Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2260
Peer-review started: November 3, 2022
First decision: December 26, 2022
Revised: February 8, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: April 6, 2023
Processing time: 146 Days and 23.3 Hours
Contrast-induced encephalopathy (CIE) is a rare transient, reversible abnormality in the structure or function of the nervous system caused by the intravascular use of contrast agents. CIE can present with a range of neurological manifestations, including focal neurological deficits (hemiplegia, hemianopia, cortical blindness, aphasia, and parkinsonism) and systemic symptoms (confusion, seizures, and coma). However, if not accurately diagnosed and treated in a timely manner, CIE can cause irreversible damage to patients, especially critically ill patients.
A male in his 50 s, 2 h after digital subtraction angiography, had a progressive disorder of consciousness, mixed aphasia, bilateral pupillary sluggish light reflex, and right limb weakness. Seven hours after the procedure, he developed unconsciousness, high fever (39.5 °C), seizures, hemiplegia, neck stiffness (+), and right Babinski signs (+). computed tomography (CT) findings 2 h postprocedure were very confusing and led us to misdiagnose the patient with subarachnoid hemorrhage. Brain CT was performed again 7 h after the procedure. Compared with the CT 2 h after the procedure, the CT 7 h after the procedure showed that the manifestations of subarachnoid hemorrhage in the left cerebral hemisphere had disappeared and were replaced by brain tissue swelling, and the cerebral sulci had disappeared. Combined with the clinical manifestations of the patient and after the exclusion of subarachnoid hemorrhage and cerebrovascular embolism, we diagnosed the patient with CIE, and intravenous fluids were given for adequate hydration, as well as mannitol, albumin dehydration, furosemide and the glucocorticoid methylprednisolone. After 17 d of active treatment, the patient was discharged with no sequelae.
CIE should be taken seriously, but it is easily misdiagnosed, and once CIE is diagnosed, rapid, accurate diagnosis and treatment are critical steps. Whether a follow-up examination using a contrast agent can be performed should be closely evaluated, and the patient should be fully informed of the associated risks.
Core Tip: Contrast-induced encephalopathy (CIE) is a rare disease induced by the injection of contrast agents. In this case, unilateral CIE was caused by a long time of internal carotid arteriogram on left side and a large amount of contrast agent. The onset was misdiagnosed as subarachnoid hemorrhage at an early stage, and he was discharged without sequelae after 18 d of diagnosis and treatment of CIE. The main treatment measures are corticosteroids, dehydration and diuresis, and adequate hydration. Rapid, accurate diagnosis and treatment are critical steps. Whether the follow-up examination using contrast agent can be done should be closely evaluated and fully informed.
- Citation: Zhang ZY, Lv H, Wang PJ, Zhao DY, Zhang LY, Wang JY, Hao JH. Unilateral contrast-induced encephalopathy with contrast medium exudation: A case report. World J Clin Cases 2023; 11(10): 2260-2266
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2260.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2260
Contrast-induced encephalopathy (CIE) is a rare, transient, reversible abnormality in the structure or function of the nervous system caused by the intravascular use of contrast agents. It can present with a range of neurological manifestations, including focal neurological deficits (hemiplegia, hemianopia, cortical blindness, aphasia, and parkinsonism) and systemic symptoms (confusion, seizures, and coma)[1,2]. In general, the severity of the clinical manifestations of CIE varies, ranging from mild headache symptoms to severe clinical manifestations such as coma and even death. We report a case of severe unilateral contrast-induced encephalopathy characterized by acute unilateral hemispheric neurological dysfunction. The incidence of CIE is less than 1%, and unilateral concentration of lesions is less commonly reported. Compared with bilateral lesions, unilateral lesions are more confusing and need to be distinguished from subarachnoid hemorrhage and cerebrovascular embolism or spasm. This case is special in that the lesion was concentrated on one side (left side), the symptoms lasted for a long time, and the condition was misdiagnosed during the initial course of treatment. Our report aims to improve the awareness of this condition so as to provide early diagnosis and active treatment and effectively prevent the occurrence of irreversible damage to patients.
A male in his 50 s was admitted to the hospital because of “headache for 1 d”.
The patient had episodic headache 1 d before without obvious causes. At that time, he recorded his blood pressure at 178/100 mmHg, and he took “valsartan”. He had no rotatory vision and no nausea or vomiting.
A middle-aged male with a 10-year history of hypertension who was taking “valsartan” orally for treatment. He had a 3-year history of diabetes and was treated with oral “metformin” with unknown glycemic control.
The patient and his family had no relevant medical history.
Vital signs: R 22 times/min P 90 times/min H 170 cm T 36.2 °C W 75 kg BP 118/81 mmHg. The patient was conscious, with fluent speech and equal-sized and round pupils with a diameter of 3 mm. The presence of light reflex, flexible eye movements in all directions, bilateral nasolabial fold symmetry, and tongue protrusion to the left was noted. Other cranial nerves were normal. His limb muscle strength was grade 5 and muscle tension was acceptable, tendon reflexes (++), and bilateral pathological signs were negative. His sense of depth and lightness was normal, and motor function was normal.
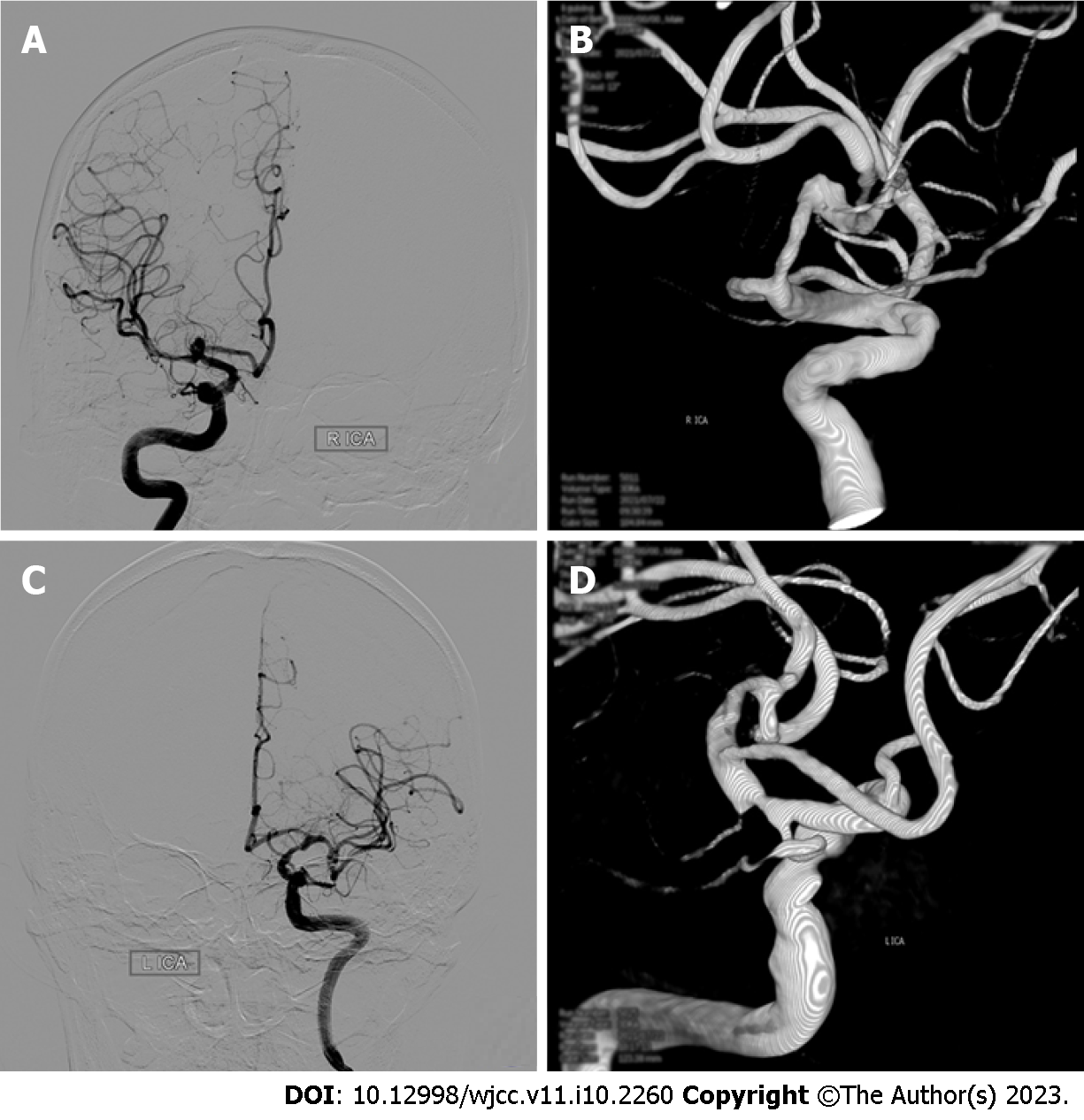
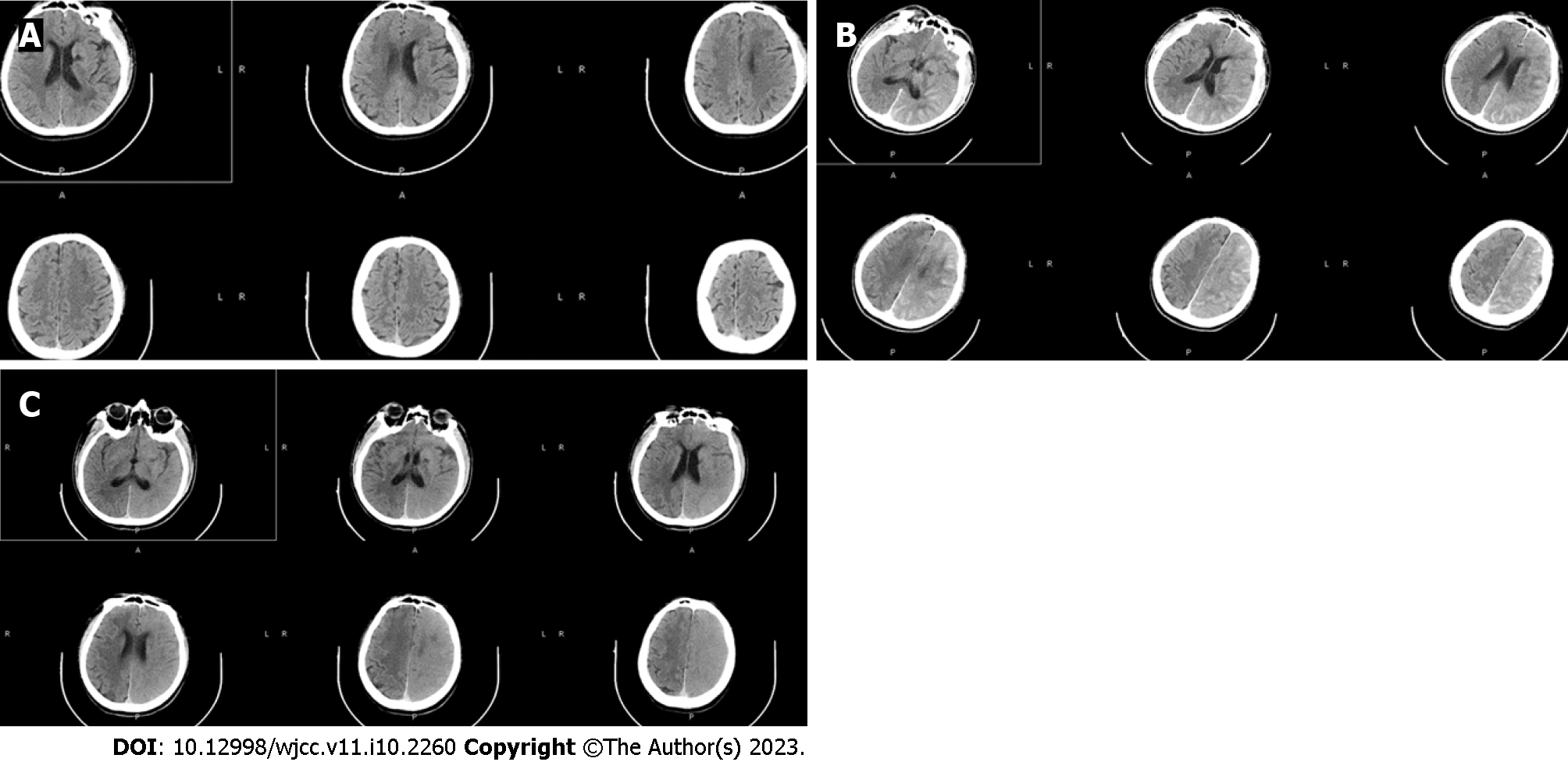
Digital subtraction angiography (DSA) confirmed the diagnosis of a middle cerebral artery aneurysm (Figure 1). Two hours later, the patient suddenly had slurred speech, accompanied by right limb weakness. An emergency CT scan (within 15 min of onset) showed that the density of the interhemispheric cistern, the left fissure cistern, and the left part of the sulcus was increased (Figure 2). Subarachnoid hemorrhage was considered first and treated accordingly. Seven hours after DSA, the patient’s condition significantly deteriorated, and the nervous system examination findings are shown in Table 1. A re-examination of the brain computed tomography (CT) showed significant changes: the temporal, parietal, and occipital lobes of the brain were swollen, the high-density shadows in the sulci and gyri had disappeared, and the sulci and gyri had become shallow and disappeared (Figure 2).
| Preoperative | 2 h after DSA | 7 h | 3 d | 6 d | On discharge | |
| Vital signs | R 22 times/min; P 90 times/min; H 170 cm; T 36.2 °C; W 75 kg; BP 118/81 mmHg | High fever (39.5 °C) | ||||
| Nervous system examination findings | The patient was conscious, with fluent speech and equal-sized and round pupils with a diameter of 3 mm. The presence of light reflex, flexible eye movements in all directions, bilateral nasolabial fold symmetry, and tongue protrusion to the left was noted. Other cranial nerves were normal. His limb muscle strength was grade 5 and muscle tension was acceptable, tendon reflexes (++), and bilateral pathological signs were negative. His sense of depth and lightness was normal, and motor function was normal | He suddenly had slurred speech, accompanied by right limb weakness | He developed unconsciousness, high fever (39.5 °C), seizures, hemiplegia of the right limb, neck stiffness (+), and right Babinski signs (+) | His consciousness gradually improved, he was able to open eyes voluntarily, and he could answer simple questions even though he was not fluent in speech. His pupils were equal in size and round, with a diameter of 3 mm. His light reflex was sluggish, his eyes moved flexibly in all directions, and his bilateral nasolabial folds were symmetrical. His muscle tension of the four limbs was acceptable, the left limb movement was acceptable, the right limb muscle strength was grade 3, tendon reflexes (+), and right pathological signs (+) | The patient was clearly conscious, but his speech was still not fluent. The seizures did not recur.The muscle strength of the healthy limb recovered to grade 4 | The patient was conscious and in good spirits. He had fluent speech. Both pupils were of equal size and were round, 3 mm in diameter. The presence of the light reflex, flexible eye movements in all directions, bilateral nasolabial fold symmetry, and tongue protrusion to the left (same as preprocedure) was noted. The remaining cranial nerves were not (see exception). The muscle strength of the limbs was grade 5, the muscle tension was acceptable, the tendon reflex (+), and the bilateral Babinski signs were negative |
The patient was diagnosed as contrast-induced encephalopathy.
CIE was considered at this time, and then the patient was given intravenous fluid for full hydration, including 20% mannitol 250 mL Q6H, albumin 10 g bid for dehydration and furosemide 20 mg bid to accelerate the excretion of contrast media and reduce cerebral edema; the glucocorticoid methylprednisolone 40 mg bid was given to protect the blood-brain barrier. However, the patient was still restless, with limb convulsions and grand mal seizures. After intravenous injection of diazepam 10 mg, the seizures stopped; continuous intravenous pump dexmedetomidine was continued, and continuous intravenous pump sodium valproate 1.2 g was added to combat epilepsy symptoms.
Three days after the treatment, the patient’s consciousness gradually improved, and the nervous system examination findings are shown in Table 1. A repeat cranial CT showed that the swelling of brain tissue was significantly reduced, and there was no significant difference in the density of the bilateral cerebral hemispheres (Figure 3). On the 6th d, the patient was clearly conscious, but his speech was still not fluent, and his muscle strength level was 4. On the 17th d, there was no obvious abnormality in cranial magnetic resonance imaging (MRI) examination (Figure 3). When the patient was discharged on the 18th postprocedure day, there were no sequelae.
The first documented case of CIE was reported in 1970, and the patient presented with transient cortical blindness after coronary angiography[3]. According to a systematic review published in December 2020, the total number of CIE cases recorded after coronary angiography was 75[4].
It has been reported in the literature that contrast-induced encephalopathy usually occurs 2-12 h after the injection of contrast agent and disappears within 24-72 h, with a reported incidence of 0.06%[5]. Our department has conducted more than 3000 DSA procedures, and this is the first case of CIE. The pathogenesis of contrast-induced encephalopathy is still unclear, and potential mechanisms include: (1) Blood-brain barrier disruption: Normally, iodine compounds do not cross the blood-brain barrier. It is speculated that the integrity of the blood-brain barrier is destroyed during CIE, and the contrast agent penetrates into the central nervous system[6,7]; (2) neurotoxic effects: The blood-brain barrier is destroyed, and the contrast agent extravasation and direct stimulation of nerve cells caused by it led to neurotoxicity; however, the mechanism of the direct toxicity of contrast agents to neurons has not been elucidated; and (3) iodinated contrast agents can promote transient vasoconstriction. Animal studies have shown that iodinated contrast agents can increase the release of endothelin from endothelial cells and reduce the production of nitric oxide[8].
The clinical manifestations of CIE vary in severity, ranging from mild headache symptoms to severe clinical manifestations such as coma and even death. The clinical manifestations of CIE reported in the literature include transient cortical blindness and neurological symptoms such as restlessness, visual hallucinations, and deafness. Physical examination shows delirium state, increased muscle tone, tendon hyperreflexia, and positive pathological signs.
Based on the CT 2 h after DSA, we misdiagnosed the patient with subarachnoid hemorrhage (SAH). The disturbance of consciousness rapidly and progressively worsened 7 h after DSA. To exclude increased bleeding, new intracranial hemorrhage and cerebral infarction, the cranial CT was re-examined, and CIE was confirmed. Subarachnoid hemorrhage-like changes on CT in CIE have not been reported before. CIE imaging contrast enhancement, bleeding 40-60 (HU), CIE 100-300 (HU). MRI: T2, DWI and FLAIR high signal, MRI-derived apparent diffusion coefficient (ADC) in acute ischemic stroke ADC decreased, normal in CIE, can serve to differentiate from intracranial hemorrhage[9]. The diagnosis of CIE has to be based on both clinical symptoms and neurological signs: It appears within minutes to hours after the injection of an iodinated contrast agent. It usually recovers completely within 48-72 h. Other pathological processes, such as cerebral ischemia, etc., should be excluded. In this case, the neurological deficit lasted for a significantly longer time, and the symptoms began to resolve on the 3rd d after DSA. On the 6th d, there was still a lack of fluency in speech and poor calculation skills, but there were no sequelae at the 3-mo follow-up.
In this case, the lesions in this patient were mainly concentrated on the left side. This patient was a male with a history of hypertension, hyperlipidemia, and diabetes. Combined with intracranial aneurysm, the patient is prone to blood-brain barrier damage and belongs to the CIE risk group. In addition to common risk factors, the surgeon considered that the procedure time of angiography on the left side was longer than that on the right side, and the amount of contrast agent used was higher than that on the right side.
The patient did not undergo further interventional therapy for the aneurysm after DSA. Regarding the risk of CIE recurrence, Law reported a patient with binocular vision impairment following a previous cardiac catheterization who was prescribed antihistamines and corticosteroids as prophylaxis before a second procedure, but seizures and hemianopia occurred after the second angiography using iodixanol; these symptoms resolved after 24 h[10]. Spina reported a 65-year-old man with transient limb weakness after angiography. After a second coronary angiography two years later, he showed complete aphasia postprocedure despite preprocedure corticosteroids[11]. However, patients with a previous history of CIE who undergo repeat cardiac catheterization without adverse reactions after limiting the amount of contrast agent used and providing adequate hydration have also been reported. Therefore, the relevant reports show inconsistent conclusions on the recurrence of CIE.
In view of the large number of procedures in which contrast agents are used every year worldwide, CIE should be taken seriously. In patients with neurological and psychiatric symptoms after angiography, the possibility of CIE needs to be considered, but CIE must be distinguished from acute cerebrovascular accidents, including subarachnoid hemorrhage. Treatment measures include mainly corticosteroids, dehydration and diuresis, and adequate hydration. Patients with a history of contrast-induced encephalopathy should be carefully evaluated before any subsequent intravascular injection of contrast media and should be fully informed of the relevant risks.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fernandes SA, Brazil; Pitton Rissardo J, Brazil; Tavan H, Iran S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sawaya RA, Hammoud R, Arnaout S, Alam S. Contrast-induced encephalopathy following coronary angioplasty with iohexol. South Med J. 2007;100:1054-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Frontera JA, Pile-Spellman J, Mohr JP. Contrast-induced neurotoxicity and selective cortical injury. Cerebrovasc Dis. 2007;24:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Fischer-Williams M, Gottschalk PG, Browell JN. Transient cortical blindness. An unusual complication of coronary angiography. Neurology. 1970;20:353-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kariyanna PT, Aurora L, Jayarangaiah A, Das S, Gonzalez JC, Hegde S, McFarlane IM. Neurotoxicity Associated with Radiological Contrast Agents Used during Coronary Angiography: A Systematic Review. Am J Med Case Rep. 2020;8:60-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | de Bono D. Complications of diagnostic cardiac catheterisation: results from 34,041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London. Br Heart J. 1993;70:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Sterrett PR, Bradley IM, Kitten GT, Janssen HF, Holloway LS. Cerebrovasculature permeability changes following experimental cerebral angiography. A light- and electron-microscopic study. J Neurol Sci. 1976;30:385-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Lalli AF. Contrast media reactions: data analysis and hypothesis. Radiology. 1980;134:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Yanagisawa M, Inoue A, Ishikawa T, Kasuya Y, Kimura S, Kumagaye S, Nakajima K, Watanabe TX, Sakakibara S, Goto K. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988;85:6964-6967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 343] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Yu J, Dangas G. Commentary: New insights into the risk factors of contrast-induced encephalopathy. J Endovasc Ther. 2011;18:545-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Law S, Panichpisal K, Demede M, John S, Marmur JD, Nath J, Baird AE. Contrast-Induced Neurotoxicity following Cardiac Catheterization. Case Rep Med. 2012;2012:267860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Spina R, Simon N, Markus R, Muller DW, Kathir K. Recurrent contrast-induced encephalopathy following coronary angiography. Intern Med J. 2017;47:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |