Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2254
Peer-review started: October 13, 2022
First decision: December 13, 2022
Revised: January 16, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: April 6, 2023
Processing time: 167 Days and 22.9 Hours
Neonatal hyperinsulinism can result from perinatal stress, genetic disorders, or syndromes, which can lead to persistent or intractable hypoglycemia in newborns. Mutations in the ABCC8 gene result in abnormal functioning of potassium channel proteins in pancreatic β-cells, leading to an overproduction of insulin and congenital hyperinsulinemia.
We report a case of a high-birth-weight infant with postnatal hypoglycemia and hyperinsulinemia, whose mother had pregestational diabetes mellitus with poor glycemic control and whose sister had a similar history at birth. Whole-exome sequencing revealed a new mutation in the ABCC8 gene in exon 8 (c.1257T>G), which also occurred in his sister and mother; thus, the patient was diagnosed with neonatal hyperinsulinism with an ABCC8 mutation. With oral diazoxide treatment, the child’s blood glucose returned to normal, and the pediatrician gradually discontinued treatment because of the child’s good growth and development.
We report a new mutation locus in the ABCC8 gene. This mutation locus warrants attention for genetic disorders and long-term prognoses of hypoglycemic children.
Core Tip: Neonatal hyperinsulinemia can have multiple causes. Persistent hypoglycemia caused by a genetic mutation is difficult to correct by conventional glucose and hydrocortisone infusion treatments, which may lead to adverse outcomes. In this case, the newborn had a family history of hypoglycemia and hyperinsulinism, was exposed to perinatal stress, and exhibited a mutation locus in the ABCC8 gene in exon 8 (c.1257T>G), which has not been previously reported. With diazoxide treatment, the child recovered well, and family genetic examinations showed a good prognosis.
- Citation: Liu MT, Yang HX. Neonatal hyperinsulinism with an ABCC8 mutation: A case report. World J Clin Cases 2023; 11(10): 2254-2259
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2254.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2254
Neonatal hyperinsulinemia occurs in three types: Perinatal-stress-related, monogenic, and syndromic (e.g., Beckwith-Wiedemann syndrome). Primarily resulting from single gene defects, congenital hyperinsulinism (CHI) can cause infants to be large for their gestational age and can lead to persistent or intractable hypoglycemia, seizures, and potentially hypoglycemic encephalopathy. This condition can arise from mutations in common genes such as ABCC8 and KCN1, which can be autosomal recessive or dominant[1]. In the general population, the prevalence of CHI is approximately 1/50000-1/28000, reaching up to 1/2700 in consanguineous couples[2].
The ABCC8 gene, located on chromosome 11p15,1, encodes the sulfonylurea receptor 1 subunit of the pancreatic β-cell ATP-sensitive potassium channel protein (KATP), which regulates the flux of K+ ions and binds glucose via membrane electrical activity and insulin release. Mutations in the ABCC8 gene result in abnormal functioning of the potassium channel protein, such as the deletion of membrane surface proteins and closure of potassium channels, causing pancreatic β-cells to overproduce insulin[3,4], which results in CHI. Prognoses vary widely, ranging from adverse neurodevelopment and the need for lifelong medication to situations requiring no intervention[5].
Consequently, a better understanding of neonatal hypoglycemia and hyperinsulinism is needed. Quickly identifying the underlying genetic abnormalities in patients and improving genetic testing may be helpful for future treatments and prognoses. However, many reported genetic abnormalities remain undetected, and their genetic characteristics are unclear. Herein, we report a case of neonatal hyperinsulinism in a macrosomia male newborn with an ABCC8 mutation located in exon 8 (c.1257T>G), which has not been previously reported. The effects of this mutation require further elucidation, but we propose that this genetic mutation combined with perinatal stress caused severe hyperinsulinism and hypoglycemia in this patient. We also present a review of previous studies, summarize clinical features and treatment methods, and identify potential prognostic factors for this mutation.
A large-for-gestational-age male newborn was brought to the neonatal intensive care unit because of mild asphyxia, with an APGAR score of 7/10/10. After two hours, the infant presented intractable hypoglycemia and hyperinsulinism.
This term male infant was born to nonconsanguineous parents on February 22, 2021, with a birth weight of 5020 g. This baby represented his mother’s second gestation. The mother was 38 years old and was diagnosed with pregestational diabetes mellitus at 25 wk of gestation, with an oral glucose tolerance test value of 6.28-12.63-14.11 mmol/L, which was unsatisfactorily controlled by diet and treated by insulin. Her glycosylated hemoglobin changed from 6.10% (2020-12-16) to 6.40% (2021-1-20) and 6.10% (2021-2-18), and her glycosylated albumin changed from 17.52% (2020-12-16) to 15.19% (2020-12-28), 14.88% (2021-1-20), and 13.70% (2021-2-18).
The patient’s mother had previously developed gestational diabetes (controlled with diet) during her first gestation, which occurred eight years ago.
The patient’s older sister was also large for her gestational age, with a birth weight of 4000 g. She presented severe hypoglycemia with brain damage after birth, but is currently in good condition.
The child showed an appearance of macrosomia with a ruddy complexion and good crying but a poor mental response. The oxygen saturation level was 70%-89%. The patient’s breath sounds were mildly rough in both lungs. A pair of ears was visible on the right. The remainder of the physical examination showed no obvious abnormalities.
The child had a minimum postnatal terminal glucose level of 2.70 mmol/L, minimum intravenous glucose level of 2.66 mmol/L, maximum insulin level of 122.60 µIU/mL, and C-peptide level of 17.08 ng/mL during this period. His blood and urine amino acid and organic acid metabolic screening showed no abnormalities.
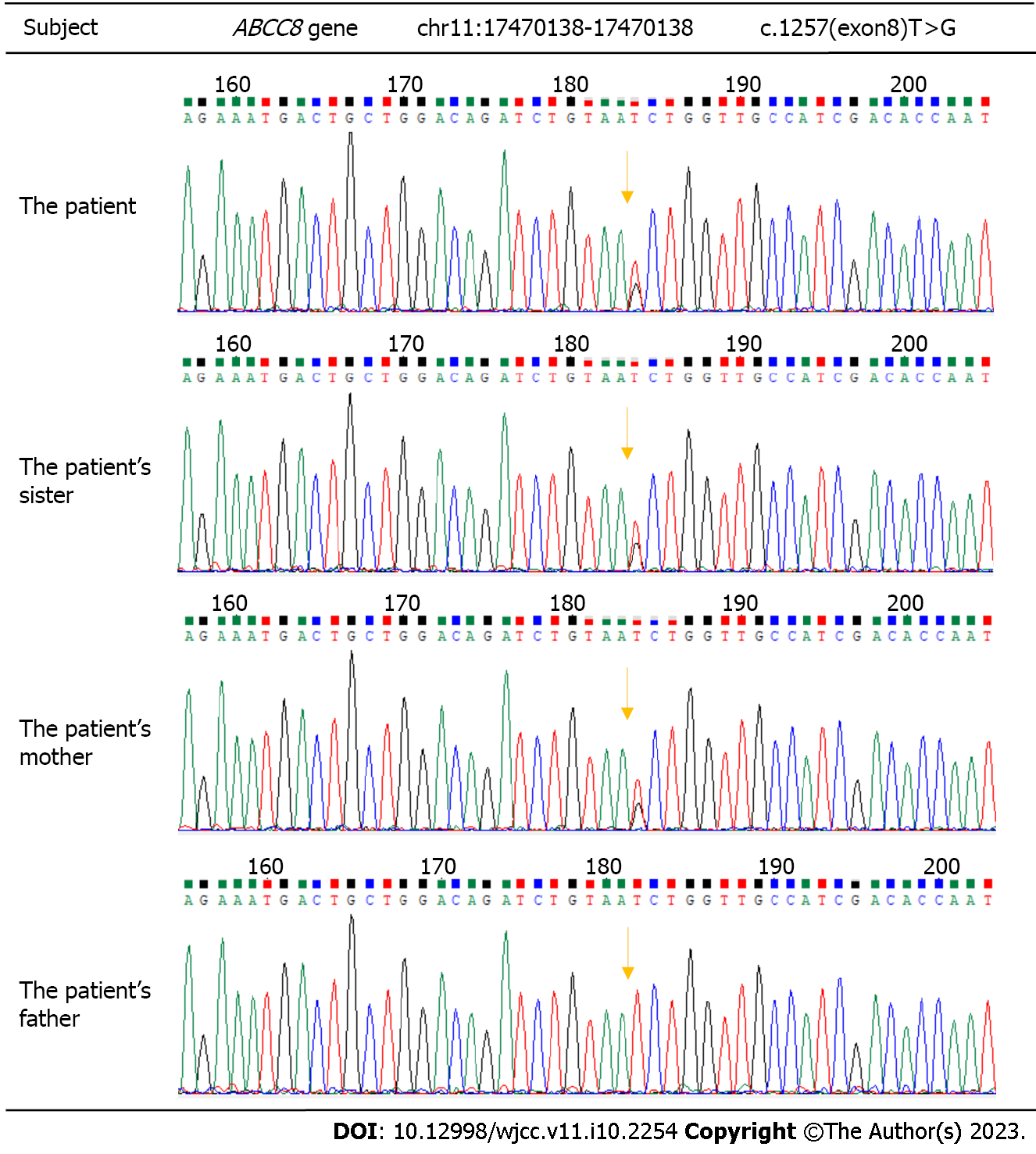
The persistent hypoglycemia and hyperinsulinemia state of the child could not be explained by perinatal stress alone; thus, whole-exome sequencing was performed and identified a missense heterozygous mutation in the ABCC8 gene in exon 8 (c.1257T>G), resulting in amino acid p.N419K (p.Asn419Lys). Unsurprisingly, genetic examinations of the family revealed similar mutations in the mother and sister but no variation in the father (Figure 1).
On day 1, the patient’s postnatal electrocardiogram showed mild abnormalities: Multifocal spike and spike-wave issuance during sleep QS. Postnatal re-examinations on days 4 and 12 showed normal neonatal electroencephalography findings. A cranial ultrasound showed mild enhancement of cerebral white matter echogenicity; the ultrasound was later repeated and showed a reduction compared with the previous ultrasound. The ultrasound showed mild enhancement of bilateral paraventricular white matter echogenicity 11 d after birth. Cranial MRI did not show any significant abnormalities (Table 1).
| Time | Incidents |
| 8 yr ago | The patient’s mother was diagnosed gestational diabetes (controlled with diet) during her first gestation, and deliver a girl with a birth weight of 4000 g, who presented severe hypoglycemia with brain damage after birth |
| At the mother’s 25th wk of gestation | The mother was diagnosed with pregestational diabetes mellitus and was treated by insulin |
| 20 min after birth | The patient was diagnosed mild asphyxia, with an APGAR score of 7/10/10, and was brought to the neonatal intensive care unit |
| 2 h after birth | The infant presented intractable hypoglycemia and hyperinsulinism |
| During hospitalization | He was initially treated with glucose and hydrocortisone infusion. Next, he was treated with octreotide and glucagon. whole-exome sequencing was performed because the upper treatments are ineffective. Thus, he was treated with diazoxide, which was effective |
| 25 d after birth | The patient was discharged from the hospital and continued to take diazoxide orally, and the medication was tapered off |
| 30 d after birth | The whole-exome sequencing identified a missense heterozygous mutation in the ABCC8 |
| Now | The patient’s blood glucose level is average, and his growth and development are acceptable. His mother’s fasting glucose level is impaired |
The child was diagnosed with neonatal hyperinsulinism with an ABCC8 mutation.
The child was given endotracheal intubation and mechanical ventilation and was withdrawn from ventilator assistance 23 h after birth. He was initially treated with glucose and hydrocortisone infusion, but his blood glucose was still not well controlled. Next, he was treated with octreotide and glucagon, which did not provide satisfactory results; thus, he was treated with diazoxide, which was effective. The patient’s condition was stable 25 d after birth, and he was discharged from the hospital and continued to take diazoxide orally.
After discharge, the patient was treated at a local hospital, and the medication was tapered off. Currently, his blood glucose level is average, and his growth and development are acceptable. His mother’s fasting glucose level is impaired.
A variety of CHI cases caused by mutations in the ABCC8 gene have been previously reported in multiple countries. Snider et al[6] reviewed genetic variants in 417 children with CHI and reported 160 mutations in the ABCC8 gene, 91 of which resulted in single-amino-acid changes. The mode of inheritance of CHI due to mutations in the ABCC8 gene is primarily autosomal recessive. However, cases of paternal gene mutation with maternal chromosome deletion in the tissue of the pancreatic lesion often respond poorly to diazoxide treatment and require surgical resection treatment. The features of dominant KATP hyperinsulinemia differ substantially from those of recessive inheritance, including a retention of normal subunit transport and impaired channel activity. This mild hypoglycemic phenotype can usually be effectively treated by diazoxide and may even go undetected in the absence of clinical symptoms. This phenotype also has a better prognosis, with the hypoglycemic component resolving spontaneously, and in some cases, treatment can be stopped altogether[7]. From a histopathological viewpoint, CHI is roughly divided into diffuse and focal types, with surgical lesion resection being the treatment of choice for the latter. For diffuse CHI, which appears to predominantly arise from paternally inherited mutations[8], a pharmacological approach is considered as the first line of treatment[5].
In this case, the child’s mother had gestational diabetes mellitus with poor glycemic control and macrosomia. The intrauterine fetus was exposed to high glucose levels during development, which led to an excessive activation of pancreatic islet cells and overproduction of insulin. Research has shown that these conditions result in a significantly higher incidence of hypoglycemia among newborns; thus, it was considered that the child was born with hypoglycemia and high insulin due to perinatal stress and his mother’s poor glycemic control during pregnancy. However, theoretically, the mother’s blood glucose did not reach a sufficiently high level to cause the degree of hyperinsulinemia observed in the child. Moreover, the child’s sister showed similar symptoms at birth, despite her mother having reasonable glycemic control during her pregnancy; therefore, familial CHI could not be excluded. The child was found to have an ABCC8 missense heterozygous mutation [c.1257(exon8)T>G]. Both his mother and sister had similar mutations, and diazoxide was effective in treating the child’s hypoglycemia with a good follow-up prognosis. Hence, it was considered that maternal autosomal dominant inheritance may have led to CHI.
Similar cases of macrosomia combined with transient CHI have been attributed to dominant mutations in ABCC8[9,10]. A mild form of CHI channel activity with a reduced KATP pathway in infancy can be effectively treated with the potassium channel opener diazoxide, with a good prognosis; however, by the time these patients reach adulthood, this mutation leads to decreased insulin secretion capacity and glucose intolerance, and long-term prognoses may be associated with diabetes[10-12]. Kassem et al[13] studied 23 children with CHI and found that the natural disease course may lead to progressive glucose intolerance and diabetes mellitus. It has also been recently reported that multifarious ABCC8 genetic variants cause early-onset diabetes in Chinese individuals[14]. This condition may be caused by increased β-cell apoptosis and slow progressive loss of insulin secretory function. These findings suggest that attention should be given to the long-term glycemic situation of this child and his sister, although the effects of the mutation locus in exon 8 (c.1257T>G) are not yet clear.
Upon searching the Human Gene Mutation Database, we found no previous reports related to mutations at the c.1257 Locus similar to the mutation in this child; thus, the effect of such mutations on protein expression requires further investigation. A total of approximately 180 variants of unknown significance (VUSs) have been reported for the ABCC8 gene[3], accounting for approximately 15% of the clinically reported variants found in CHI. It has been proposed that variant-induced splicing alterations may be the primary mechanism of inheritance. Therefore, functional analysis may be required for further pathogenicity assessment to clarify VUSs or phenotype-associated ABCC8 mutations[15].
This case report provides insights for perinatal medicine practitioners when neonatal hypoglycemia or hyperinsulinemia is not easily corrected. Apart from considering the association with maternal glycemic control, the possibility of a single gene defect should be considered to inform the long-term prognosis of the child and subsequent pregnancies of the mother.
Neonatal hyperinsulinemia can arise from multiple causes. Genetic causes such as ABCC8 mutations should be considered when a persistent hypoglycemic state cannot be explained by perinatal stress alone. In such cases, genetic testing is recommended. Moreover, genetic testing may help in developing individual treatment plans and predicting outcomes and future impacts for these newborns and their families.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lang FC, United States; Redkar RG, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | James C, Kapoor RR, Ismail D, Hussain K. The genetic basis of congenital hyperinsulinism. J Med Genet. 2009;46:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Yau D, Laver TW, Dastamani A, Senniappan S, Houghton JAL, Shaikh G, Cheetham T, Mushtaq T, Kapoor RR, Randell T, Ellard S, Shah P, Banerjee I, Flanagan SE. Using referral rates for genetic testing to determine the incidence of a rare disease: The minimal incidence of congenital hyperinsulinism in the UK is 1 in 28,389. PLoS One. 2020;15:e0228417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | De Franco E, Saint-Martin C, Brusgaard K, Knight Johnson AE, Aguilar-Bryan L, Bowman P, Arnoux JB, Larsen AR, Sanyoura M, Greeley SAW, Calzada-León R, Harman B, Houghton JAL, Nishimura-Meguro E, Laver TW, Ellard S, Del Gaudio D, Christesen HT, Bellanné-Chantelot C, Flanagan SE. Update of variants identified in the pancreatic β-cell K(ATP) channel genes KCNJ11 and ABCC8 in individuals with congenital hyperinsulinism and diabetes. Hum Mutat. 2020;41:884-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 4. | Stanley CA. Perspective on the Genetics and Diagnosis of Congenital Hyperinsulinism Disorders. J Clin Endocrinol Metab. 2016;101:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | Banerjee I, Salomon-Estebanez M, Shah P, Nicholson J, Cosgrove KE, Dunne MJ. Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia. Diabet Med. 2019;36:9-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 6. | Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, Ganapathy K, Bhatti T, Stanley CA, Ganguly A. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98:E355-E363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 234] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, Ganguly A, Shyng SL, Stanley CA. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008;118:2877-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Bellanné-Chantelot C, Saint-Martin C, Ribeiro MJ, Vaury C, Verkarre V, Arnoux JB, Valayannopoulos V, Gobrecht S, Sempoux C, Rahier J, Fournet JC, Jaubert F, Aigrain Y, Nihoul-Fékété C, de Lonlay P. ABCC8 and KCNJ11 molecular spectrum of 109 patients with diazoxide-unresponsive congenital hyperinsulinism. J Med Genet. 2010;47:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Kole MB, Ayala NK, Clark MA, Has P, Esposito M, Werner EF. Factors Associated With Hypoglycemia Among Neonates Born to Mothers With Gestational Diabetes Mellitus. Diabetes Care. 2020;43:e194-e195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Kapoor RR, Flanagan SE, James CT, McKiernan J, Thomas AM, Harmer SC, Shield JP, Tinker A, Ellard S, Hussain K. Hyperinsulinaemic hypoglycaemia and diabetes mellitus due to dominant ABCC8/KCNJ11 mutations. Diabetologia. 2011;54:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, Laakso M. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Hussain K, Cosgrove KE. From congenital hyperinsulinism to diabetes mellitus: the role of pancreatic beta-cell KATP channels. Pediatr Diabetes. 2005;6:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 266] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Li M, Gong S, Han X, Zhang S, Ren Q, Cai X, Luo Y, Zhou L, Zhang R, Liu W, Zhu Y, Zhou X, Sun Y, Li Y, Ma Y, Ji L. Genetic variants of ABCC8 and phenotypic features in Chinese early onset diabetes. J Diabetes. 2021;13:542-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Saint-Martin C, Cauchois-Le Mière M, Rex E, Soukarieh O, Arnoux JB, Buratti J, Bouvet D, Frébourg T, Gaildrat P, Shyng SL, Bellanné-Chantelot C, Martins A. Functional characterization of ABCC8 variants of unknown significance based on bioinformatics predictions, splicing assays, and protein analyses: Benefits for the accurate diagnosis of congenital hyperinsulinism. Hum Mutat. 2021;42:408-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |