Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2213
Peer-review started: January 18, 2023
First decision: January 31, 2023
Revised: February 1, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 6, 2023
Processing time: 71 Days and 3 Hours
Primary hyperparathyroidism (pHPT) is the third most common endocrine disease. The surgical procedure aims for permanent cure, but recurrence has been reported in 4%-10% of pHPT patients. Preoperative localization imaging is highly valuable. It includes ultrasound, computed tomography (CT), single-photon-emission CT, sestamibi scintigraphy and magnetic resonance imaging. The operation has been defined as successful when postoperative continuous eucalcemia exists for more than the first six months. Ongoing hypercalcemia during this period is defined as persistence, and recurrence is defined as hypercalcemia after six months of normocalcemia. Vitamin D is a crucial factor for a good outcome. Intraoperative parathyroid hormone (PTH) monitoring can safely predict the outcomes and should be suggested. PTH ≤ 40 pg/mL or the traditional decrease ≥ 50% from baseline minimizes the likelihood of persistence. Risk factors for persistence are hyperplasia and normal parathyroid tissue on histopathology. Risk factors for recurrence are cardiac history, obesity, endoscopic approach and low-volume center (at least 31 cases/year). Cases with double adenomas or four-gland hyperplasia have a greater likelihood of persistence/ recurrence. A 6-mo calcium > 9.7 mg/dL and eucalcemic parathyroid hormone elevation at 6 mo may be associated with recurrence necessitating long-term follow-up. 18F-fluorocholine positron emission tomography and 4-dimensional CT in persistent and recurrent cases can be valuable before reoperation. With these novel advances in preoperative imaging and localization as well as intraoperative PTH measurement, the recurrence rate has dropped to 2.5%-5%. Six-month serum calcium ≥ 9.8 mg/dL and parathyroid hormone ≥ 80 pg/mL indicate a risk of recurrence. Negative sestamibi scintigraphy, diabetes and elevated osteocalcin levels are predictors of multiglandular disease, which brings an increased risk of persistence and recurrence. Bilateral neck exploration was considered the gold-standard diagnostic method. Minimally invasive parathyroidectomy and neck exploration are both effective surgical techniques. Multidisciplinary diagnostic and surgical management is required to prevent persistence and recurrence. Long-term follow-up, even up to 10 years, is necessary.
Core Tip: Surgical intervention can permanently cure primary hyperparathyroidism. Advances in preoperative imaging and localization as well as intraoperative parathormone monitoring have significantly reduced the rates of persistence and recurrence. The criteria for reoperation must be stricter and based on biochemical confirmation of the diagnosis, review of preexisting data, and positive imaging findings. However, diagnosis and treatment are multidisciplinary tasks. The final decision about reoperation and its procedure should be made by an experienced endocrine surgeon. In any case, preoperative assessment of recurrent laryngeal nerve function and intraoperative measurement of parathyroid hormone are necessary.
- Citation: Pavlidis ET, Pavlidis TE. Update on the current management of persistent and recurrent primary hyperparathyroidism after parathyroidectomy. World J Clin Cases 2023; 11(10): 2213-2225
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2213.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2213
Basic prerequisites in parathyroid surgery are the ability to identify the parathyroids, the deep knowledge of their anatomical location and its wide spectrum of variations, and the ability to distinguish between a normal and pathological gland. The superior parathyroids in 85% of cases are located within a radius of 1 cm from the inferior angle of the thyroid, while inferior parathyroids in more than 50% of cases are located in the lower third of the junction between the posterior end of the thyroid gland and the anterior part of the thymus gland or within it, within the mediastinum, or even within the thyroid parenchyma. The exact anatomical location of the parathyroid glands comes in six recognized types, as shown in Table 1[1]. The anatomical location of the supernumerary parathyroid is located below the thyroid in the remnants of the thymus (two-thirds of cases) or near the thyroid, usually between the other two parathyroids (one-third)[1,2].
| Type | Location |
| A1 | Attached to the thyroid capsule |
| A2 | Partially or completely embedded in the thyroid gland but outside the capsule |
| A3 | Within the thyroid parenchyma |
| B1 | Peripheral, existence of a natural gap space between parathyroid and thyroid gland |
| B2 | Within the thymus gland |
| B3 | Blood supply from thymic or mediastinal vessels |
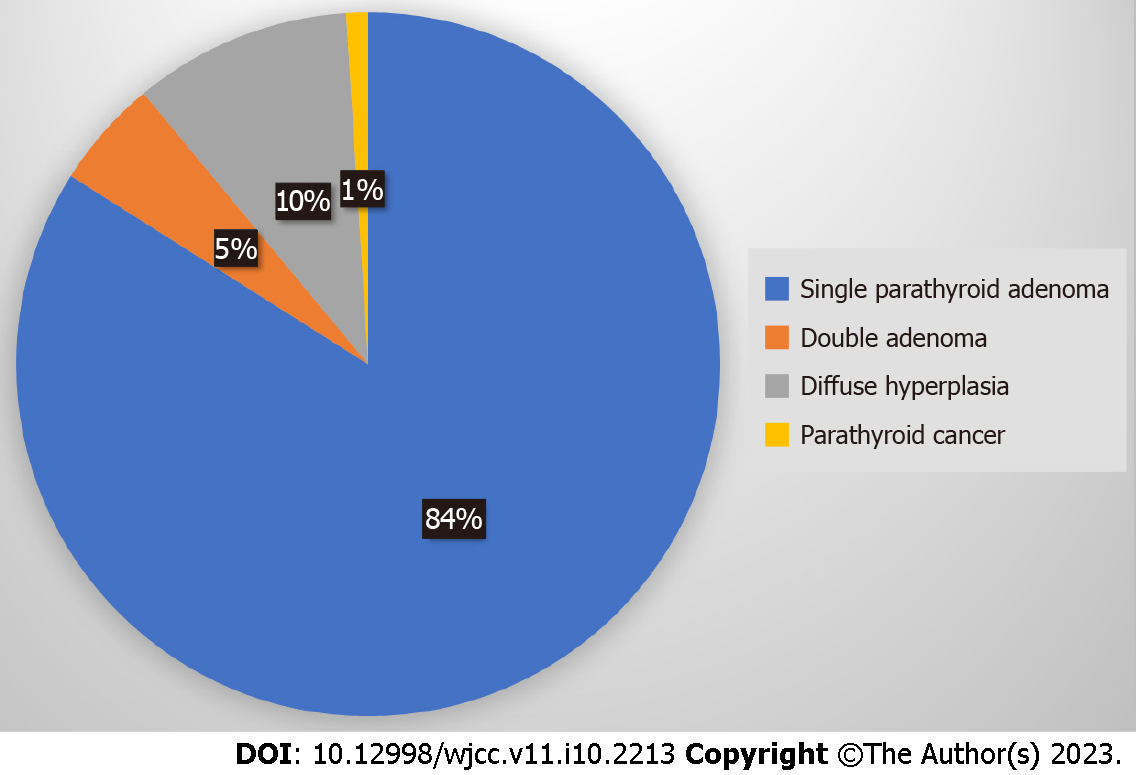
Primary hyperparathyroidism (pHPT) is the third most common endocrine disease[3], with ongoing incidence and 100000 new cases annually in the United States[4]. It is sporadic (90%-95%) and hereditary (5%-10%) and is associated with multiple endocrine neoplasia (MEN) syndromes. Its causes include mainly single parathyroid adenoma (PA) (80%-85%), double adenoma (4%-5%), diffuse hyperplasia (10%-15%) and parathyroid cancer (< 1%), as shown schematically in Figure 1[5]. Cure has been defined as the restoration of calcium levels (normal homeostasis) lasting six months at a minimum[3].
Cure rates currently range between 95% and 99%[3,6,7]. However, normocalcemia before the initial operation exists in 10% of pHPT[8,9]. Patients with pHPT with normal calcium levels but persistently high PTH values after parathyroidectomy should be evaluated for possible secondary HPT or recurrent disease[7].
Persistent hyperparathyroidism (P-HPT) is defined as the condition where calcium either does not return to normal values or rises again within 6 mo of the initial parathyroidectomy for pHPT[8]. It is mainly due to failure to remove an overactive parathyroid (adenoma or unrecognized parathyroid hyperplasia)[8,10,11]. Recurrent hyperparathyroidism (R-HPT) occurs when calcium increases beyond normal limits after at least 6 mo of normal values after the initial parathyroidectomy for pHPT. It is due to untreated parathyroid hyperplasia, parathyroid carcinoma (PC) or metastasis, autograft hyperplasia or parathyromatosis[3,6,12]. The latter (benign diffused multiple nodules of hyperfunctioning parathyroid tissue in the neck and superior mediastinum) is a very rare cause of R-HPT[13,14].
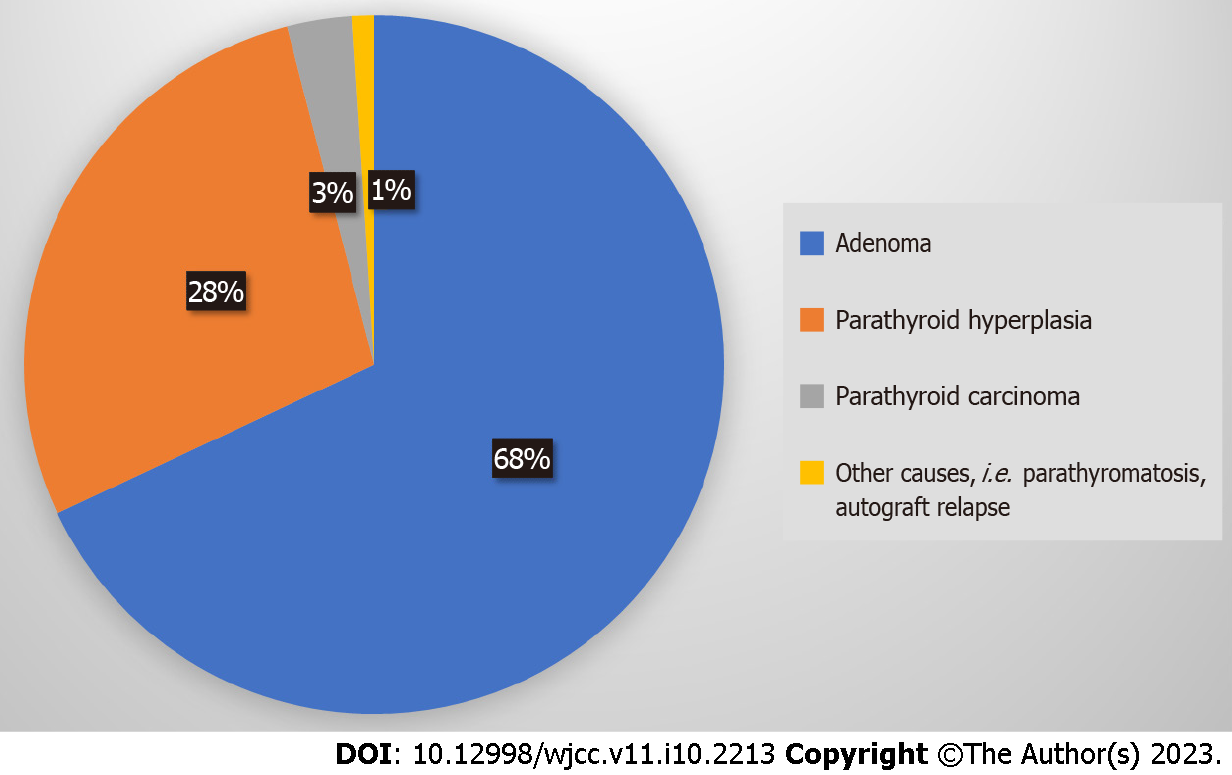
The causes of P-HPT/R-HPT are adenoma (68%), parathyroid hyperplasia (28%), PC (3%), and other causes (1%, parathyromatosis, autograft relapse), as shown schematically in Figure 2[9]. Cases with double adenomas or four-gland hyperplasia have a higher likelihood of P-HPT/R-HPT[15]. The ectopic locations of PA (approximately 0.3%-8%) include the hypoglossal nerve, the posterior triangle of the neck, the axilla, the mediastinum, the pericardium[16], the thymus gland[2] and thyroid gland[17].
Risk for persistence constitutes hyperplasia and normal parathyroid tissue on the histopathological report[3]. Additionally, measuring parathyroid hormone and calcium in the early postoperative period (after two weeks) may better predict later recurrent pHPT[18]. However, a postoperative increase in PTH does not always indicate P-HPT/R-HPT; rather, it may be due to vitamin D insufficiency, mild renal failure, hyperfunction of previously suppressed parathyroid glands, unrecognized familial hypercalciuric hypocalcemia, and increased bone redistribution of calcium[18]. Subsequently, preoperative 25-hydroxyvitamin D level must be assessed especially in patients without severe hypercalcemia, and must be corrected if low for a good outcome[19].
The incidence of P-HPT/R-HPT was approximately 30% in 1990. Today, with the ongoing advances in preoperative parathyroid imaging, implementation of intraoperative PTH monitoring (IPM), and avoidance of bilateral neck exploration (BNE) to locate the parathyroids, this percentage ranges between 2.5%-5%[12]; however, in recent reports, it was higher than traditionally described, reaching up to 10% or even 14%[8]. The predictors of P-HPT/R-HPT occurrence are shown in Table 2[12]. Transient hypercalcemia in the early postoperative period does not predict persistent pHPT, since it exists at a rate of almost 10% after successful parathyroidectomy, which in most cases is normalized within two weeks[20].
| No. | Predictor |
| 1 | Age > 70 |
| 2 | Obesity |
| 3 | ASA score 3 |
| 4 | Low hospital case volume (n < 50/yr) |
| 5 | Inadequate surgeon’s experience |
| 6 | Ambiguous scintigraphy results with Sestamibi |
| 7 | Primary disease (single adenoma < double adenoma < parathyroid hyperplasia) |
| 8 | Surgical strategy (not recognizing the pathological gland, not applying bilateral cervical exploration in case of inadequate PTH reduction intraoperatively) |
In this minireview, we evaluate the current management options for failure after surgery for pHPT, highlighting the updated knowledge by selecting and focusing on the most relevant articles from PubMed.
Multidisciplinary diagnostic and surgical management is required to prevent persistence and recurrence. The preoperative localization imaging of the initial operation is of great importance. It can include ultrasound (US), computed tomography (CT), single-photon emission CT (SPECT) and sestamibi scintigraphy[21].
The preoperative evaluation of P-HPT/R-HPT includes confirmation of the diagnosis, review of preexisting data, stricter indications for reintervention, and diagnostic imaging[7]. Confirmation of the diagnosis can be made by the following: (1) All biochemical data before and after the initial operation should be rechecked; (2) checking for medications that affect calcium metabolism (e.g., lithium, thiazide diuretics); (3) detailed checks for elevated PTH from another cause, as mentioned above (e.g., renal failure, vitamin D deficiency); (4) taking a detailed family history for MEN (MEN1, MEN2A); and (5) investigation of possible parathyroid cancer and/or parathyromatosis[7].
The review of preexisting data is based on previous imaging scans, details of the operative report, histopathological findings and even re-evaluation of paraffin blocks[12].
Diagnostic imaging includes US, sestamibi scintigraphy +/- SPECT, 4-dimensional (4D) CT or magnetic resonance imaging (MRI) and elective venous catheterization. The introduction of an iodine-containing contrast is contraindicated in cases where there is a decrease in the filtration function of the kidneys[12].
Normal parathyroid glands are typically not visible on US due to their small size (2-3 mm). The advantages of US include no exposure to radiation, low cost, easy availability and simultaneous checking of the thyroid gland. Its disadvantages are decreased resolution with high body mass index, which depends on the sonographer’s experience, limited visibility in parathyroid hyperplasia and low-lying upper or inferior glands[7]. A meta-analysis has shown a sensitivity of up to 79%[22]. This falls to 35% in cases of parathyroid hyperplasia[23]. Specificity in some centers reaches 96%[24]. In combination with sestamibi or 4D-CT, US has the optimal cost benefit ratio[25]. The combination of US and sestamibi significantly increases the sensitivity for identifying an adenoma compared to each method alone; however, it remains low in cases of parathyroid hyperplasia, at 30%-60%[7].
Sestamibi scintigraphy is based on the differential uptake and retention of the radiotracer in mitochondria-rich cells. Its advantages include assessment of deep-neck-located or ectopic parathyroid even in the mediastinum, low radiation exposure and ability to assess parathyroid tissue autografts. Its disadvantages are weaker assessment of the thyroid gland, possible false-positive results from thyroid nodules and reduced effectiveness in parathyroid hyperplasia[7]. There are several protocols that use sestamibi (dual-phase, I131 subtraction, SPECT, and 4-D SPECT-CT imaging) with various strengths and weaknesses[26].
SPECT imaging provides 4-D imaging with a sensitivity of 79% (range 45%-92%). It is useful for detecting posteriorly located adenomas of superior parathyroids that are usually obscured by thyroid uptake. According to a meta-analysis, its sensitivity is reduced significantly in cases of parathyroid hyperplasia (44%) or double adenoma (33%)[23]. Additionally, the sensitivity of SPECT depends on the weight of the affected gland. If it is more than 500 mg, the sensitivity will be 93%, whereas if it is less than 500 mg, it will fall to 51%[27]. Typically, a PA is characterized by the rapid uptake of contrast medium and thus the relative elimination of its distribution to the thyroid and surrounding tissues. The advantages of 4D-CT include the accurate anatomical detail and functional condition of the parathyroid whether it has a normal or ectopic position, its being the only method that can identify normal parathyroids and its greater sensitivity for parathyroid hyperplasia compared to other methods (62.5%-85.7%). Its disadvantages are the cost, greater exposure to radiation, limited availability and accessibility, and the requirement of specialized personnel to interpret the results[7]. According to another meta-analysis, it has a sensitivity of 89%[22]. In a direct comparison, the sensitivity of 4D-CT (88%) is superior to that of US (57%) and sestamibi (65%)[28].
For the initial imaging of parathyroids, US of the neck is recommended to identify parathyroid disease and assess possible coexisting thyroid pathology. An experienced clinician should decide which other imaging modality to use, depending on the capabilities available in each center or region[7]. In reoperative parathyroid imaging, at least one positive preoperative imaging is necessary to plan the reoperation, as abnormal glands may also be ectopic. Otherwise, there is a fourfold greater likelihood of failure. Some surgeons require one positive imaging test, while others require two positive imaging tests before reoperation[29]. 4D-CT has a higher sensitivity (88%) than Sestamibi scintigraphy (54%) in reintervention[30]. In a study of 90 patients with reoperation, there was 90% concordance between 4D-CT and surgical findings, while the concordance between other imaging and surgical findings was only 63%, with a shorter operating time (76 min vs 114 min). Its efficiency in reoperations, especially in difficult undiagnosed-by-sestamibi cases and hyperplasia, along with its growing use in recent years, makes it the first choice imaging method for P-pHPT and R-pHPT[31].
The two most commonly used preoperative diagnostic tools are neck US and scintigraphy. The latter is typically performed using the radiotracer technetium-99m-sestamibi in combination with SPECT plus 4D-CT if needed, which may be more cost-effective[25]. In cases of P-pHPT/R-pHPT, positron emission tomography (PET)/CT can detect a hyperfunctioning parathyroid with accuracy and has been proven to be better than 4D-CT. Therefore, 18F-fluorocholine PET/CT has been recently advocated before reoperation[32-34].
A recent study demonstrated that in addition to US and myocardial perfusion imaging (MIBI), noncontrast 3-Tesla MRI further increased the preoperative localization of pHPT (sensitivity of 92.0%)[35].
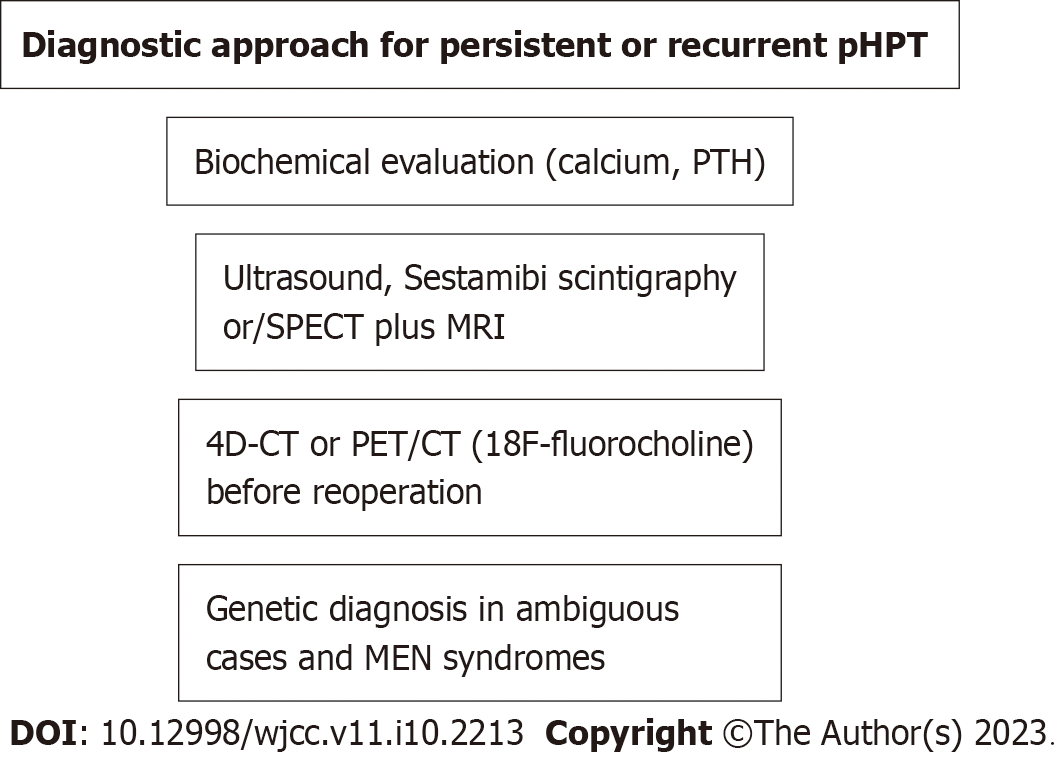
A scheme of the diagnostic approach is shown in Figure 3.
Despite the above, although it can be risky, no preoperative routine imaging protocols to reduce the cost, only intraoperative PTH measurement, have been advocated for pHPT with a skilled surgeon and without previous neck surgery[36].
While noninvasive imaging methods are preferred, invasive methods play a selective role. In contrast to what happens in the initial surgery, in reoperation, US-guided fine needle aspiration of the suspected parathyroid gland preoperatively may be useful by providing cytology and PTH levels. Its potential complications are hematoma, parathyromatosis and the spread of cancer cells. Thus, it is only recommended in selected cases[7,9].
Selective venous catheterization is rarely used and is impossible if there has been ligation of the thyroid and parathyroid vessels. Catheterization of the femoral vein and assessment of PTH in venous blood drained from the cervix and mediastinum can identify the side where the pathological gland is located. Its sensitivity in P-HPT/R-HPT is 83.3% for solitary adenomas and 91.6% for parathyroid hyperplasia. However, it is expensive, requires expertise and is associated with inguinal hematoma and vascular injuries[7].
There are rare cases of atypical tumors[37] or atypical PA (APA) with an ambiguous potential for malignancy that have a good prognosis in the vast majority, but mutations in the CDC73 gene are related to high P-HPT/R-HPT[38,39]. Flow-cytometric DNA analysis has been recommended in such cases[40], especially in the context of MEN syndromes[41]. CDC73 gene mutations are not involved in the tumorigenesis of sporadic APA[38]. KMT2D might be a novel candidate driver gene that can be used as a diagnostic biomarker for PA. However, CDC73 mutations can be an early developmental event from PA to PC[42]. Germline mutations in CDKIs (the CDKN1B gene and CDKN2C gene) should be included in the genetic testing of patients with pHPT[43].
In resected specimen biopsy, hypercellularity of an otherwise normal parathyroid does not indicate multiglandular disease related to hyperfunction[44].
In a multicenter study, two preoperative mathematical models predicted adenoma, atypical adenoma and PC with reliability, thus determining the choice of surgical plan[45].
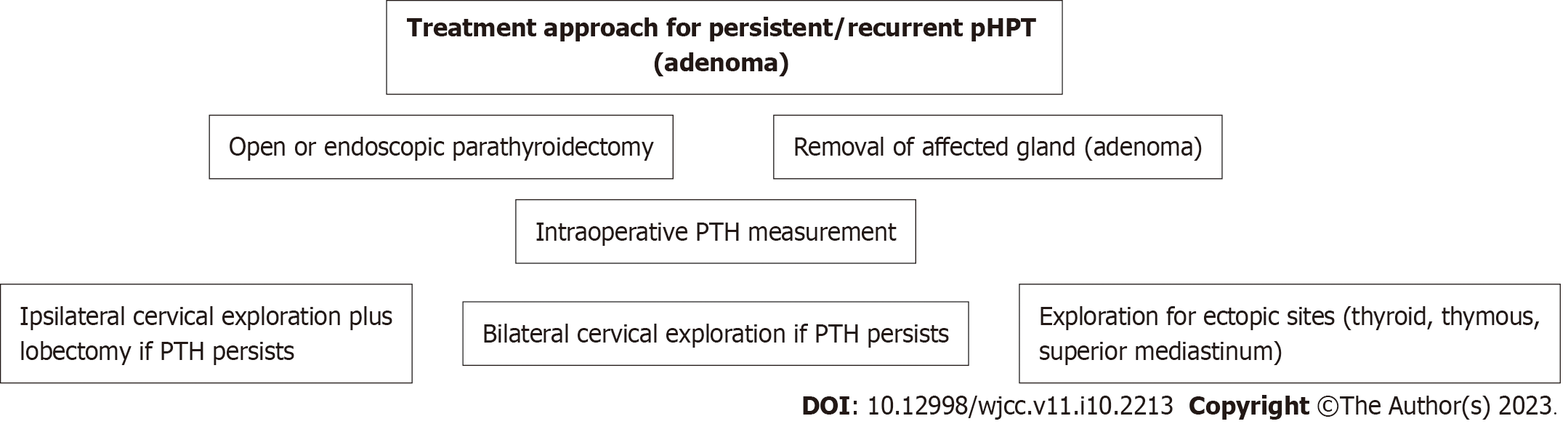
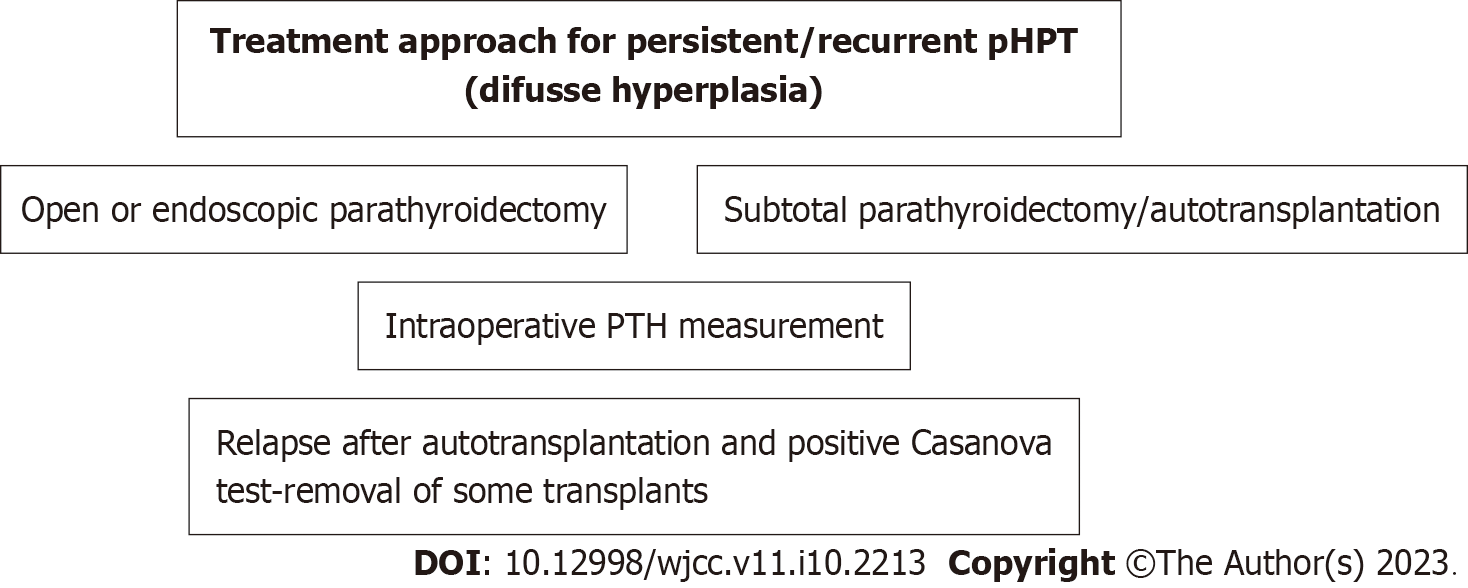
The surgical operation aims for permanent cure[21,46]. However, recurrence has been reported in 4%-10% of surgery patients[8]. The extent of surgical resection in pHPT depends on its cause. Removal of the enlarged gland is the indicated surgical technique in adenoma, the most common case. The surgical strategy in diffuse hyperplasia includes either subtotal parathyroidectomy (removal of 3.5 glands) or total parathyroidectomy and autotransplantation of thin pieces (n = 10-15) of parathyroid tissue in the forearm. The operative strategy in carcinoma includes the unblock removal of the affected gland with the corresponding thyroid lobe and surrounding tissues[9]. A scheme of the treatment approach is shown in Figures 4 and 5.
Ipsilateral cervical exploration has been recommended because the majority of cases are due to an adenoma, there is a possibility of preoperative identification of the responsible gland, and it is possible to quickly determine the parathyroid hormone levels. Its advantages ensure a limited extent of the surgical wound, shorter operation and reoperation on the contralateral intact side if needed. Its disadvantages include the limited effectiveness in the presence of supernumerary glands and the possibility of double adenoma[4,9]. With the current advancements in diagnostic adjuncts for accurate abnormal parathyroid localization, surgery has moved from BNE to a focused open parathyroidectomy or unilateral neck exploration as well as to a minimally invasive parathyroidectomy (MIP) that necessitates even more accurate preoperative localization[22,25,47-49].
The success rate of surgical treatment is more than 95% in experienced hands[4,10]. The causes of its failure are shown in Table 3[9,12]. In the following studies, the recurrences occurred a long time after the first intervention. This means that the tumors probably developed de novo during the follow-up period and were not missed during the first operation.
| No. | Cause |
| 1 | Failure to recognize histopathological lesion |
| 2 | Supernumerary (5th) parathyroid |
| 3 | Ectopic site of responsible parathyroid gland |
| 4 | Carcinoma or spread of adenoma parts |
| 5 | Inadequate experience of the surgeon |
| 6 | Insufficient cooperation with pathologist |
A study from the United States including 345 patients who underwent surgical intervention for pHPT, either single-gland resection (79%) or bilateral cervical exploration (38%), found that persistent hypercalcemia (9%) and recurrent hypercalcemia (14%) were transient mainly for other reasons, while only 3.2% of them required reoperation (median time to recurrence 12.2 years)[8]. Likewise, a more recent study from the United Kingdom including 404 patients with pHPT who underwent successful MIP had a long-term follow-up (median 6.5 years) with normocalcemia in 96% of cases; 3.85% had biochemical recurrence after 5 years (median 7.7 years), and only one patient had persistent hypercalcemia[50]. Another recent study from the United States including 261 patients after successful parathyroidectomy for pHPT reported a 10.7% late recurrence rate (up to 17 years, median time 6.5 years) and an 89.3% cure rate of recurrences. Multivariate analysis found calcium ≥ 9.7 mg/dL and normocalcemic PTH increase as independent risk factors for late recurrence at six months[4]. Another retrospective trial including 196 patients found recurrence of 14.8% (median time 6.3 years), but 34.5% of recurrences occurred later (> 10 years), without a difference between open parathyroidectomy and MIP[51]. The above findings necessitate a long-term postoperative follow-up. Additionally, concerns about the efficacy of preoperative US and sestamibi scintigraphy alone provide more argument in favor of additional BNE again.
A recent large study from Taiwan including 522 patients after parathyroidectomy (mostly MIP) with a median follow-up of 2.5 years found, by multivariate analysis, that age > 66.5 years, serum calcium ≥ 9.8 mg/dL and parathyroid hormone ≥ 80 pg/mL at 6 mo were risk factors for recurrence (rate of 2.5%); after MIP, the discrepancy of at least one preoperative imaging study with intraoperative findings was a risk factor[47].
A recent nationwide French study including 13247 patients after focused parathyroidectomy by either open surgery (88.7%) or MIP (11.3%) found that the cure rate was 97.3% for the former and 96.5% for the latter; the need for reoperation was 2.8% at two years. The predictive risk factors for failure of the initial parathyroidectomy and the need for reoperation shown by the multivariate analysis were cardiac history (congestive heart failure, arrhythmias, and valvular disease), obesity, endoscopic approach, and surgery at a low-volume center (at most 31 cases per year)[46].
A meta-analysis of 70 studies with 9643 patients undergoing MIP and 38 studies with 2471 patients undergoing open BNE confirmed the effectiveness of both operative methods for the management of pHPT. Comparing BNE vs MIP, the cure rates were 98% vs 97%, bleeding rates were 0.9% vs 0.1% [statistically significant (SS)], postoperative (short-term and long-term) hypocalcemia 13.6% vs 2.3% (SS), laryngeal nerve injury 0.9% vs 0.3% (SS), infection 0.5% vs 0.5% and mortality 0.5% vs 0.1%, respectively[52].
In patients with histologically large normal parathyroid and pHPT, a controversial issue, excision exhibits a beneficial effect on PTH levels despite the higher risk of P-HPT/R-HPT than in adenoma or hyperplasia; thus, these patients need stricter biochemical postoperative follow-up[53].
Stricter indications for reoperation for P-HPT/R-HPT have been recommended because it may cause fibrosis, alteration of normal anatomy and complications. These indications are determined by the presence of severe features, i.e., persistent clinical musculoskeletal or neuropsychiatric symptoms, nephrolithiasis, nephrocalcinosis and osteoporosis[8,9,17,47]. According to the guidelines of the American Association of Endocrine Surgeons, in the above indications are added to serum calcium > 1 mg/dL above the normal upper value and young age (< 50 years)[47].
It has been reported that reoperation is linked with significantly lower cure rates (82%-98%) and higher rates of hypoparathyroidism (5%-8%) and recurrent laryngeal nerve palsy (15%) than initial parathyroidectomy[7]. Thus, rechecking nerve function preoperatively is mandatory. Intraoperative neuromonitoring is also a useful tool[9].
The cervical endoscopic approach for adenoma located at the posterior mediastinum is a current preferable safe choice[54].
A normocalcemic PTH increase may occur after parathyroidectomy for pHPT. This may be caused by higher preoperative PTH, vitamin D deficiency, and lower creatinine clearance. Postoperative calcium and vitamin D administration have been recommended in such cases[55].
The size of an adenoma (dwarf < 300 mg or giant > 3000 mg) does not affect the outcomes of parathyroidectomy, which is indicated for all dwarf adenomas, while giant adenomas do not have malignant behavior[56].
It has been postulated that the success of parathyroidectomy is reflected in a decrease in the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio by its modulatory effect on systemic inflammation[57].
Multiglandular disease (its predictors by multivariable analysis are negative sestamibi scintigraphy, diabetes and elevated osteocalcin) is associated with a higher risk of intraoperative complications and P-pHPT/R-pHPT. The parathyroid glands are associated with glucose metabolism. PTH, insulin and osteocalcin interact by modulating insulin secretion and peripheral lipolysis[58].
After parathyroid tissue autotransplantation, usually in the forearm for diffuse hyperplasia and pHPT, graft recurrence indicates reoperation that is difficult, requiring much caution, but first, a recurrence in the neck or mediastinum must be excluded[12]. For this, the Casanova diagnostic test is useful. A tourniquet is applied to the arm, causing ischemia for 15 min, and PTH is measured. The test is positive when the PTH level is decreased to > 50% of the preischemic value at 10 min after the end of ischemia. A decrease of < 20% indicates a negative test[59].
The use of cinacalcet reduces PTH production and is limited in persistent-recurrent pHPT. It is mostly used in patients with high surgical risk or as short-term operative preparation in patients with high calcium levels (≥ 12 mg/dL). Antiresorptive drugs can also be used in the conservative management of patients with P-pHPT/R-pHPT[60]. Other than surgery, which is the gold standard for P-pHPT/R-pHPT, microwave ablation or ethanol ablation has been reported, but they might have a place mainly in parathyroid cancer spread or parathyromatosis[12]. In a recent comparative study, microwave ablation had comparable results with parathyroidectomy but a shorter procedure time and smaller incision[61]. Likewise, radiofrequency ablation was safe and effective, ensuring a cure rate of 98% and a recurrent HPT rate of 2%[62].
Several useful intraoperative tools facilitate accurate parathyroid assessment, but no tool can replace the surgeon's judgment and experience. The following constitute the available choices[7-9].
The confirmation of parathyroid tissue removal is achieved either by IPM, which is preferable, or by frozen section histopathological examination or ex vivo aspiration of the resected parathyroid tissue to confirm the presumed parathyroid tissue intraoperatively[7]. IPM is considered to indicate successful parathyroidectomy when 10 min after excision it is decreased by ≥ 50% from its highest value before excision (Miami sole criterion, often used) or the latter plus a drop to a normal value (dual criteria)[63,64]. IPM ≤ 40 pg/mL minimizes persistence as the final PTH will fall ≤ 65 pg/mL[65]. Intraoperative PTH monitoring can safely predict the outcome (accuracy at six months 98% at least), even in patients with normohormonal HPT (8%)[6]. In a comparative study, patients who underwent reoperation with the use of IPM and without it showed normal calcium levels postoperatively in 94% and 74%, respectively[66]. A recent meta-analysis of 28 studies with a total of 13323 patients documented that IPM reduces both persistent and recurrent pHPT and so must be used[67]. However, IPM in MIP in patients with positive and concordant preoperative ultrasonography and sestamibi scintigraphy is still under debate because, on the one hand, it costs more and increases the operating time and conversion rate; on the other hand, IPM may contribute to an increase in the cure rate to 97.6% from 93.3%[68].
Visual identification of parathyroid can be achieved by near-infrared autofluorescence angiography (infrared spectroscopy)[69,70] or indocyanine green (ICG) angiography[70-72], both with high sensitivity of up to 82% and 81%, respectively[70].
The anatomical localization of the parathyroids by intraoperative US, bilateral jugular venous sampling, or radioprobe guidance is another available choice[73]. Identification using trackers is based on carbon nanoparticles (when they are collected and deposited in the lymph nodes, the thyroid, and the drainage area of the lymph node will be painted black)[74] or radioprobe guidance[9,75]. It is based on the use of technetium-99m sestamibi for intraoperative identification of hyperactive parathyroids[9]. The tracer is injected 1-2 h before surgery. The surgeon with a handheld gamma camera detects the gamma radiation. Confirmation of resection of parathyroid tissue is indicated by high radioisotope concentrations. However, it has low specificity because the radioisotope is also taken up by the thyroid and cardiac tissue.
Recurrent laryngeal nerve monitoring is valuable for identification and preservation, thus avoiding injury and palsy[76,77].
Autotransplantation and cryopreservation should be considered when hypoparathyroidism occurs after parathyroidectomy or when the target parathyroid in reoperation is the only viable parathyroid tissue remaining[78].
Much progress has been made in the management of pHPT over the last few years. Parathyroidectomy for pHPT can offer a permanent cure, and its success depends on accurate preoperative localization. Preoperative ultrasonography and technetium-99m sestamibi scintigraphy-SPECT in addition to 4D-CT or PET/CT should be done if needed. This in combination with intraoperative parathyroid hormone monitoring and other novel localization adjuncts has contributed greatly to accurate parathyroid assessment, leading to a significant decrease in persistent and recurrent hyperparathyroidism and providing the best outcomes. Multidisciplinary diagnostic and therapeutic management is highly valuable to prevent persistence and recurrence. Surgery may be either open or endoscopic. Long-term follow-up, even up to 10 years, is imperative to detect late recurrence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gorbacheva AM, Russia; Moshref RH, Saudi Arabia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Zhu J, Tian W, Xu Z, Jiang K, Sun H, Wang P, Huang T, Guo Z, Zhang H, Liu S, Zhang Y, Cheng R, Zhao D, Fan Y, Li X, Qin J, Zhao W, Su A. Expert consensus statement on parathyroid protection in thyroidectomy. Ann Transl Med. 2015;3:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 2. | Reitz RJ 3rd, Dreimiller A, Khil A, Horwitz E, McHenry CR. Ectopic and supernumerary parathyroid glands in patients with refractory renal hyperparathyroidism. Surgery. 2021;169:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Olguνn Joseau S, Arias A, Garzσn A, Peretti E, Guzmαn L, Ruggieri M. Risk factors for surgical failure in patients undergoing surgery for primary hyperparathyroidism. Cir Esp (Engl Ed). 2022;100:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Mallick R, Nicholson KJ, Yip L, Carty SE, McCoy KL. Factors associated with late recurrence after parathyroidectomy for primary hyperparathyroidism. Surgery. 2020;167:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Aygόn N, Uludaπ M. Surgical Treatment of Primary Hyperparathyroidism: Which Therapy to Whom? Sisli Etfal Hastan Tip Bul. 2019;53:201-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Stuart H, Azab B, Roque OP, Pasieka J, Lew JI. Intraoperative parathormone monitoring to predict operative success in patients with normohormonal hyperparathyroidism. Can J Surg. 2022;65:E468-E473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Wilhelm SM, Wang TS, Ruan DT, Lee JA, Asa SL, Duh QY, Doherty GM, Herrera MF, Pasieka JL, Perrier ND, Silverberg SJ, Solσrzano CC, Sturgeon C, Tublin ME, Udelsman R, Carty SE. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016;151:959-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 612] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 8. | Szabo Yamashita T, Mirande M, Huang CT, Kearns A, Fyffe-Freil R, Singh R, Foster T, Thompson G, Lyden M, McKenzie T, Wermers RA, Dy B. Persistence and Recurrence of Hypercalcemia after Parathyroidectomy Over Five Decades (1965-2010) in a Community-based Cohort. Ann Surg. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 9. | Nawrot I, Chudziρski W, CiΉζka T, Barczyρski M, Szmidt J. Reoperations for persistent or recurrent primary hyperparathyroidism: results of a retrospective cohort study at a tertiary referral center. Med Sci Monit. 2014;20:1604-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Erinjeri NJ, Udelsman R. Volume-outcome relationship in parathyroid surgery. Best Pract Res Clin Endocrinol Metab. 2019;33:101287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Kota SK, Kota SK, Jammula S, Bhargav PRK, Sahoo AK, Das S, Talluri SC, Kongara S, S Krishna SV, Modi KD. Persistent Elevation of Parathormone Levels after Surgery for Primary Hyperparathyroidism. Indian J Endocrinol Metab. 2020;24:366-372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 12. | Guerin C, Paladino NC, Lowery A, Castinetti F, Taieb D, Sebag F. Persistent and recurrent hyperparathyroidism. Updates Surg. 2017;69:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Haciyanli M, Karaisli S, Gucek Haciyanli S, Atasever A, Arikan Etit D, Gur EO, Acar T. Parathyromatosis: a very rare cause of recurrent primary hyperparathyroidism - case report and review of the literature. Ann R Coll Surg Engl. 2019;101:e178-e183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Jain M, Krasne DL, Singer FR, Giuliano AE. Recurrent primary hyperparathyroidism due to Type 1 parathyromatosis. Endocrine. 2017;55:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Mazotas IG, Yen TWF, Doffek K, Shaker JL, Carr AA, Evans DB, Wang TS. Persistent/Recurrent Primary Hyperparathyroidism: Does the Number of Abnormal Glands Play a Role? J Surg Res. 2020;246:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ademiluyi A, Jackson N, Betty S, Ademiluyi A, Appiah-Pippim J, Bonhomme K. Missing in action. J Community Hosp Intern Med Perspect. 2021;11:856-858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | McIntyre CJ, Allen JL, Constantinides VA, Jackson JE, Tolley NS, Palazzo FF. Patterns of disease in patients at a tertiary referral centre requiring reoperative parathyroidectomy. Ann R Coll Surg Engl. 2015;97:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Ryder CY, Jarocki A, McNeely MM, Currey E, Miller BS, Cohen MS, Gauger PG, Hughes DT. Early biochemical response to parathyroidectomy for primary hyperparathyroidism and its predictive value for recurrent hypercalcemia and recurrent primary hyperparathyroidism. Surgery. 2021;169:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Carsote M, Paduraru DN, Nica AE, Valea A. Parathyroidectomy: is vitamin D a player for a good outcome? J Med Life. 2016;9:348-352. [PubMed] |
| 20. | Lai V, Yen TW, Doffek K, Carr AA, Carroll TB, Fareau GG, Evans DB, Wang TS. Delayed Calcium Normalization After Presumed Curative Parathyroidectomy is Not Associated with the Development of Persistent or Recurrent Primary Hyperparathyroidism. Ann Surg Oncol. 2016;23:2310-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Van Den Heede K, Bonheure A, Brusselaers N, Van Slycke S. Long-term outcome of surgical techniques for sporadic primary hyperparathyroidism in a tertiary referral center in Belgium. Langenbecks Arch Surg. 2022;407:3045-3055. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. 2012;19:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 23. | Ruda JM, Hollenbeak CS, Stack BC Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 490] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 24. | Carlier T, Oudoux A, Miralliι E, Seret A, Daumy I, Leux C, Bodet-Milin C, Kraeber-Bodιrι F, Ansquer C. 99mTc-MIBI pinhole SPECT in primary hyperparathyroidism: comparison with conventional SPECT, planar scintigraphy and ultrasonography. Eur J Nucl Med Mol Imaging. 2008;35:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Wang TS, Cheung K, Farrokhyar F, Roman SA, Sosa JA. Would scan, but which scan? Surgery. 2011;150:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Neumann DR, Obuchowski NA, Difilippo FP. Preoperative 123I/99mTc-sestamibi subtraction SPECT and SPECT/CT in primary hyperparathyroidism. J Nucl Med. 2008;49:2012-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Jones JM, Russell CF, Ferguson WR, Laird JD. Pre-operative sestamibi-technetium subtraction scintigraphy in primary hyperparathyroidism: experience with 156 consecutive patients. Clin Radiol. 2001;56:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Rodgers SE, Hunter GJ, Hamberg LM, Schellingerhout D, Doherty DB, Ayers GD, Shapiro SE, Edeiken BS, Truong MT, Evans DB, Lee JE, Perrier ND. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery. 2006;140:932-40; discussion 940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Hessman O, Stεlberg P, Sundin A, Garske U, Rudberg C, Eriksson LG, Hellman P, Akerstrφm G. High success rate of parathyroid reoperation may be achieved with improved localization diagnosis. World J Surg. 2008;32:774-81; discussion 782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Mortenson MM, Evans DB, Lee JE, Hunter GJ, Shellingerhout D, Vu T, Edeiken BS, Feng L, Perrier ND. Parathyroid exploration in the reoperative neck: improved preoperative localization with 4D-computed tomography. J Am Coll Surg. 2008;206:888-95; discussion 895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Cham S, Sepahdari AR, Hall KE, Yeh MW, Harari A. Dynamic Parathyroid Computed Tomography (4DCT) Facilitates Reoperative Parathyroidectomy and Enables Cure of Missed Hyperplasia. Ann Surg Oncol. 2015;22:3537-3542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Ramalho D, Rocha G, Oliveira JM, Oliveira MJ. Fluorine-18 Fluorocholine Positron Emission Tomography/Computed Tomography in Primary Hyperparathyroidism: A Case Report and Review of Literature. Cureus. 2022;14:e21958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 33. | Latge A, Riehm S, Vix M, Bani J, Ignat M, Pretet V, Helali M, Treglia G, Imperiale A. (18)F-Fluorocholine PET and 4D-CT in Patients with Persistent and Recurrent Primary Hyperparathyroidism. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Christakis I, Khan S, Sadler GP, Gleeson FV, Bradley KM, Mihai R. (18)Fluorocholine PET/CT scanning with arterial phase-enhanced CT is useful for persistent/recurrent primary hyperparathyroidism: first UK case series results. Ann R Coll Surg Engl. 2019;101:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Kawai Y, Iima M, Yamamoto H, Kawai M, Kishimoto AO, Koyasu S, Yamamoto A, Omori K, Kishimoto Y. The added value of non-contrast 3-Tesla MRI for the pre-operative localization of hyperparathyroidism. Braz J Otorhinolaryngol. 2022;88 Suppl 4:S58-S64. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Dombrowsky A, Weiss D, Bushman N, Chen H, Balentine CJ. Can imaging studies be omitted in patients with sporadic primary hyperparathyroidism? J Surg Res. 2018;231:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Tang AL, Aunins B, Chang K, Wang JC, Hagen M, Jiang L, Lee CY, Randle RW, Houlton JJ, Sloan D, Steward DL. A multi-institutional study evaluating and describing atypical parathyroid tumors discovered after parathyroidectomy. Laryngoscope Investig Otolaryngol. 2022;7:901-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 38. | Saponaro F, Pardi E, Mazoni L, Borsari S, Torregrossa L, Apicella M, Frustaci G, Materazzi G, Miccoli P, Basolo F, Marcocci C, Cetani F. Do Patients With Atypical Parathyroid Adenoma Need Close Follow-up? J Clin Endocrinol Metab. 2021;106:e4565-e4579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Ying W, Zhen-Long Z, Xiao-Jing C, Li-Li P, Yan L, Ming-An Y. A study on the causes of operative failures after microwave ablation for primary hyperparathyroidism. Eur Radiol. 2021;31:6522-6530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Gawrychowska A, Kowalski G, Nabrdalik M, Bu³a G, Lackowska B, Bednarczyk A, Werbowski M, Polczyk J, Gawrychowski J. Flow cytometric DNA analysis of parathyroid benign lesions. Endokrynol Pol. 2021;72:44-50. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 41. | Gorbacheva AM, Eremkina AK, Mokrysheva NG. [Hereditary syndromal and nonsyndromal forms of primary hyperparathyroidism]. Probl Endokrinol (Mosk). 2020;66:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Tao X, Xu T, Lin X, Xu S, Fan Y, Guo B, Deng X, Jiao Q, Chen L, Wei Z, Chen C, Yang W, Zhang Z, Yu X, Yue H. Genomic profiling reveals the variant landscape of sporadic parathyroid adenomas in Chinese population. J Clin Endocrinol Metab. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 43. | Mazarico-Altisent I, Capel I, Baena N, Bella-Cueto MR, Barcons S, Guirao X, Albert L, Cano A, Pareja R, Caixΰs A, Rigla M. Novel germline variants of CDKN1B and CDKN2C identified during screening for familial primary hyperparathyroidism. J Endocrinol Invest. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | McCoy KL, Yip L, Dhir M, Langenborg K, Seethala RR, Carty SE. Histologic hypercellularity in a biopsied normal parathyroid gland does not correlate with hyperfunction in primary hyperparathyroidism. Surgery. 2021;169:524-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Krupinova JA, Elfimova AR, Rebrova OY, Voronkova IA, Eremkina AK, Kovaleva EV, Maganeva IS, Gorbacheva AM, Bibik EE, Deviatkin AA, Melnichenko GA, Mokrysheva NG. Mathematical model for preoperative differential diagnosis for the parathyroid neoplasms. J Pathol Inform. 2022;13:100134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 46. | Donatini G, Marciniak C, Lenne X, Clιment G, Bruandet A, Sebag F, Miralliι E, Mathonnet M, Brunaud L, Lifante JC, Tresallet C, Mιnιgaux F, Theis D, Pattou F, Caiazzo R; on the behalf of AFCE Study Group. Risk Factors of Redo Surgery After Unilateral Focused Parathyroidectomy: Conclusions From a Comprehensive Nationwide Database of 13,247 Interventions Over 6 Years. Ann Surg. 2020;272:801-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Shirali AS, Wu SY, Chiang YJ, Graham PH, Grubbs EG, Lee JE, Perrier ND, Fisher SB. Recurrence after successful parathyroidectomy-Who should we worry about? Surgery. 2022;171:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Jinih M, O'Connell E, O'Leary DP, Liew A, Redmond HP. Focused Versus Bilateral Parathyroid Exploration for Primary Hyperparathyroidism: A Systematic Review and Meta-analysis. Ann Surg Oncol. 2017;24:1924-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Norlιn O, Wang KC, Tay YK, Johnson WR, Grodski S, Yeung M, Serpell J, Sidhu S, Sywak M, Delbridge L. No need to abandon focused parathyroidectomy: a multicenter study of long-term outcome after surgery for primary hyperparathyroidism. Ann Surg. 2015;261:991-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Patel N, Mihai R. Long-term Cure of Primary Hyperparathyroidism After Scan-Directed Parathyroidectomy: Outcomes From A UK Endocrine Surgery Unit. World J Surg. 2022;46:2189-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Lou I, Balentine C, Clarkson S, Schneider DF, Sippel RS, Chen H. How long should we follow patients after apparently curative parathyroidectomy? Surgery. 2017;161:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Singh Ospina NM, Rodriguez-Gutierrez R, Maraka S, Espinosa de Ycaza AE, Jasim S, Castaneda-Guarderas A, Gionfriddo MR, Al Nofal A, Brito JP, Erwin P, Richards M, Wermers R, Montori VM. Outcomes of Parathyroidectomy in Patients with Primary Hyperparathyroidism: A Systematic Review and Meta-analysis. World J Surg. 2016;40:2359-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 53. | Krawitz R, Glover A, Koneru S, Jiang J, Di Marco A, Gill AJ, Aniss A, Sywak M, Delbridge L, Sidhu S. The Significance of Histologically "Large Normal" Parathyroid Glands in Primary Hyperparathyroidism. World J Surg. 2020;44:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Rubio-Manzanares Dorado M, Pino-Diaz V, Padillo-Ruνz J, Martos-Martνnez JM. Prevertebral cervical approach to posterior mediastinum parathyroid adenomas. Surg Endosc. 2022;36:6319-6325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 55. | Xie LD, Wang N, Zhang JP, Wang X, Chen XP, Zhang B, Bu S. [Normocalcemic with elevated post-operative parathormone in primary hyperpara-thyroidism: 9 case reports and literature review]. Beijing Da Xue Xue Bao Yi Xue Ban. 2021;53:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Abdel-Aziz TE, Gleeson F, Sadler G, Mihai R. Dwarfs and Giants of Parathyroid Adenomas-No Difference in Outcome After Parathyroidectomy. J Surg Res. 2019;237:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 57. | Yang PS, Liu CL, Liu TP, Chen HH, Wu CJ, Cheng SP. Parathyroidectomy decreases neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. J Surg Res. 2018;224:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Thier M, Daudi S, Bergenfelz A, Almquist M. Predictors of multiglandular disease in primary hyperparathyroidism. Langenbecks Arch Surg. 2018;403:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Anamaterou C, Lang M, Schimmack S, Rudofsky G, Bόchler MW, Schmitz-Winnenthal H. Autotransplantation of parathyroid grafts into the tibialis anterior muscle after parathyroidectomy: a novel autotransplantation site. BMC Surg. 2015;15:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Walker MD, Shane E. Hypercalcemia: A Review. JAMA. 2022;328:1624-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 61. | Wei Y, Zhao ZL, Cao XJ, Peng LL, Li Y, Wu J, Yu MA. Microwave ablation versus parathyroidectomy for the treatment of primary hyperparathyroidism: a cohort study. Eur Radiol. 2022;32:5821-5830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 62. | Peng CZ, Chai HH, Zhang ZX, Hu QH, Zeng Z, Cui AL, Pang HS, Ruan LT. Radiofrequency ablation for primary hyperparathyroidism and risk factors for postablative eucalcemic parathyroid hormone elevation. Int J Hyperthermia. 2022;39:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 63. | Wang TS, Pasieka JL, Carty SE. Techniques of parathyroid exploration at North American endocrine surgery fellowship programs: what the next generation is being taught. Am J Surg. 2014;207:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Irvin GL 3rd, Carneiro DM, Solorzano CC. Progress in the operative management of sporadic primary hyperparathyroidism over 34 years. Ann Surg. 2004;239:704-8; discussion 708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 65. | Claflin J, Dhir A, Espinosa NM, Antunez AG, Cohen MS, Gauger PG, Miller BS, Hughes DT. Intraoperative parathyroid hormone levels ≤40 pg/mL are associated with the lowest persistence rates after parathyroidectomy for primary hyperparathyroidism. Surgery. 2019;166:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Prescott JD, Udelsman R. Remedial operation for primary hyperparathyroidism. World J Surg. 2009;33:2324-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Medas F, Cappellacci F, Canu GL, Noordzij JP, Erdas E, Calς PG. The role of Rapid Intraoperative Parathyroid Hormone (ioPTH) assay in determining outcome of parathyroidectomy in primary hyperparathyroidism: A systematic review and meta-analysis. Int J Surg. 2021;92:106042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Akgόn IE, άnlό MT, Aygun N, Kostek M, Uludag M. Contribution of intraoperative parathyroid hormone monitoring to the surgical success in minimal invasive parathyroidectomy. Front Surg. 2022;9:1024350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 69. | Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ, Ladurner R. Intraoperative Near-Infrared Autofluorescence and Indocyanine Green Imaging to Identify Parathyroid Glands: A Comparison. Int J Endocrinol. 2019;2019:4687951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Liang TJ, Wang KC, Wang NY, Chen IS, Liu SI. Indocyanine Green Angiography for Parathyroid Gland Evaluation during Transoral Endoscopic Thyroidectomy. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Spartalis E, Ntokos G, Georgiou K, Zografos G, Tsourouflis G, Dimitroulis D, Nikiteas NI. Intraoperative Indocyanine Green (ICG) Angiography for the Identification of the Parathyroid Glands: Current Evidence and Future Perspectives. In Vivo. 2020;34:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 72. | Papavramidis TS, Anagnostis P, Chorti A, Pliakos I, Panidis S, Koutsoumparis D, Michalopoulos A. Do Near-Infrared Intra-Operative Findings Obtained Using Indocyanine Green Correlate with Post-Thyroidectomy Parathyroid Function? Endocr Pract. 2020;26:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Aygόn N, Uludaπ M. Intraoperative Adjunct Methods for Localization in Primary Hyperparathyroidism. Sisli Etfal Hastan Tip Bul. 2019;53:84-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 74. | Huang K, Luo D, Huang M, Long M, Peng X, Li H. Protection of parathyroid function using carbon nanoparticles during thyroid surgery. Otolaryngol Head Neck Surg. 2013;149:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Bononi M, Viviana F, De Feo MS, Sollaku S, Pani A, Falconi R, Pani R, Cavallaro G, Brozzetti S, De Vincentis G. Gonioprobe, an Innovative Gamma-probe to Guide Parathyroid Radioguided Surgery: First Clinical Experiences with Navigator and Lock-ontarget Functions. Curr Radiopharm. 2021;14:161-169. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 76. | Ghani U, Assad S. Role of Intraoperative Nerve Monitoring During Parathyroidectomy to Prevent Recurrent Laryngeal Nerve Injury. Cureus. 2016;8:e880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Zhu Y, Gao DS, Lin J, Wang Y, Yu L. Intraoperative Neuromonitoring in Thyroid and Parathyroid Surgery. J Laparoendosc Adv Surg Tech A. 2021;31:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Aiti A, Rossi M, Alviano F, Morara B, Burgio L, Cioccoloni E, Cavicchi O, Pasquinelli G, Bonsi L, Buzzi M. Parathyroid Tissue Cryopreservation: Does the Storage Time Affect Viability and Functionality? Biopreserv Biobank. 2019;17:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |