pathology Branch of Chinese Medical Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rolla G, Italy; Teragawa H, Japan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Ständer S. Atopic Dermatitis. N Engl J Med. 2021;384:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 2. | Hamie L, Haddad I, Nasser N, Kurban M, Abbas O. Primary Localized Cutaneous Amyloidosis of Keratinocyte Origin: An Update with Emphasis on Atypical Clinical Variants. Am J Clin Dermatol. 2021;22:667-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lee DD, Huang CK, Ko PC, Chang YT, Sun WZ, Oyang YJ. Association of primary cutaneous amyloidosis with atopic dermatitis: a nationwide population-based study in Taiwan. Br J Dermatol. 2011;164:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Chia B, Tan A, Tey HL. Primary localized cutaneous amyloidosis: association with atopic dermatitis. J Eur Acad Dermatol Venereol. 2014;28:810-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Akar A, Taştan HB, Demiriz M, Erbil H. Lack of effect of cyclosporine in lichen amyloidosis associated with atopic dermatitis. Eur J Dermatol. 2002;12:612-614. [PubMed] |
| 6. | Gooderham MJ, Hong HC, Eshtiaghi P, Papp KA. Dupilumab: A review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78:S28-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 7. | Halling AS, Loft N, Silverberg JI, Guttman-Yassky E, Thyssen JP. Real-world evidence of dupilumab efficacy and risk of adverse events: A systematic review and meta-analysis. J Am Acad Dermatol. 2021;84:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 8. | Liu P, Zhao Y, Mu ZL, Lu QJ, Zhang L, Yao X, Zheng M, Tang YW, Lu XX, Xia XJ, Lin YK, Li YZ, Tu CX, Yao ZR, Xu JH, Li W, Lai W, Yang HM, Xie HF, Han XP, Xie ZQ, Nong X, Guo ZP, Deng DQ, Shi TX, Zhang JZ. Clinical Features of Adult/Adolescent Atopic Dermatitis and Chinese Criteria for Atopic Dermatitis. Chin Med J (Engl). 2016;129:757-762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Shanon J. Cutaneous amyloidosis associated with atopic disorders. Dermatologica. 1970;141:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Fujisawa T, Shu E, Ikeda T, Seishima M. Primary localized cutaneous nodular amyloidosis that appeared in a patient with severe atopic dermatitis. J Dermatol. 2012;39:312-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Aoki K, Ohyama M, Mizukawa Y. A case of lichen amyloidosis associated with atopic dermatitis successfully treated with dupilumab: A case report and literature review. Dermatol Ther. 2021;34:e15005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 12. | Goh MS, Yun JS, Su JC. Management of atopic dermatitis: a narrative review. Med J Aust. 2022;216:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 13. | Guttman-Yassky E, Bissonnette R, Ungar B, Suárez-Fariñas M, Ardeleanu M, Esaki H, Suprun M, Estrada Y, Xu H, Peng X, Silverberg JI, Menter A, Krueger JG, Zhang R, Chaudhry U, Swanson B, Graham NMH, Pirozzi G, Yancopoulos GD, D Hamilton JD. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:155-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 466] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 14. | Kang MJ, Kim HS, Kim HO, Park YM. A case of atopic dermatitis-associated lichen amyloidosis successfully treated with oral cyclosporine and narrow band UVB therapy in succession. J Dermatolog Treat. 2009;20:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Koh WS, Oh EH, Kim JE, Ro YS. Alitretinoin treatment of lichen amyloidosis. Dermatol Ther. 2017;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 16. | Chu H, Shin JU, Lee J, Park CO, Lee KH. Successful treatment of lichen amyloidosis accompanied by atopic dermatitis by fractional CO(2) laser. J Cosmet Laser Ther. 2017;19:345-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Oiso N, Yudate T, Kawara S, Kawada A. Successful treatment of lichen amyloidosus associated with atopic dermatitis using a combination of narrowband ultraviolet B phototherapy, topical corticosteroids and an antihistamine. Clin Exp Dermatol. 2009;34:e833-e836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Moniaga CS, Tominaga M, Takamori K. The Pathology of Type 2 Inflammation-Associated Itch in Atopic Dermatitis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Schreml S. Is targeting interleukin-31 a cure for the itch? Br J Dermatol. 2016;174:1192-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |