Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2282
Peer-review started: December 22, 2022
First decision: January 20, 2023
Revised: February 1, 2023
Accepted: March 9, 2023
Article in press: March 9, 2023
Published online: April 6, 2023
Processing time: 98 Days and 6.4 Hours
Bronchopleural fistula (BPF) is a relatively rare, but severe complication of pulmonary tuberculosis. It is associated with significant mortality; however, its management remains a major therapeutic challenge.
We present a 24-year-old man with BPF resulting from severe pulmonary tuberculosis combined with mixed infections. The damaged right upper lobe and concomitant empyema were demonstrated via computed tomography. After undergoing open-window thoracostomy and tuberculosis treatment for 4 mo, decortication and right upper lobectomy were subsequently performed, leading to the resolution of tuberculosis and other concurrent pulmonary infections. Follow-up, 6 mo after surgery, failed to reveal any evidence of infection recurrence resulting in a good prognosis.
The disease course of tuberculous BPF is particularly challenging. Surgical intervention serves as an effective and safe therapeutic strategy for BPF.
Core Tip: Bronchopleural fistula (BPF) is a relatively rare but severe complication of pulmonary tuberculosis. However, no consensus has been reached on the ideal treatment modality. This article presents a patient with a BPF resulting from severe pulmonary tuberculosis accompanied by mixed infections. Strict preoperative evaluation of surgical indications, including standard preoperative anti-tuberculosis treatment and controlling the infection through an open-window thoracostomy during surgery, can achieve a satisfactory long-term prognosis for the treatment of BPF through this three-pronged approach.
- Citation: Shen L, Jiang YH, Dai XY. Successful surgical treatment of bronchopleural fistula caused by severe pulmonary tuberculosis: A case report and review of literature. World J Clin Cases 2023; 11(10): 2282-2289
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2282.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2282
Tuberculosis is ranked as the second leading infectious killer after coronavirus disease 2019. In 2021, 10.6 million people were diagnosed with active tuberculosis, out of which 1.6 million cases resulted in death[1]. Bronchopleural fistula (BPF) is a relatively rare but life-threatening complication of tuberculosis, with a mortality rate varying between 18% to 67%[2]. BPF most commonly manifests as a postoperative complication of pulmonary resection, particularly pneumonectomy[3]. Other common causes of BPF include lung necrosis complicating infection, persistent spontaneous pneumothorax, chemoradiotherapy, and tuberculosis[4]. The treatment of BPF involves various surgical methods, drug therapy, or bronchoscopic procedures. However, due to limited studies on the condition, there is a lack of consensus concerning its optimal treatment. The purpose of this article is to describe an effective treatment strategy for BPF caused by serious pulmonary tuberculosis.
A 24-year-old man was referred to our hospital with a productive cough accompanied by expectoration of purulent sputum, weight loss, and exertional polypnea. The patient was treated for tuberculosis but he subsequently developed fever and chest pain, 2 mo after being discharged from our hospital.
When the severity of the clinical symptoms increasingly progressed, the patient was readmitted to our hospital for further evaluation and treatment.
The patient had no significant medical history.
The patient denied smoking previously and had no past history of lung disease. His family history was unremarkable.
On physical examination, the patient was in poor general condition. The patient’s body mass index was around 20. Lung auscultation indicated decreased breath sounds in the right lower lung. Chest wall collapse was evident upon visual inspection.
Laboratory analysis evidenced that sputum smears for acid-fast bacilli, sputum culture, GeneXpert Mtuberculosis/RIF, and polymerase chain reaction were all positive for tuberculosis. Furthermore, multidrug-resistant Klebsiella pneumoniae and Aspergillus fumigatus were isolated in the bacterial culture of the effusion and sputum. The blood markers were as follows: White blood count: 13.67 × 109/L (81.1% neutrophils and 5.6% lymphocytes); C-reactive protein: 89.54 mg/L; ESR: 34 mm/h; procalcitonin: 0.22 ng/mL. The patient's liver and kidney functions, electrolytes, myocardial enzymes, and coagulation were normal.
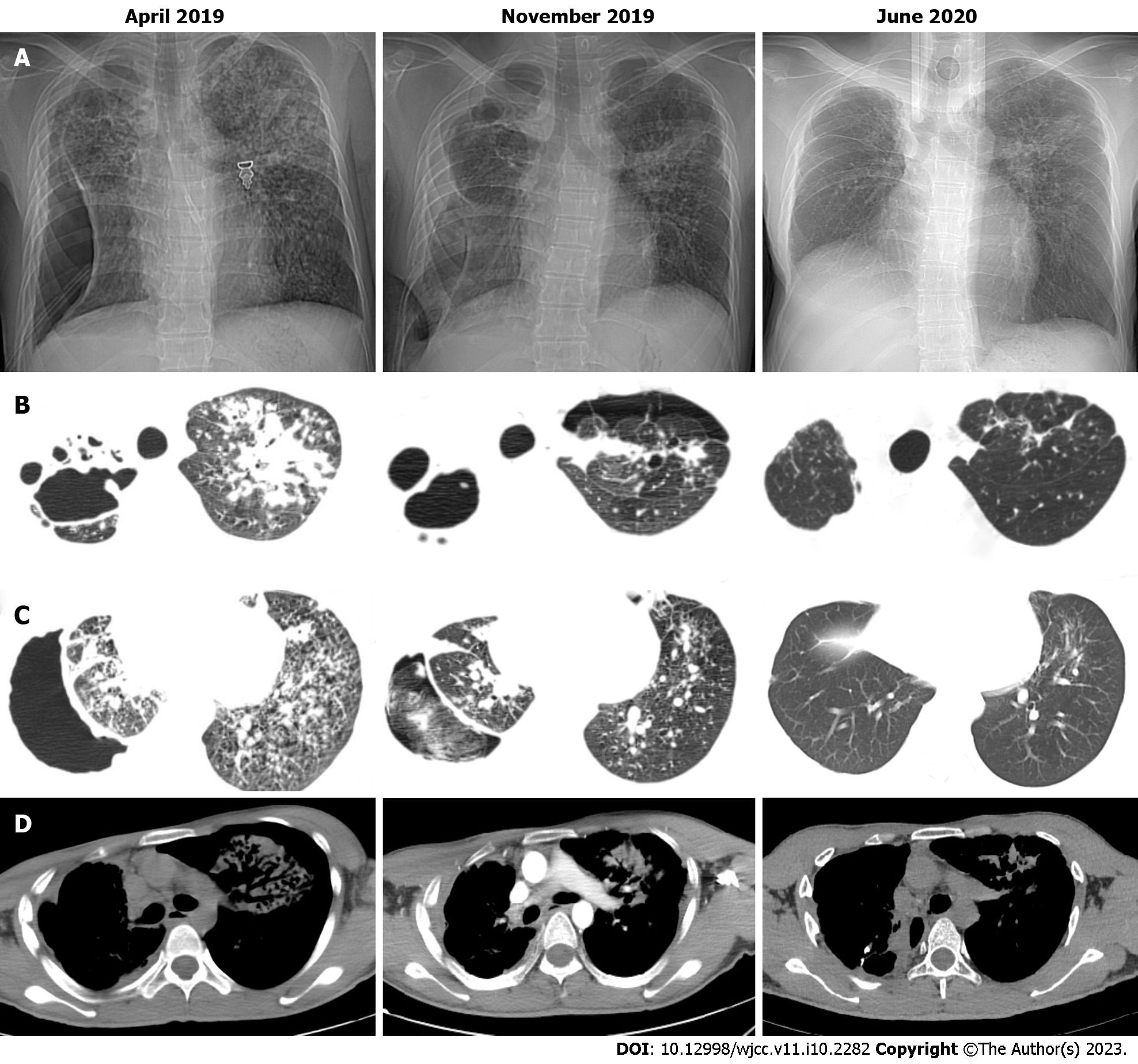
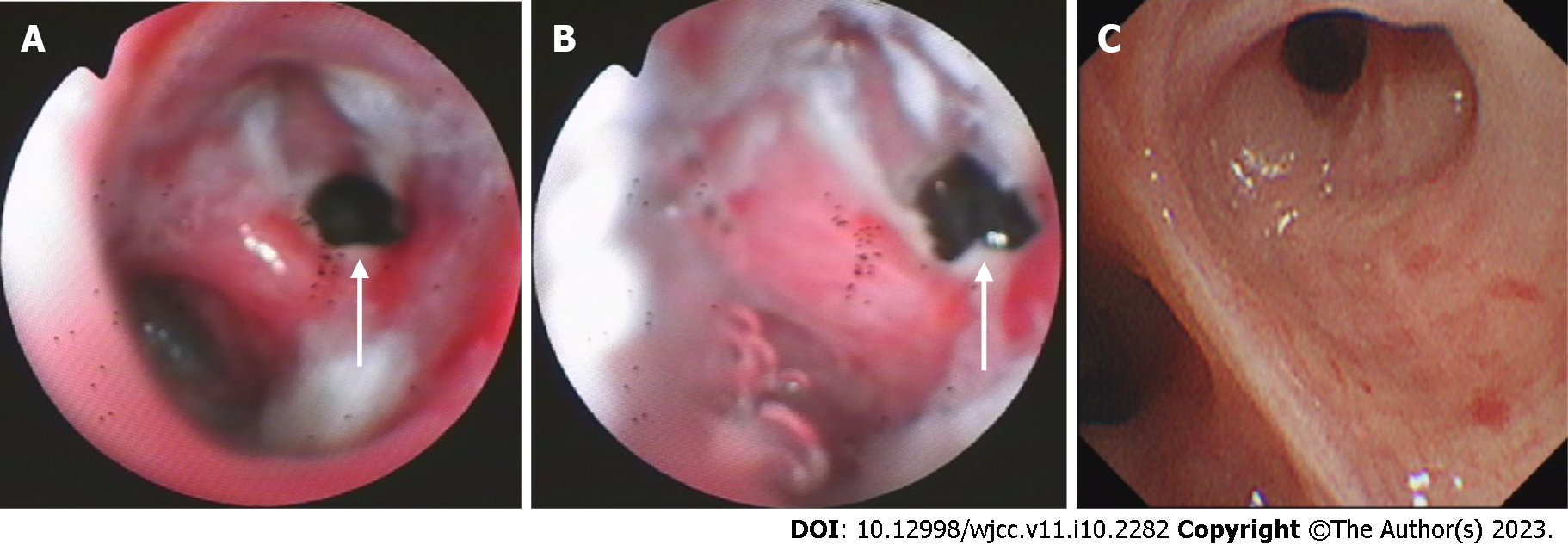
The chest computed tomography (CT) scan showed bilateral pulmonary infection with cavitary lesions (Figure 1A). The right lung was completely compressed and atelectatic with severe air leakage; pleural thickening of the right upper lung indicated right lung damage (Figure 1B). The coarctation of the right intercostal space is noted on the chest CT (Figure 1D). Bronchoscopy at admission (Figure 2A) and 1 mo after the anti-tuberculosis treatment showed a 5 mm fistula of the right upper bronchus (Figure 2B), along with erythema, erosion, and hyperplasia of the apical bronchus, and a small amount of necrotic material.
This patient was diagnosed with BPF, endobronchial tuberculosis, pulmonary tuberculosis, tuberculosis lung damage, and pleural empyema.
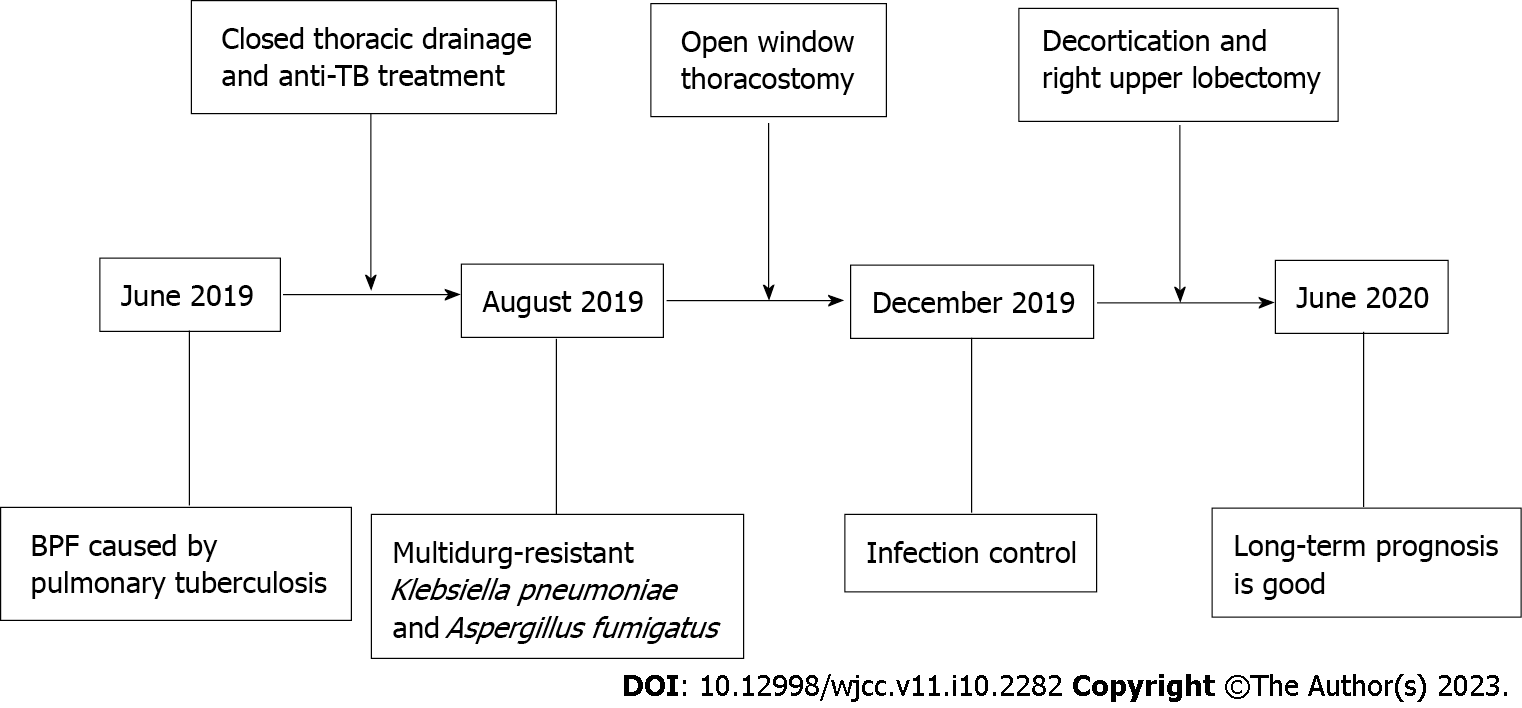
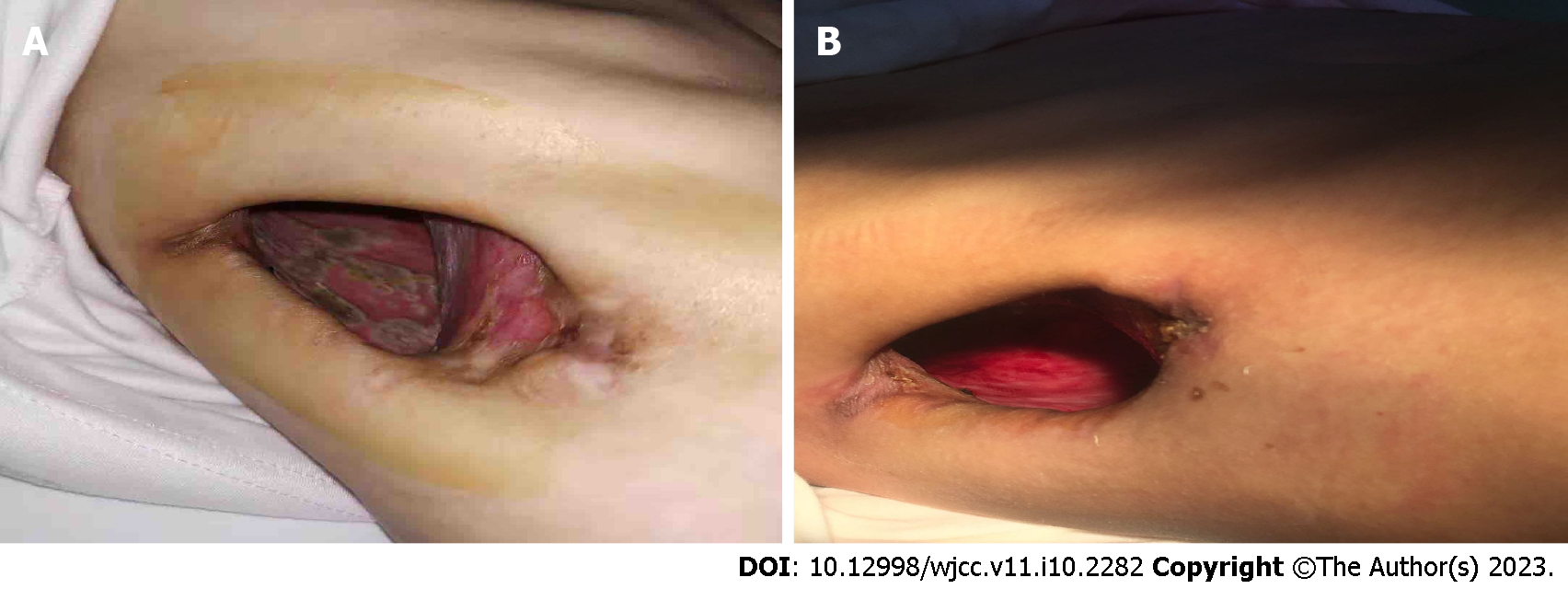
The timeline of management is shown in Figure 3. Closed thoracic drainage and anti-tuberculosis therapy were conducted after the patient’s hospital admission. The patient’s anti-tuberculosis treatment program included the following medications taken once daily: Isoniazid 300 mg qd; rifampicin 450 mg qd; ethambutol 750 mg qd; levofloxacin 600 mg qd; amikacin 600 mg qd and linezolid 200 mg qd. The patient was subsequently discharged to continue treatment at home. Despite anti-tuberculosis treatment for 2 mo, the disease progressed leading to readmission. A previously installed chest tube from the initial hospitalization was removed due to a complicated pleural infection. Due to difficulty finding an appropriate location for closed drainage, the patient underwent a right open-window thoracostomy to improve fluid drainage. The 7th and 8th partial ribs were removed to construct a window, 10 cm in diameter. Surgery confirmed the presence of a thick pleural membrane surrounding the right lung and a 5 mm fistula with air leakage from the right upper lung. In addition, mold was found on the pleural surface (Figure 4A). To address the pleural empyema, gauzes soaked in iodine were packed into the pleural space through the window, once or twice a day after surgery. Voriconazole and intrapleural irrigation of amphotericin B were administered for antifungal treatment. Amphotericin B (10 mg) was diluted with 5% glucose solution to form a total volume of approximately 50 mL. Subsequently, the aspergillosis-infected pleura was irrigated with this solution by packing amphotericin-soaked gauze into the emphysematous space. Irrigation was performed once daily for 1 mo.
Infection was controlled remarkably after surgery, through continuous treatment with antibiotics and antifungal agents over 4 mo. The patient underwent right upper lobectomy accordingly. Intraoperatively, thick, fibrotic pleural membranes were found and decortication was conducted. Bronchial stump reinforcement was performed through the mediastinal pleura. Finally, the thoracic window was closed. In order to prevent recurrence, postoperative antifungal treatment was continued for 3 mo, and anti-tuberculosis treatment for 6 months.
Improvement of symptoms and postoperative examination demonstrated the positive effects of the therapeutic strategy. Repeat cultures of sputum and bronchoalveolar lavage fluid were negative. Chest CT at 6 mo after the treatment revealed diminished lung lesions. (Figure 1B). Repeat bronchoscopy also demonstrated improvement of the right upper bronchial tuberculosis and the absence of any abnormality in the middle and lower bronchus (Figure 2C). The pleural effusion were resolved and no lesions were found on the pleural surface (Figure 4B). The patient’s physical strength returned after dietary intervention. The right lung expanded well and the chest wall deformity resolved 6 mo after the operation (Figure 1C). The patient showed a favorable long-term prognosis and no evidence of recurrence at follow-up.
Many therapeutic strategies have been implemented in the treatment of BPF[5-11], but a consensus on the best treatment modality has yet to be reached. The current therapeutic options can be complementary, and the treatment needs to be individualized[12]. Herein, we described the successful treatment of tuberculous BPF through surgery. Favorable surgical outcomes for BPF depend on the selection and timing of the operation, standard preoperative anti-tuberculosis treatment, full thoracic drainage, and infection control.
Clinical treatment of tuberculous BPF complicated with multiple infections is a huge challenge. We believe that preoperative standard anti-tuberculosis treatment combined with full thoracic drainage is crucial[13,14]. For patients with Mycobacterium tuberculosis-positive sputum before surgery, a drug-resistant tuberculosis test is needed to choose effective anti-tuberculosis drugs. In our case, however, drug therapy for inflammation caused by Klebsiella pneumoniae spp. and Aspergillus fumigatus was not effective. Full drainage of the thoracic cavity was eventually achieved through open-window thoracostomy, which led to the significant improvement of chronic inflammation. Open-window thoracostomy is a simple, certain, and last resort drainage procedure for thoracic empyema[15,16]. It is the best means to drain purulent effusion caused by empyema from the pleural space and is an excellent interim treatment for BPF[17,18]. Furthermore, intrapleural irrigations of amphotericin B have been proven effective in preventing the recurrence of lung aspergillomas[19,20]. As prolonged air leaks caused by BPF create ventilatory abnormalities through the ineffective gas exchange, packing iodine-soaked gauzes into the empyema-affected pleural space through an open window can add positive intrapleural pressure during inspiration. This procedure could decrease the air leaks caused by BPF. Klebsiella pneumoniae spp. and Aspergillus fumigatus are opportunistic infections associated with immunodeficiency. Accordingly, nutritional supplementation also plays a very important role in recuperation from these infections.
The main challenge in BPF lies in closing the fistula and eliminating the cavitation. A variety of surgical and bronchoscopic strategies for treating BPF have been described, although none of these approaches has been successful or suitable for all patients. According to Li et al[10], the scope of bronchoscopy makes it difficult to treat peripheral BPF. The size of BPF > 8 mm is generally unsuitable for bronchoscopic treatment[12,21]. The degree of infection, timing of diagnosis, underlying disease, localization and the size of the fistula may be the critical factors in choosing between surgical and bronchoscopic procedures for treatment[8,22,23]. In our case, as the bronchial stump was affected by tuberculosis and the right upper lung was damaged, we did not select the minimally invasive bronchoscopic method as a treatment strategy. In a patient with lobar lung damage combined with empyema, especially in patients with BPF, aggressive radical therapy with lobectomy or thoracoplasty is necessitated[24,25]. However, the effectiveness of surgery in the treatment of drug-resistant tuberculosis remains unclear[26]. The advantages of bronchial stump reinforcement in preventing BPF are also still controversial, and no consensus has been reached on the ideal buttressing tissue to use[27-29]. Patients with empyema and bronchial tuberculosis undergoing lung resections are at increased risk for BPF recurrence. Besides, preoperative bronchoscopy is used to exclude endobronchial tuberculosis. Hence, we used the mediastinal pleura to cover the bronchial stump. However, as these procedures carries a high risk of mortality and recurrence[30], it is not universally recommended and its indications should be carefully considered. In our patient, chest CT and bronchoscopic images were reviewed regularly to assess the progression of pulmonary and bronchial lesions, preoperatively. The disease course is even more complicated for patients with tuberculous BPF. The key to the treatment of pulmonary tuberculosis complicated with BPF is to have a continual and effective anti-tuberculosis treatment. The preoperative anti-tuberculosis treatment regimen is complex. Clinicians should be ready to adjust the regimen at any time according to its therapeutic effects and adverse reactions.
In summary, the treatment of tuberculous BPF has been a challenging issue in thoracic surgery. Surgery is the modality of choice for tuberculous BPF, but it has many complications. Strict preoperative evaluation of surgical indications is required, including standard preoperative anti-tuberculosis treatment with medical therapy and full thoracic drainage to control the infection. The proper selection of patients for surgical intervention can achieve a satisfactory long-term prognosis, and the procedures are safe and efficient. Further research is required to obtain more definitive evidence.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keikha M, Iran; Skrzypczak PJ, Poland S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization 2022. [cited December 10, 2022]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. |
| 2. | Hollaus PH, Lax F, el-Nashef BB, Hauck HH, Lucciarini P, Pridun NS. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg. 1997;63:1391-6; discussion 1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Okuda M, Go T, Yokomise H. Risk factor of bronchopleural fistula after general thoracic surgery: review article. Gen Thorac Cardiovasc Surg. 2017;65:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Sato M, Saito Y, Fujimura S, Usuda K, Takahashi S, Kanma K, Imai S, Suda H, Nakada T, Hashimoto K. [Study of postoperative bronchopleural fistulas--analysis of factors related to bronchopleural fistulas]. Nihon Kyobu Geka Gakkai Zasshi. 1989;37:498-503. [PubMed] |
| 5. | Giller DB, Kesaev OS, Koroev VV, Enilenis II, Shcherbakova GV, Romenko MA, Ratobylsky GV, Pekhtusov VA, Martel II. [Surgical treatment of bronchopleural complications after lung resection and pleurectomy in patients with tuberculosis]. Khirurgiia (Mosk). 2021;39-46. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Misaki N, Yokomise H. [Surgical Approach for Treatment of Postoperative Bronchopleural Fistula and Pyothorax]. Kyobu Geka. 2021;74:856-861. [PubMed] |
| 7. | Bai Y, Li Y, Chi J, Guo S. Endobronchial closure of the bronchopleural fistula with the ventricular septal defect occluder: a case series. BMC Pulm Med. 2021;21:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Song X, Gu Y, Wang H, Zhang L. The efficacy of endobronchial valves for the treatment of bronchopleural fistula: a single-arm clinical trial. J Thorac Dis. 2022;14:712-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Siddique A, Sabbah BN, Arabi T, Shakir IM, Abdulqawi R, AlKattan K, Ahmed MH. Treatment of bronchial anastomotic fistula using autologous platelet-rich plasma post lung transplantation. J Cardiothorac Surg. 2022;17:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Li X, Wang S, Yin M, Li X, Qi Y, Ma Y, Li C, Wu G. Treatment of peripheral bronchopleural fistula with interventional negative pressure drainage. Ther Adv Respir Dis. 2022;16:17534666221111877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Fruchter O, Kramer MR, Dagan T, Raviv Y, Abdel-Rahman N, Saute M, Bruckheimer E. Endobronchial closure of bronchopleural fistulae using amplatzer devices: our experience and literature review. Chest. 2011;139:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005;128:3955-3965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Sikander N, Ahmad T, Mazcuri M, Ali N, Thapaliya P, Nasreen S, Abid A. Role of Anti-Tuberculous Treatment in the Outcome of Decortication for Chronic Tuberculous Empyema. Cureus. 2021;13:e12583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Piccolo F, Popowicz N, Wong D, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J Thorac Dis. 2015;7:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 15. | Hiramatsu M, Atsumi J, Shiraishi Y. Surgical Management of Mycobacterial Infections and Related Complex Pleural Space Problems: From History to Modern Day. Thorac Surg Clin. 2022;32:337-348. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Fukui T, Matsukura T, Wakatsuki Y, Yamawaki S. Simple chest closure of open window thoracostomy for postpneumonectomy empyema: a case report. Surg Case Rep. 2019;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Dantis K, Kumar Dewan R. Surgical outcomes and the factors affecting lung expansion following open window thoracostomy in chronic tuberculous empyema with destroyed lung. Asian Cardiovasc Thorac Ann. 2022;30:696-705. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Massera F, Robustellini M, Della Pona C, Rossi G, Rizzi A, Rocco G. Open window thoracostomy for pleural empyema complicating partial lung resection. Ann Thorac Surg. 2009;87:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Chen QK, Jiang GN, Ding JA. Surgical treatment for pulmonary aspergilloma: a 35-year experience in the Chinese population. Interact Cardiovasc Thorac Surg. 2012;15:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47:45-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 595] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 21. | Shekar K, Foot C, Fraser J, Ziegenfuss M, Hopkins P, Windsor M. Bronchopleural fistula: an update for intensivists. J Crit Care. 2010;25:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Salik I, Vashisht R, Abramowicz AE. Bronchopleural Fistula. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 23. | Brennan PG, Hsu DS, Banks KC, Maxim CL, Hornik B, Velotta JB. Vertical rectus abdominis myocutaneous free flap repair of post-pneumonectomy bronchopleural fistula: a case report. AME Case Rep. 2022;6:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Okabayashi K, Yamazaki K, Hamatake D, Yoshida Y, Shirakusa T. [Pleuropneumonectomy for pulmonary tuberculosis and chronic tuberculous empyema]. Kyobu Geka. 2004;57:1033-1037. [PubMed] |
| 25. | Bai L, Hong Z, Gong C, Yan D, Liang Z. Surgical treatment efficacy in 172 cases of tuberculosis-destroyed lungs. Eur J Cardiothorac Surg. 2012;41:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ghazvini K, Keikha M. The elimination of drug-resistant tuberculosis from a pulmonary resection surgery perspective. Int J Surg. 2022;104:106790. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Ceylan KC, Batıhan G, Kaya ŞÖ. Novel method for bronchial stump coverage for prevents postpneumonectomy bronchopleural fistula: pedicled thymopericardial fat flap. J Cardiothorac Surg. 2022;17:286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Sfyridis PG, Kapetanakis EI, Baltayiannis NE, Bolanos NV, Anagnostopoulos DS, Markogiannakis A, Chatzimichalis A. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg. 2007;84:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Skrzypczak P, Roszak M, Kasprzyk M, Dyszkiewicz W, Kamiński M, Gabryel P, Piwkowski C. The technique of stump closure has no impact on post-pneumonectomy bronchopleural fistula in the non-small cell lung cancer-a cross-sectional study. J Thorac Dis. 2022;14:3343-3351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Bribriesco A, Patterson GA. Management of Postpneumonectomy Bronchopleural Fistula: From Thoracoplasty to Transsternal Closure. Thorac Surg Clin. 2018;28:323-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |