Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2290
Peer-review started: December 2, 2022
First decision: January 17, 2023
Revised: January 30, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 6, 2023
Processing time: 118 Days and 2.3 Hours
Hypoparathyroidism, which can be sporadic or a component of an inherited syndrome, is the most common cause of hypocalcemia. If hypocalcemia is accompanied by other electrolyte disturbances, such as hypokalemia and hypomagnesemia, then the cause, such as renal tubular disease, should be carefully identified.
An 18-year-old female visited our clinic because of short stature and facial deformities, including typical phenotypes, such as low ear position, depression of the nasal bridge, small hands and feet, and loss of dentition. The lab results suggested normal parathyroid hormone but hypocalcemia. In addition, multiple electrolyte disturbances were found, including hypokalemia, hypocalcemia and hypomagnesemia. The physical signs showed a short fourth metatarsal bone of both feet. The X-ray images showed cortical thickening of long bones and narrowing of the medulla of the lumen. Cranial computed tomography indicated calcification in the bilateral basal ganglia. Finally, the genetic investigation showed a de novo heterogenous mutation of “FAM111A” (c. G1706A:p.R569H). Through a review of previously reported cases, the mutation was found to be the most common mutation site in Kenny-Caffey syndrome type 2 (KCS2) cases reported thus far (16/23, 69.6%). The mutation was slightly more prevalent in females than in males (11/16, 68.8%). Except for hypocalcemia, other clinical manifestations are heterogeneous.
As a rare autosomal dominant genetic disease of hypoparathyroidism, the clinical manifestations of KCS2 are atypical and diverse. This girl presented with short stature, facial deformities and skeletal deformities. The laboratory results revealed hypocalcemia as the main electrolyte disturbance. Even though her family members showed normal phenotypes, gene detection was performed to find the mutation of the FAM111A gene and confirmed the diagnosis of KCS2.
Core Tip: The FAM111A mutation was more likely to be a de novo mutation. Since the patient was an adult at the time of consultation, long-term follow-up treatment and observation of electrolyte status is necessary. Moreover, the fertility of the patient and the genetics of her offspring should be determined.
- Citation: Yuan N, Lu L, Xing XP, Wang O, Jiang Y, Wu J, He MH, Wang XJ, Cao LW. Clinical and genetic features of Kenny-Caffey syndrome type 2 with multiple electrolyte disturbances: A case report. World J Clin Cases 2023; 11(10): 2290-2300
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2290.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2290
Calcium ions (Ca2+) are essential to life, participating in many pathophysiological processes, such as the synthesis and release of neurotransmitters, the synthesis and secretion of hormones, blood coagulation, muscle contraction, nerve activity and bone mineralization. Under normal circumstances, parathyroid hormone and vitamin D maintain normal serum calcium concentrations. One of the most common causes of hypocalcemia is hypoparathyroidism, which can be transient or persistent. There are also various causes, of which the most common cause of chronic hypoparathyroidism is thyroid surgery, accounting for approximately 75% of cases[1]. Nonoperative causes include (1) autoimmunity: Autoimmune polyendocrine syndrome type 1; and (2) genetic disorders: Familial isolated hypoparathyroidism, autosomal dominant hypocalcemia, and complex disorders, which include hypothyroidism and abnormal magnesium metabolism. Hypothyroidism can be part of the following complex disorders: DiGeorge syndrome, hypoparathyroidism, sensorineural deafness, renal dysplasia (HDR) syndrome, Kenny-Caffey syndrome (KCS), Kearns-Sayre syndrome, mitochondrial encephalomyopathy with lactic acidosis and stroke-like attack syndrome, mitochondrial trifunctional protein deficiency syndrome, and Sanjad-Sakati syndrome (SSS). Hypoparathyroidism is often difficult to diagnose because of its complicated and heterogeneous manifestations, which require careful differential diagnosis depending on clinical manifestations, laboratory examinations and imaging features. DiGeorge syndrome type 1, or 22q11.2 deletion syndrome, is an autosomal dominant genetic disease caused by heterozygous microdeletions on chromosome 22; it can lead to immunodeficiency, abnormal cardiac outflow tracts and hypoparathyroidism. Deletion of the transcription factor TBX1 is necessary for parathyroid development and is considered to be a cause of hypoparathyroidism[2]. HDR syndrome is an autosomal dominant genetic disease caused by GATA3 gene deletion mutations. GATA3 is a transcription factor that plays an important role in parathyroid organogenesis[3]. KCS is a rare disease characterized by short stature, osteosclerotic bone dysplasia and hypoparathyroidism. The autosomal recessive form (type 1) is caused by the mutation of tubulin-specific chaperone protein E (TBCE)[4], whereas the autosomal dominant form (type 2) is caused by a FAM111A gene mutation[5]. This study presents a rare case of Kenny-Caffey syndrome type 2 (KCS2) with short stature, skeletal deformities and multiple electrolyte disturbances confirmed by genetic analysis. This study was approved by the Ethics Committee of Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical College, and written informed consent was obtained from the patient.
The proband was an 18-year-old female who was hospitalized with abnormal behavior for 3 d beginning October 19, 2021.
Three days before she visited the local psychiatric hospital, she suddenly refused to eat, accompanied by glazed eyes and with no definitive causes.
She was born by cesarean section at the 33rd week of her mother’s second pregnancy. Her weight was 3.8 kg [+1 standard deviation (SD)], and her height was 48 cm (-1 SD) at birth. Fontanel closure was delayed until she was 5 years old. During her childhood, she was always shorter than other children of the same age, but her body weight was overweight. She had occasional muscle tetany and limb numbness. Her studying ability in school was slightly poorer than that of her classmates. Menarche started at the age of 13 years, followed by irregular periods (cycle of 28-90 d).
Her parents were not consanguineous and were in good health. The growth and development of her two sisters were normal, and there was no family history of hereditary diseases.
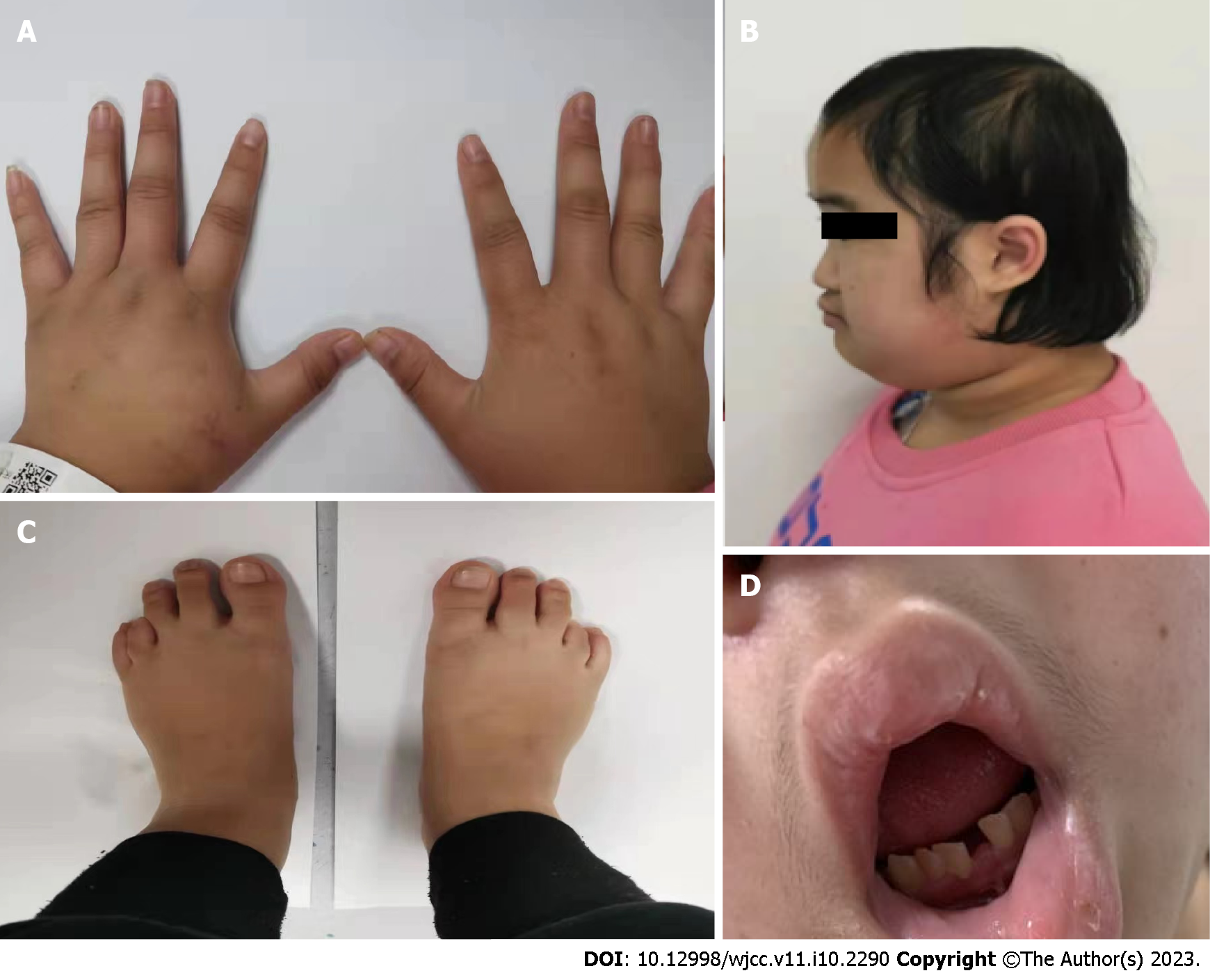
The patient’s blood pressure was 104/78 mmHg; her height was 132 cm (-5 SD), weight was 40 kg (-2 SD), waist circumference was 80 cm, and head circumference was 48 cm; and some peculiar clinical characteristics were observed, including cheek freckles, high anterior hairline, sparse scalp hair, prominent forehead, depressed nasal bridge, low-set ears, partially absent dentition, small mandible, slightly higher mandibular arch, increased quilt hair, stubby limbs, small hands and feet and short bilateral 4th toes (Figure 1). She had no cubitus valgus, no nail thickening or roughness and no scoliosis. Gynecological examination showed breast development at Tanner stage 4 and pubic hair at Tanner stage 4. Her Mini-Mental State Examination (MMSE) score was 28 points after her symptoms improved.
The patient had low calcium, low potassium, low magnesium, and high phosphorus levels. Her serum aspartate aminotransferase (AST) level was 94 U/L (reference range: 0 to 40), and her serum alanine transaminase (ALT) level was 70 U/L (reference range: 7 to 40). Serum potassium was 2.57 mmol/L (reference range: 3.5 to 5.3), chlorine was 96.4 mmol/L (reference range: 99.0 to 110.0), corrected calcium level was 1.06 mmol/L (reference range: 2.11 to 2.52), magnesium was 0.45 mmol/L (reference range: 0.75-1.02), and phosphorus was 1.86 mmol/L (reference range: 0.85 to 1.51). Details of laboratory findings are listed in Table 1. Therefore, she was referred to our hospital for further diagnosis and treatment.
| Tests | Measured value | Reference range |
| Serum potassium (mmol/L) | 2.56 | 3.5-5.3 |
| 24-h urinary potassium (mmol) | 46.82 | 25-100 |
| PH | 7.40 | 7.35-7.45 |
| Blood HCO3- (mmol/L) | 25 | 22-27 |
| ALD (pg/mL)1 | 355.586 | 40-310 |
| Renin (pg/mL)1 | 300.452 | 4-38 |
| Angiotensin II (pg/mL)1 | 329.740 | 49-310 |
| Serum cortisol (μg/dL)2 | 18.33 | 6.02-18.4 |
| Serum ACTH (pg/mL)2 | 13.72 | 7.2-63.3 |
| PTH (pg/mL) | 10 | 15-65 |
| 25-hydroxyvitamin D (ng/mL) | 17.61 | 30-100 |
| Corrected serum calcium (mmol/L) | 1.12 | 2.11-2.52 |
| Serum P (mmol/L) | 1.86 | 0.85-1.51 |
| Serum Mg (mmol/L) | 0.69 | 0.75-1.02 |
| 24-h urinary calcium (mmol/L) | 3.66 | < 6.75 |
| FT4 (pmol/L) | 16.2 | 12-22 |
| FT3 (pmol/L) | 4.43 | 3.1-6.8 |
| TSH (uIU/mL) | 0.8 | 0.27-4.2 |
| LH (uIU/mL) | 4.08 | 2.4-12.6 |
| FSH (uIU/mL) | 3.57 | 3.5-12.5 |
| PRL (uIU/mL) | 644.2 | 102-496 |
| E2 (pg/mL) | 52.93 | 12.4-233 |
| P (nmol/L) | 0.72 | 0.181-2.84 |
| T (nmol/L) | 1.76 | 0.29-1.67 |
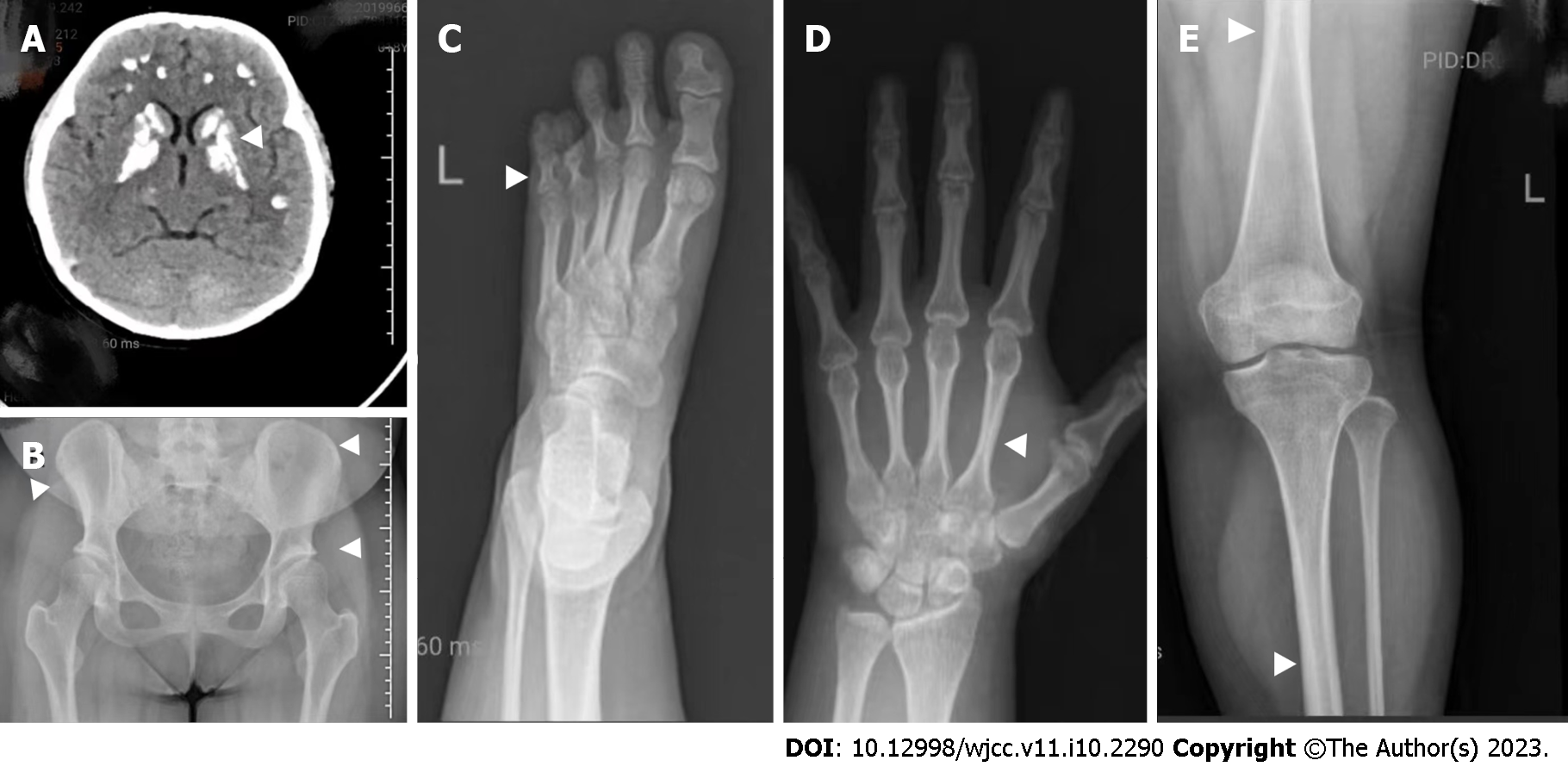
Ophthalmological examination revealed hyperopia. Visual acuity was 0.15 in the left eye and 0.8 in the right eye. No evidence of cataract or papilledema was observed. Her hearing test results were normal. Cranial computed tomography showed sporadic symmetrical calcifications in the cerebellar hemisphere, frontotemporal parietal lobe, basal ganglia, and thalamus. Thyroid ultrasonography showed a cystic-solid mixed nodule measuring approximately 2.3 mm × 1.2 mm in the right lobe, with a Thyroid Imaging Reporting and Data System grade of 2. X-rays of the left knee, anteroposterior pelvis, left hand, and both feet showed a smaller right ilium compared to the contralateral side, shallow acetabular fossa on both sides, and no obvious abnormalities in the bone structure of the remaining pelvis or the structures of the sacroiliac and hip joints on either side (Figure 2). No abnormal density was observed in the pelvis. The phalanges of the left little finger were short, with thickening of the cortex of the tubular bone and narrowing of the medulla. The 4th and 5th metatarsal bones and the corresponding phalanges of both feet were short and small. Ultrasonography of the pelvic cavity showed an anteverted uterus measuring approximately 2.7 cm × 1.9 cm × 3.0 cm. Uniform endometrial echogenicity was observed with no obvious tumor-like structures. The endometrial thickness was approximately 0.4 cm. The bilateral ovaries were normal in size, with no obvious space-occupying lesions in the bilateral adnexal areas.
Peripheral blood samples of the patient as well as her parents (who had no abnormal manifestations) were collected, and DNA analysis was performed after obtaining informed consent. This study was conducted according to the principles of the Declaration of Helsinki with the approval of the ethics committee of Nanchong Central Hospital. The samples were subjected to Sanger sequencing by polymerase chain reaction (PCR) and whole-exome sequencing.
First, DNA was extracted from the blood samples. Libraries were then constructed, and sequencing was performed on the Illumina platform. After obtaining the original sequencing results, analysis was performed with the given reference sequence or genome (GRCh37/hg19). The analysis included the following three steps: (1) Quality assessment of the sequencing data; (2) variation detection; and (3) variation screening and prediction of disease associations. Burrows–Wheeler Aligner was used to align the sequenced fragments to the reference genome, and Picard software was used to remove sequences generated by PCR duplication. After analyzing the patient gene sequencing results for single-nucleotide variants (SNVs) and insertions-deletions with reference to the hg19 genome, somatic mutation predictions were made on paired samples using MuTect, followed by annotation of the SNVs using the ANNOVAR program combined with the refGene annotation in the UCSC Genome Browser. Finally, the effects of SNV mutation sites in the exonic region on protein translation were annotated using SIFT and PolyPhen.
Sample collection: Peripheral venous blood was collected from the patient and her family members, anticoagulated with sodium citrate, and stored at -20 °C for subsequent use.
Genomic DNA extraction: Genomic DNA was extracted using a blood DNA extraction kit (QIAamp DNA Blood Mini Kit) per the manufacturer’s instructions.
PCR amplification: Exon 5 of FAM111A was amplified by PCR (Applied Biosystems Veriti® 96-Well Thermal Cycler, Applied Biosystems, United States; ABI3130XL genetic analyzer, Applied Biosystems, United States), and sequencing was performed. The primers were as follows: Forward, TGCTTGTGCTGTGATCCCTC; reverse, ATGCCTATGAAATAACAACTCC. The total volume of the PCR was 30 μL, including 3 μL of template DNA, 1.5 μL each of the forward and reverse primers, 15 μL of 2 × HieffTM PCR Master Mix (With Dye, Yeasen, China), and 9 μL of ddH2O.
Sequencing of the PCR products and analysis of DNA mutations: All sequencing results were compared against the known sequencing results of the genomic DNA of FAM111A in the NCBI Blast database.
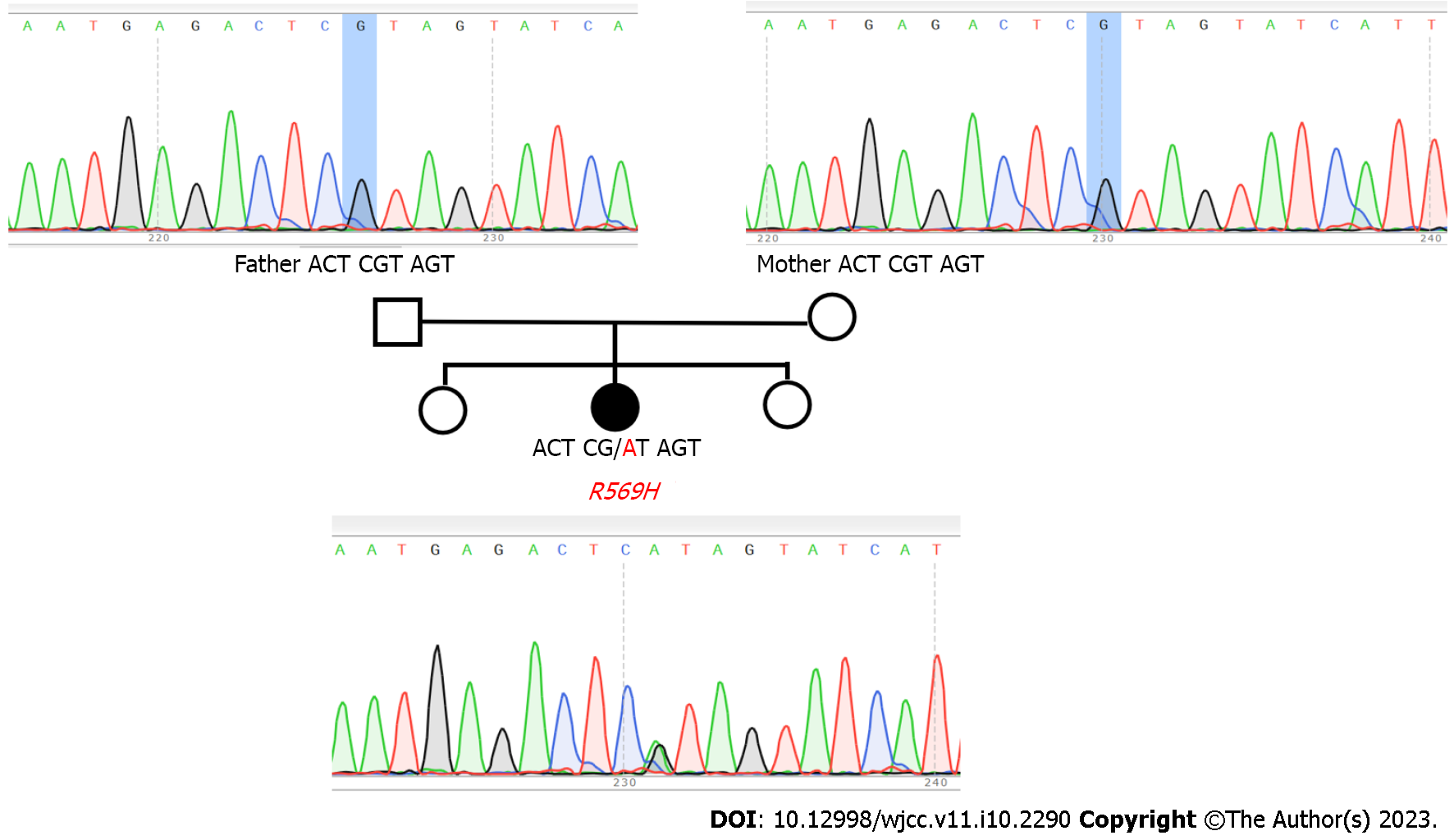
First, whole-exome sequencing showed a missense mutation in exon 5 (c.1706G>A) of FAM111A (NM_001142519) of the patient (Figure 3), which resulted in an amino acid substitution (R569H). The American College of Medical Genetics and Genomics rating of the variant was a variant of uncertain significance. No mutation in TBCE was observed. Neither of her parents, who showed no clinical manifestations, carried this gene mutation, indicating that this was a de novo mutation of a novel nonsynonymous coding variant, consistent with three cases of novel KCS2 mutations reported in Japan[6].
The patient was treated with calcium carbonate D3 (600 mg qd) and calcitriol (0.25 μg qd) after the diagnosis of primary hypoparathyroidism.
After intravenous treatment with magnesium sulfate improved the hypomagnesemia, the regimen was changed to potassium magnesium aspartate (2 tablets tid for continued magnesium supplementation). Follow-up after 2 mo of treatment showed a 24 h urinary calcium concentration of 1.69 mmoL (urine volume: 1300 mL), ALT concentration of 51 U/L, AST concentration of 47.2 U/L, blood potassium concentration of 3.41 mmol/L, blood calcium concentration of 2.10 mmol/L, blood phosphorus concentration of 2.0 mmol/L, and blood magnesium concentration of 0.57 mmol/L. The patient was generally in good condition, with no numbness of the extremities or muscle twitching.
In addition to previously reported cases, the clinical features and gene mutation sites of the patient with KCS2 are summarized in Table 2[5-17]. The 23 patients included 10 males and 13 females. The main clinical manifestations were as follows: (1) Signs such as short stature, polydactyly, developmental delay, abnormal liver function, eye abnormalities (hyperopia and pseudopapilledema), and normal intelligence; (2) hypocalcemia, hypomagnesemia, hyperphosphatemia, and low parathyroid hormone (PTH) level, with some patients showing abnormal liver function and hypothyroidism; and (3) calcification in the basal ganglia, with thickening of the cortex and medullary stenosis. Abnormal head circumference was also observed, combined with delayed closure of the anterior fontanelle. Sanger sequencing analysis of FAM111A showed that c.1706G>A (p.R569H) is the hotspot in most reported cases (16/23, 69.6%) and is more common in women (11/16, 68.8%). However, only three female patients were followed up after the age of 18 years (22, 25, 40 and 56 years of age), two of whom carried de novo mutations (the other was unknown).
| Year | Country | Fellow-up age | Sex | Inheritance patterns | Nucleotide change | Amino acid alteration | Ref. | |
| 1 | 2013 | Switzerland | 40 yr | F | De novo | c.1706G>A | p.R569H | [5] |
| 2 | India | 17 yr | M | NA | c.1706G>A | p.R569H | [5] | |
| 3 | Germany | 10 yr | M | NA | c.1706G>A | p.R569H | [5] | |
| 4 | Italy | 6 mo | F | De novo | c.1706G>A | p.R569H | [5] | |
| 5 | India | 7 yr | M | NA | c.1531T>C | p.Y511H | [5] | |
| 6 | 2014 | Japan | 10 yr | F | De novo | c.1706G>A | p.R569H | [6] |
| 7 | Japan | 16 yr | M | De novo | c.1706G>A | p.R569H | [6] | |
| 8 | Japan | 22 yr | F | De novo | c.1706G>A | p.R569H | [6] | |
| 9 | Japan | 38 yr | M | NA | c.1706G>A | p.R569H | [6] | |
| 10 | 2014 | Canada | 3 yr | F | AD | c.1706G>A | p.R569H | [7] |
| 11 | Canada | 25 yr | F | NA | c.1706G>A | p.R569H | [7] | |
| 12 | 2014 | America | NA | F | De novo | c.1706G>A | p.R569H | [8] |
| 13 | 2017 | Australia | 6 yr | F | NA | c.1622C> A | p.S541Y | [9] |
| 14 | 2019 | China | 10 yr | F | NA | c.1706G>A | p.R569H | [10] |
| 15 | 2020 | Brazil | 18 yr | M | De novo | c.1706G>A | p.R569H | [11] |
| 16 | 2020 | China | 23 yr | M | De novo | c.1621T>C | p.S541P | [12] |
| 17 | China | 23 yr | M | De novo | c.1621T>C | p.S541P | [12] | |
| 18 | 2020 | America | 20 mo | M | De novo | c.968G>A | p.G323E | [13] |
| 19 | 2020 | Brazil | 10 yr | F | De novo | c.1706G>A | p.R569H | [14] |
| 20 | 2021 | India | 9 yr | F | NA | g.58920847G>A | p.R569H | [15] |
| 21 | 2021 | Turkey | NA | M | AR | c.976T>A | NA | [16] |
| c.1714_1716del | ||||||||
| 22 | 2021 | China | 18 yr | F | De novo | c.1706G>A | p.R569H | Our patient |
| 23 | 2023 | Japan | 56 yr | F | NA | c.1706G>A | p.R569H | [17] |
In 1966, Kenny and Linarelli[18] reported a mother and child with severely short stature and hypo
Patients with KCS2 may also have different clinical manifestations due to different mutation sites in FAM111A. Even for mutations at the same site, the described phenotypes may differ. This report describes a case of a young woman who presented with a 3-d history of psychosis, in addition to severely short stature, thickening of the tubular cortex and medullary stenosis, delayed anterior fontanelle closure, hyperopia, missing dentition, hypokalemia, hypochloremia, uneven hip bone size, and overweight on top of hypocalcemia as a result of hypothyroidism. The patient did not have scoliosis. She also showed normal intelligence, with no familial genetic background. Whole-exome sequencing was performed on the proband based on the high suspicion given her clinical manifestations and laboratory imaging results. The relevant loci were also sequenced in samples from her parents. The results showed that the patient had a missense point mutation (c.1706G>A, p.R569H) in FAM111A, with no abnormal mutations in her parents. Therefore, the patient was considered to have a de novo mutation, which was consistent with previous reports[6].
The patient also showed microcephaly. Cavole et al[11] reported a case in Brazil of an 18-year-old male with a head circumference of 33 cm (ninth percentile) and suggested that microcephaly may be due to a biological spectral overlap between FAM111A and the TBCE locus in SSS. However, no TBCE mutation was detected in the present case. Moreover, when the patient’s electrolyte levels improved, her language expression was clear, and she showed no intellectual impairment according to the MMSE. Therefore, there was no evidence for SSS, which was consistent with the description of patients with KCS2 showing no intellectual abnormalities. A report of a pair of adult twin males in China described macrocephaly, ankylosing spondylitis, and scoliosis among the clinical manifestations of KCS2[12]. However, these characteristics were not observed in the present case. Inconsistent reports regarding the head circumference of the upper third of the cephalofacial deformity of patients with KCS2 have been noted, suggesting that imaging findings of calvarial thinning, sclerosis, or hypoplasia are more useful for the confirmation of the diagnosis than measurements of head circumference[13].
Few studies have reported reproductive function in adult female patients with KCS2, with two previous cases mentioning fertility. The mother reported by Kenny had normal intelligence and had menarche at 12 years of age, followed by regular menstruation[18]. She had three pregnancies. The first pregnancy led to the delivery of a normal boy without any skeletal abnormalities via cesarean section due to her small pelvis. The second pregnancy with a baby girl was terminated at 7 mo, while the third pregnancy, a son who had the same phenotype as his mother, was delivered by cesarean section at 37 wk. Although the woman had not received genetic testing, KCS2 was highly likely based on her intelligence. Menstruation was not described for the Canadian mother with KCS2 reported with her daughter[7]. Based on previous reports, KCS2 may have little effect on the gonadal axis. However, since most of the previous patients were minors, additional reports on the effect of KCS2 on menstruation and pregnancy are required for analysis and summary. In the present case, the age of menarche was 13 years, with no obvious abnormalities compared to other young women of the same age and race. However, the menstruation cycle of the patient was extremely irregular. Her testosterone level was slightly increased, which indicated possible polycystic ovary syndrome (PCOS). Pelvic ultrasound did not find features of polycystic ovarian changes. Further assessments, including basal body temperature and oral glucose tolerance tests, have been recommended to determine the etiology of hyperandrogenism during follow-up. Previous studies have suggested that, despite the equivalent prevalence of KCS2 between male and female patients, male patients have microorchidism and infertility, resulting in no father-to-son cases reported to date[11,12]. For patients with KCS2, in addition to hypocalcemia, which requires long-term calcium supplementation, and biochemical disorders as a result of hypoparathyroidism, genetic counseling is required before pregnancy[12]. Previous case reports rarely mentioned the presence of menstrual irregularity in female patients. According to the current literature, it is unclear whether KCS2 is related to female hyperandrogenemia.
The differences between this patient and previous reports include the following: (1) Sudden onset of psychiatric disorder. The patient was admitted to the hospital with hypokalemia, hypomagnesemia, hypocalcemia, and hyperphosphatemia. However, her urinary calcium level was not low. Her hypocalcemia, hypokalemia, and hypomagnesemia were corrected after treatment, suggesting the presence of electrolyte abnormalities, especially the possible presence of a syndrome including hypoparathyroidism, which has been reported in patients with severe hypocalcemia accompanied by hypomagnesemia. The fourth KCS2 patient mentioned in a report by Isojima et al[6] from Japan had hypoparathyroidism secondary to hypomagnesemia, as confirmed by magnesium load and calcium restriction tests. The serum calcium levels of the patient were corrected when the calcium supplementation regimen was changed from vitamin D and calcium lactate to magnesium supplementation with magnesium sulfate. Support therapy for KSC2 may be associated with complex electrolyte disorders, and the correction of low magnesium levels may be a critical step in the treatment of this condition; and (2) this patient was more obese than patients in previous reports based on the patient's appearance, waist circumference, and body mass index. These values were inconsistent with the normal or lean body shapes in previous reports[6,9,12,14,15], suggesting that some patients with KSC2 may be obese or overweight. The patient had no acanthosis nigricans, insulin resistance, or polycystic findings on color Doppler ultrasound of the uterine appendage. PCOS was not supported despite the patient’s menstrual disorders.
Exome sequencing of the four patients with KCS2 from Japan reported by Isojima et al[6] identified FAM111A as the pathogenic gene of KCS2. One of the patients showed a heterozygous de novo mutation of FAM111A (R569H), which was the same mutation observed in the present case. The mRNA expression levels of the individual with this mutation were comparable to those of the normal controls. Thus, the FAM111A mutation may have caused atypical features of phenylalanine by altering the intermolecular protein interactions. Five patients with clinical diagnoses of KCS2 in five different countries reported by Unger et al[5] also showed de novo mutations in FAM111A, with four of them having the c.1706G>A (p.Arg569His) mutation. Pathogenic mutations of FAM111A were also identified in subsequently reported cases of KCS2 in different populations, with the R569H mutation being the most common. There were no genetic relationships among these sporadic cases (Table 1). FAM111A is located on the long arm of chromosome 11 (11q12.1) and encodes a protein whose C-terminal region is homologous to trypsin-like peptidase[14]. The C-terminus is an evolutionarily conserved region of proteins and appears to be a hotspot for mutations[9,11,22]. The cellular function of FAM111A remains unclear. Previous studies have speculated that FAM111A may be involved in transcriptional regulation owing to its cell cycle-dependent expression and nuclear localization. One plausible model to explain these findings is that FAM111A is active when unbound but inactive when bound to an as-yet unidentified chaperone[5]. A recent study suggested that alterations in peptidase activity and the acquisition of functional mechanisms are the pathophysiological mechanisms of FAM111A-related diseases. Pathogenic mutations increase the proteolytic activity of proteins, resulting in decreased DNA replication and transcription, ultimately leading to programmed cell death[22]. Furthermore, molecular simulations showed that the reported variants were not located at the putative active site but rather at or near the protein surface. Different mutation sites in FAM111A may lead to different phenotypes. For example, Müller et al[23] reported a case in the United States of a fetus whose prenatal ultrasound showed skeletal dysplasia with edema, spleen dysplasia, and cerebellum deletion, as well as C.1685A>C and p.Tyr562Ser/Y562S. FAM111A has also been proposed to lead to a phenotypic overlap with osteocraniostenosis, which is associated with more severe perinatal mortality, cerebellar hypoplasia or hypomineralization, wider metaphysis, and hypoplastic or absent spleen. FAM111A is expressed in the bone and parathyroid glands and may play a key role in regulating intracellular pathways involved in skeletal development, height growth, parathyroid development and regulation, and calcium homeostasis[12]. However, the exact mechanism remains unclear. KCS2 is an extremely rare condition. Among 173 patients with childhood-onset hypoparathyroidism reported in a 2019 single-center study by the Peking Union Medical College, samples from 23 patients were subjected to next-generation sequencing and multiplex ligation-dependent probe amplification analysis[20]. Only one patient (0.6%) was diagnosed with KCS2[15]. Most of the reported patients were children with short-term follow-up. Therefore, a detailed description of the phenotype and natural course of KCS2 in adults is lacking. In this case, due to the absence of obvious symptoms of hypocalcemia, the patient did not visit a doctor for a definite diagnosis for a long time, resulting in a delay in the treatment of her conditions. It was not until she developed a severe electrolyte disorder (hypocalcemia, hypomagnesemia, hypokalemia, and hyperphosphatemia) requiring hospital admission that the patient first saw a doctor and was diagnosed with KCS2 based on the results of genetic sequencing.
In conclusion, we detected a missense variant of FAM111A in a patient with an acute onset of psychiatric symptoms combined with short stature, electrolyte disorders including hypocalcemia and hypomagnesemia, and abnormal skeletal manifestations consistent with KCS2. The clinical features in this patient were consistent with those previously reported. Moreover, the R569H variant was similar to other reported pathogenic variants and was consistent with the proposed pathophysiological mechanisms. However, the patient showed different manifestations, including overweight, severe electrolyte disorder, and psychiatric disorders. In addition, microcephaly was a relatively rare manifestation, suggesting an overlap with SSS. This was a sporadic case, which suggested that the FAM111A mutation was more likely to be a de novo mutation. Since the patient was an adult at the time of consultation, long-term follow-up treatment and observation of electrolyte status are necessary. Moreover, the fertility of the patient and the genetics of her offspring should be determined.
The authors thank our families for their support, as well as the patient and her family, who inspired us during the clinical and genetic research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Thongon N, Thailand; Tica I, Romania S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Clarke BL, Brown EM, Collins MT, Jüppner H, Lakatos P, Levine MA, Mannstadt MM, Bilezikian JP, Romanischen AF, Thakker RV. Epidemiology and Diagnosis of Hypoparathyroidism. J Clin Endocrinol Metab. 2016;101:2284-2299. [PubMed] [DOI] [Full Text] |
| 2. | Yagi H, Furutani Y, Hamada H, Sasaki T, Asakawa S, Minoshima S, Ichida F, Joo K, Kimura M, Imamura S, Kamatani N, Momma K, Takao A, Nakazawa M, Shimizu N, Matsuoka R. Role of TBX1 in human del22q11.2 syndrome. Lancet. 2003;362:1366-1373. [PubMed] [DOI] [Full Text] |
| 3. | Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K. GATA3 haplo-insufficiency causes human HDR syndrome. Nature. 2000;406:419-422. [PubMed] [DOI] [Full Text] |
| 4. | Parvari R, Hershkovitz E, Grossman N, Gorodischer R, Loeys B, Zecic A, Mortier G, Gregory S, Sharony R, Kambouris M, Sakati N, Meyer BF, Al Aqeel AI, Al Humaidan AK, Al Zanhrani F, Al Swaid A, Al Othman J, Diaz GA, Weiner R, Khan KT, Gordon R, Gelb BD; HRD/Autosomal Recessive Kenny-Caffey Syndrome Consortium. Mutation of TBCE causes hypoparathyroidism-retardation-dysmorphism and autosomal recessive Kenny-Caffey syndrome. Nat Genet. 2002;32:448-452. [PubMed] [DOI] [Full Text] |
| 5. | Unger S, Górna MW, Le Béchec A, Do Vale-Pereira S, Bedeschi MF, Geiberger S, Grigelioniene G, Horemuzova E, Lalatta F, Lausch E, Magnani C, Nampoothiri S, Nishimura G, Petrella D, Rojas-Ringeling F, Utsunomiya A, Zabel B, Pradervand S, Harshman K, Campos-Xavier B, Bonafé L, Superti-Furga G, Stevenson B, Superti-Furga A. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013;92:990-995. [PubMed] [DOI] [Full Text] |
| 6. | Isojima T, Doi K, Mitsui J, Oda Y, Tokuhiro E, Yasoda A, Yorifuji T, Horikawa R, Yoshimura J, Ishiura H, Morishita S, Tsuji S, Kitanaka S. A recurrent de novo FAM111A mutation causes Kenny-Caffey syndrome type 2. J Bone Miner Res. 2014;29:992-998. [PubMed] [DOI] [Full Text] |
| 7. | Nikkel SM, Ahmed A, Smith A, Marcadier J, Bulman DE, Boycott KM. Mother-to-daughter transmission of Kenny-Caffey syndrome associated with the recurrent, dominant FAM111A mutation p.Arg569His. Clin Genet. 2014;86:394-395. [PubMed] [DOI] [Full Text] |
| 8. | Guo MH, Shen Y, Walvoord EC, Miller TC, Moon JE, Hirschhorn JN, Dauber A. Whole exome sequencing to identify genetic causes of short stature. Horm Res Paediatr. 2014;82:44-52. [PubMed] [DOI] [Full Text] |
| 9. | Abraham MB, Li D, Tang D, O'Connell SM, McKenzie F, Lim EM, Hakonarson H, Levine MA, Choong CS. Short stature and hypoparathyroidism in a child with Kenny-Caffey syndrome type 2 due to a novel mutation in FAM111A gene. Int J Pediatr Endocrinol. 2017;2017:1. [PubMed] [DOI] [Full Text] |
| 10. | Wang Y, Nie M, Wang O, Li Y, Jiang Y, Li M, Xia W, Xing X. Genetic Screening in a Large Chinese Cohort of Childhood Onset Hypoparathyroidism by Next-Generation Sequencing Combined with TBX1-MLPA. J Bone Miner Res. 2019;34:2254-2263. [PubMed] [DOI] [Full Text] |
| 11. | Cavole TR, Perrone E, de Faria Soares MF, Dias da Silva MR, Maeda SS, Lazaretti-Castro M, Alvarez Perez AB. Overlapping phenotype comprising Kenny-Caffey type 2 and Sanjad-Sakati syndromes: The first case report. Am J Med Genet A. 2020;182:3029-3034. [PubMed] [DOI] [Full Text] |
| 12. | Cheng SSW, Chan PKJ, Luk HM, Mok MT, Lo IFM. Adult Chinese twins with Kenny-Caffey syndrome type 2: A potential age-dependent phenotype and review of literature. Am J Med Genet A. 2021;185:636-646. [PubMed] [DOI] [Full Text] |
| 13. | Turner AE, Abu-Ghname A, Davis MJ, Shih L, Volk AS, Streff H, Buchanan EP. Kenny-Caffey Syndrome Type 2: A Unique Presentation and Craniofacial Analysis. J Craniofac Surg. 2020;31:e471-e475. [PubMed] [DOI] [Full Text] |
| 14. | Deconte D, Kreusch TC, Salvaro BP, Perin WF, Ferreira MAT, Kopacek C, da Rosa EB, Heringer JI, Ligabue-Braun R, Zen PRG, Rosa RFM, Fiegenbaum M. Ophthalmologic Impairment and Intellectual Disability in a Girl Presenting Kenny-Caffey Syndrome Type 2. J Pediatr Genet. 2020;9:263-269. [PubMed] [DOI] [Full Text] |
| 15. | Yerawar C, Kabde A, Deokar P. Kenny-Caffey syndrome type 2. QJM. 2021;114:267-269. [PubMed] [DOI] [Full Text] |
| 16. | Eren E, Tezcan Ünlü H, Ceylaner S, Tarım Ö. Compound Heterozygous Variants in FAM111A Cause Autosomal Recessive Kenny-Caffey Syndrome Type 2. J Clin Res Pediatr Endocrinol. 2023;15:97-102. [PubMed] [DOI] [Full Text] |
| 17. | Ohmachi Y, Urai S, Bando H, Yokoi J, Yamamoto M, Kanie K, Motomura Y, Tsujimoto Y, Sasaki Y, Oi Y, Yamamoto N, Suzuki M, Shichi H, Iguchi G, Uehara N, Fukuoka H, Ogawa W. Case report: Late middle-aged features of FAM111A variant, Kenny-Caffey syndrome type 2-suggestive symptoms during a long follow-up. Front Endocrinol (Lausanne). 2022;13:1073173. [PubMed] [DOI] [Full Text] |
| 18. | Kenny FM, Linarelli L. Dwarfism and cortical thickening of tubular bones. Transient hypocalcemia in a mother and son. Am J Dis Child. 1966;111:201-207. [PubMed] [DOI] [Full Text] |
| 19. | Caffey J. Congenital stenosis of medullary spaces in tubular bones and calvaria in two proportionate dwarfs--mother and son; coupled with transitory hypocalcemic tetany. Am J Roentgenol Radium Ther Nucl Med. 1967;100:1-11. [PubMed] [DOI] [Full Text] |
| 20. | Fine DA, Rozenblatt-Rosen O, Padi M, Korkhin A, James RL, Adelmant G, Yoon R, Guo L, Berrios C, Zhang Y, Calderwood MA, Velmurgan S, Cheng J, Marto JA, Hill DE, Cusick ME, Vidal M, Florens L, Washburn MP, Litovchick L, DeCaprio JA. Identification of FAM111A as an SV40 host range restriction and adenovirus helper factor. PLoS Pathog. 2012;8:e1002949. [PubMed] [DOI] [Full Text] |
| 21. | Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227-D233. [PubMed] [DOI] [Full Text] |
| 22. | Hoffmann S, Pentakota S, Mund A, Haahr P, Coscia F, Gallo M, Mann M, Taylor NM, Mailand N. FAM111 protease activity undermines cellular fitness and is amplified by gain-of-function mutations in human disease. EMBO Rep. 2020;21:e50662. [PubMed] [DOI] [Full Text] |
| 23. | Müller R, Steffensen T, Krstić N, Cain MA. Report of a novel variant in the FAM111A gene in a fetus with multiple anomalies including gracile bones, hypoplastic spleen, and hypomineralized skull. Am J Med Genet A. 2021;185:1903-1907. [PubMed] [DOI] [Full Text] |