Published online Apr 6, 2023. doi: 10.12998/wjcc.v11.i10.2201
Peer-review started: December 28, 2022
First decision: January 30, 2023
Revised: February 13, 2023
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 6, 2023
Processing time: 91 Days and 15.2 Hours
Implant-based reconstruction is the most common method of breast recon
Core Tip: Breast reconstruction can be achieved using autologous and implant-based techniques. Each method has its indications and contraindications accompanied by advantages and disadvantages. An astute plastic surgeon should have deep understanding of the nuances for each technique when consulting patients about reconstructive breast options.
- Citation: Malekpour M, Malekpour F, Wang HTH. Breast reconstruction: Review of current autologous and implant-based techniques and long-term oncologic outcome. World J Clin Cases 2023; 11(10): 2201-2212
- URL: https://www.wjgnet.com/2307-8960/full/v11/i10/2201.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i10.2201
Breast reconstruction aims to recreate the breast mound in postmastectomy patients. This can be achieved using autologous and implant-based techniques. Each method has its indications and contraindications along with advantages and disadvantages. The ultimate decision is made by the patient through an informed decision when all the options are fully explained. Here, we review implant-based and autologous reconstructive techniques along with oncologic outcomes.
The most common breast reconstruction method used by plastic surgeons is implant-based reconstruction which outpaced autologous techniques in early 21st century[1]. General advantages of implant-based reconstruction are shorter operative time, faster patient recovery and elimination of donor site morbidity. Disadvantages are capsular contracture, higher rate of infection, implant malposition, less natural feel, and breast implant illness. Complication rate and failure of implant-based reconstruction is significantly increased in patients undergoing adjuvant radiation therapy. Autologous techniques usually remain an option after implant-based reconstruction.
Prosthetic reconstructions can be performed in one or two stages and in immediate or delayed fashion. Single-stage reconstruction is favored in healthy patients with smaller breasts. A useful adjunct is use of acellular dermal matrix (ADM) which both provides better definition of the implant and maintains its position. Important risk factors for complications of prosthetic techniques are smoking, diabetes, obesity, and large breasts[2-4].
Immediate breast reconstruction preserves native skin envelop and breast borders. It minimizes trips to the operating room and improves aesthetic outcome and patient satisfaction by minimizing negative psychological impacts and improving the self-image[5]. On the other hand, delayed reconstruction is associated with fewer complications such as flap necrosis, capsular contracture, and need for prosthetic removal. As a rule, immediate reconstruction is better for patients with less comorbidity, early-stage cancer, and lower body mass index. It is common practice to delay reconstruction in patients with questionable mastectomy flaps, when close tumor surveillance is needed, and in the setting of planned adjuvant therapy[6].
Prosthetic reconstruction can be achieved via direct placement of permanent implant after mastectomy (single-stage) or placement of tissue expander and later exchange for a permanent implant (two-stage). Single-stage, a.k.a. direct-to-implant, approach has the advantage of eliminating the need for a second operation, reducing the risk of infection, and eliminating multiple office visits for device expansion. Smoking, diabetes, need for adjuvant radiation, and larger breast size are associated with increased risk of complications after single-stage reconstruction[7]. It is shown that two-stage reconstruction is associated with an overall decreased absolute rate of implant loss compared to one-stage reconstruction[8].
ADM is used as an adjunct to prostatic reconstruction to help with defining the mastectomy space, support the soft tissue, and stabilize the implant[9]. It should be noted that ADM is not yet cleared by the United States Food and Drug Administration (FDA) for use in implant-based breast reconstruction and currently wide use of ADM is off-label[10]. ADM is shown to improve aesthetic outcome in both single- and two-stage reconstruction[11]. ADM use is associated with reduced rate of capsular contracture[12]. The downside of using ADM is increased risk of postoperative complications especially infection and seroma formation[13].
There are many options for size, texture, and shape for both expander and implants selection. Expander options are relatively more limited, and they are available in different heights, base widths, projections, and capacities. Base width is the most important parameter which should match the width of the breast footprint on the chest wall. Implants, on the other hand, have more variety to choose from. They can be saline vs silicone, smooth vs texture, and round vs anatomic.
Although saline implants have a less rigorous surveillance protocol and are easier to detect when they are ruptured, they are associated with a higher rate of implant visibility and rippling. Silicone implants more closely resemble the native breast issue, but their rupture is harder to detect and can lead to capsular contracture. There are data suggesting that the rate of rupture and capsular contracture is slightly higher in silicone implants compared to saline implants[14]. On the other hand, it is shown that patients with silicone implants have higher satisfaction, and better psychological and sexual function compared to saline implants[15].
Although it might be difficult to clinically differentiate between round and anatomic-shape implants, shaped implants may result in an improved upper pole shape and better volume[16]. One should keep in mind that most shaped implants are textured which should be part of the discussion with the patient.
Selection of the plane to place the implant is usually surgeon-dependent. Subpectoral position places the implant between the pectoralis major muscle and the chest wall, whereas the prepectoral position places the implant between the skin flap and the pectoralis major muscle[17]. Historically, prepectoral position was associated with higher complications including capsular contracture and implant exposure but with improvement in surgical techniques and use of ADM (currently off-label use), these complications are significantly reduced leading to regaining popularity of the prepectoral implant placement[18]. With subpectoral positioning of the implant, it is a common practice to create an ADM sling (currently off-label use) for the exposed part of the implant below the pectoralis major muscle (dual-plane or partial subpectoral)[19]. Subpectoral implant placement has outpaced the historically higher rate of capsular contracture with prepectoral implant placement and it is also associated with a higher rate of animation deformity[17].
Quality of mastectomy flaps is the most important factor in the success of prepectoral implant placement[20,21]. Although it reduces the morbidity of subpectoral placement and animation deformity, it should never be performed in questionable mastectomy flaps[22]. In these situations, intraoperative Indocyanine Green (ICG) imaging can verify adequate blood supply to the mastectomy flaps. Active smoking, radiation to the field, and medical comorbidities also make prepectoral implant placement a less desirable option[23]. Tumor characteristics and location should be taken into account when choosing the appropriate plane for implant placement[24,25].
Need for adjuvant radiation complicates decision-making for prosthetic reconstruction techniques. Radiation is associated with increased rate of infection, flap necrosis, seroma, capsular contracture, and reconstructive failure[26,27]. Although some studies have shown no difference in the rate of complications between radiation of the expander vs the permanent implant in a two-stage reconstruction, the majority of available data have shown an increased rate of reconstructive failure following radiation of the permanent implant. Therefore, most plastic surgeons tend to increase the interval between completion of radiation and exchange to the permanent implant at about six-month interval[28-30].
Timing for radiation in the two-stage reconstruction is usually around 8 wk after tissue expander placement[28,29]. Rapid expansion starts in about 2 wk after placement of the expander and expansion is usually completed by the 6th week to have the patient ready for radiation at the 8th week[31]. Variations in timing of radiation is described with acceptable outcomes[32,33].
Complication rates of prostatic reconstruction of previously radiated breast can reach up to 50%[34,35]. Fibrosis and decreased vascularity does not respond well to expansion following radiation leading to three times increased risk of capsular contracture and reconstructive failure[36]. These risks appear to be slightly higher in post mastectomy radiation cases. Chemotherapy and hormonal therapy do not appear to increase the risk of complications following prosthetic reconstruction[37,38].
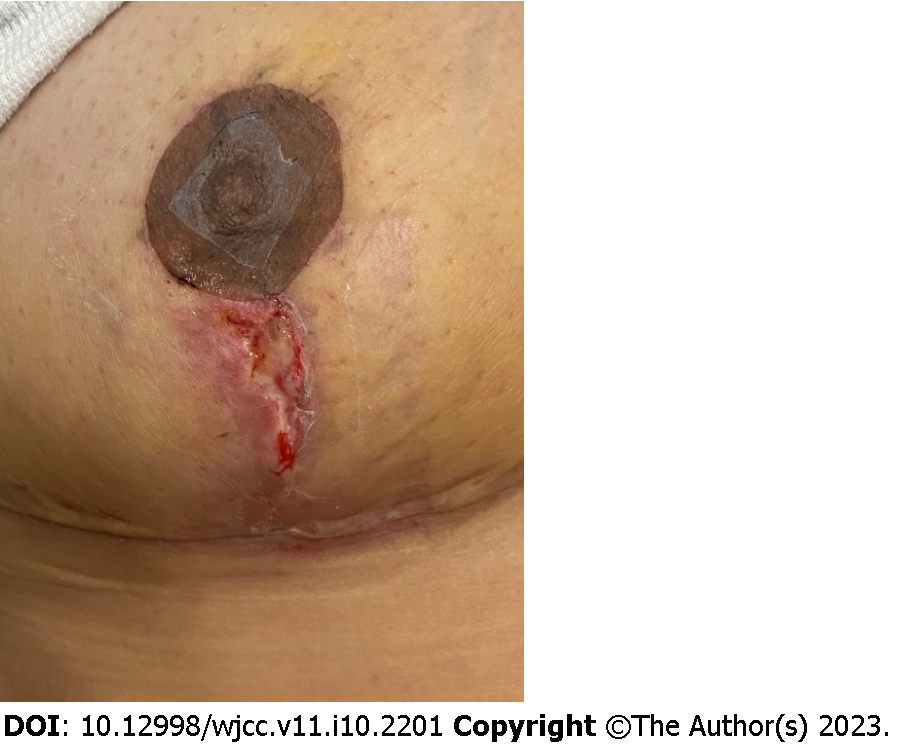
Acute hematoma occurs in the first 24-48 h after surgery in up to 3% of implant-base reconstructions[39]. Surgical drains are not useful for evacuation of blood clots and any hematoma can increase the risk of mastectomy skin flap necrosis and lead to poor aesthetic outcome. Infection rate can reach up to 8% following prosthetic reconstruction[40]. Capsular contracture can reach up to 13% in 3 years after prosthetic reconstruction and risk factors include radiation, hematoma, infection, and silicon rupture. The best way to prevent capsular contraction is targeting the risk factors at the index operation[41]. Wound dehiscence is another complication (Figure 1). Although data supports the use of preoperative antibiotic use, there is not sufficient evidence supporting outpatient use of antibiotics to prevent infection[42].
In a 10-year follow-up study of patients who underwent nipple-sparing mastectomy, of whom about 75% had implant-based reconstruction, not only the overall recurrence rate was low (3.33%) but also no demographic, tumor-specific or operative factor was found to be associated with increase recurrence[43]. This is a significant improvement from an earlier study on implant-based reconstruction after nipple-sparing mastectomy which showed a recurrence rate of 24%[44]. This is in part due to advancement in cancer treatment and better neoadjuvant and adjuvant treatments.
In a recent systematic review, locoregional recurrence was found to be 0%-7.4% after a median 5-year follow-up and no increased risk of recurrence with reconstructive method[45]. In a 7-year median follow-up study, locoregional recurrence in patients with immediate breast reconstruction was found to be 3.5% and independent from the reconstructive method[46]. In another study of at least 60-mo follow-up comparing smooth and textures implants, no significant difference was found between the two in terms of locoregional recurrence[47].
Textured implants were historically used to reduce the rate of capsular contracture and provide a more stable implant position, but studies have shown a small increased risk of breast-implant-associated anaplastic large-cell lymphoma (BIA-ALCL) with the use of textured devices[48-50]. Companies such as Allergan voluntarily recalled some textured implants (no new cases)[51]. This led to FDA scrutiny of implants and in October 2021, patient decision checklist was mandated as a condition for the sale of breast implants[52]. More recently, rare cases of breast implant capsule-associated squamous cell carcinoma (BICA-SCC) are reported which has a worse prognosis[53]. BICA-SCC can mimic BIA-ALCL but a similar diagnostic approach can be applied to distinguish between the two[54]. These should be discussed with the patient at the time of consult and during informed decision making for breast reconstruction. FDA recommends magnetic resonance imaging to detect rupture at 5 to 6 years after implant placement and then every 2 to 3 years[55]. More cohesive implants are now approved which are at an increased cohesion level compared to prior gel implants[56].
Despite the fact that the majority of patients undergo implant-base breast construction after mastectomy, autologous breast reconstruction is the only choice for certain patients and undoubtedly an option after failure of implant-based reconstruction[57,58]. Patient-related and surgeon-related factors each play a role in the choice of reconstruction. Using autologous tissue reduces postoperative risk of infection, implant rupture, capsular contractor, and malposition. Furthermore, autologous technique is the preferred method in patients expecting to receive radiation therapy or with prior irradiated breast[59-61].
Thorough history and physical examination are essential parts of the preoperative evaluation. Patient’s goals in terms of the reconstruction, and limitation of reconstructive options should be discussed in detail. Oncologic plan should be elucidated including neoadjuvant and adjuvant chemoradiation. Special attention should be paid to the tentative future donor site including prior surgeries, scars, skin quality, and infection. Although majority of patients prefer immediate reconstruction, when postmastectomy radiation is anticipated, delayed reconstruction is favored. Some microsurgeons elect to do preoperative imaging to elucidate vascular anatomy, yet many surgeons do not implement preoperative imaging and instead use pencil Doppler intraoperatively to identify the location of the perforators[62,63].
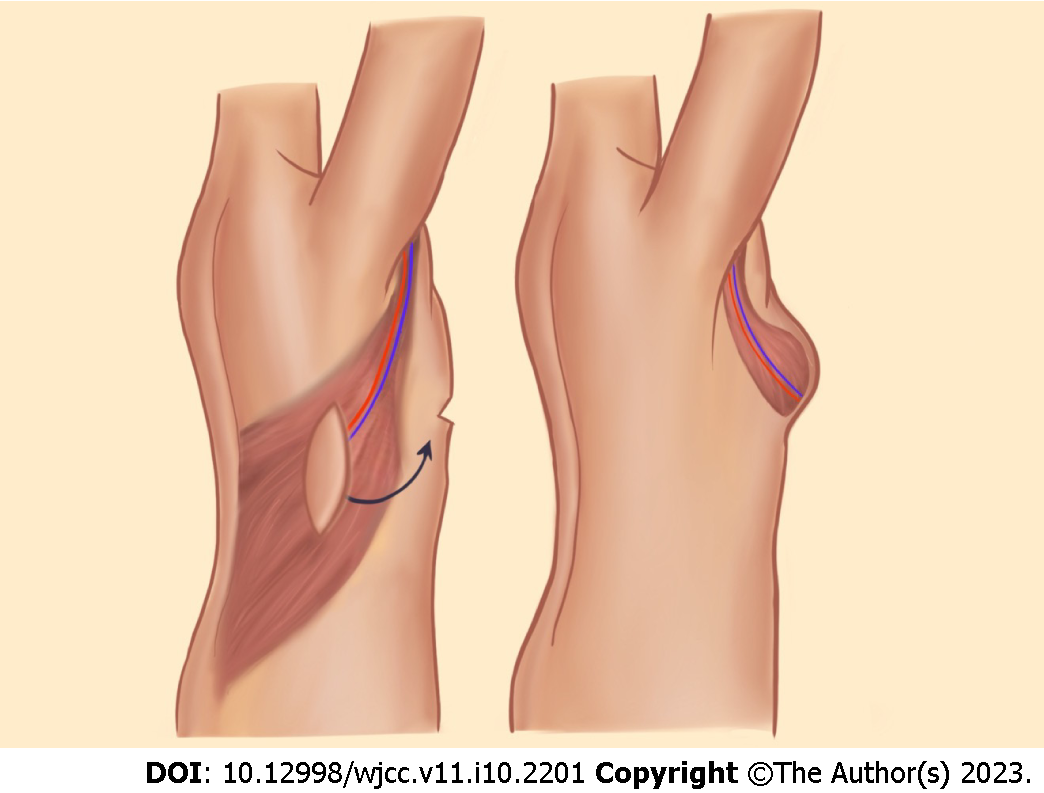
Latissimus dorsi flap: The latissimus dorsi (LD) myocutaneous flap is a well-establish pedicled flap for breast reconstruction. This flap is based on the thoracodorsal vessels, which arise from subscapular vessels that are branches of the axillary vessels (Figure 2). Medial and lateral branching of the vessels allows for muscle-sparing procedures. LD is a broad muscle that allows for a wide coverage with or without a skin paddle. The downside of using LD is that it does not typically providing sufficient volume which requires combination with prostheses. LD can also be used as a salvage option once other breast reconstruction options have failed.
Pedicled transverse rectus abdominis myocutaneous flap: The pedicled transverse rectus abdominis myocutaneous (TRAM) flap uses lower abdominal fat and is based on the superior epigastric artery which is a continuation of the internal mammary artery (Figure 3). It is elevated with the ipsilateral rectus muscle and tunneled into the mastectomy site. At times, delayed procedures should be performed by ligating the deep inferior epigastric artery so that choke vessels are recruited and superior epigastric vessels take over the perfusion of the pedicled TRAM flap[64]. Since harvesting this flap includes removal of the fascial coverage, closure requires implementation of mesh and there is an associated increased risk of incisional hernia in this patient population[65].
Commonly used free flaps are discussed below. Recipient vessels are usually internal mammary vessels, and as an alternative, thoracodorsal vessels can be used. End-to-end venous anastomosis are performed using vein couplers and end-to-end arterial anastomosis is typically handsewn using 8-0 to 10-0 nylon sutures.
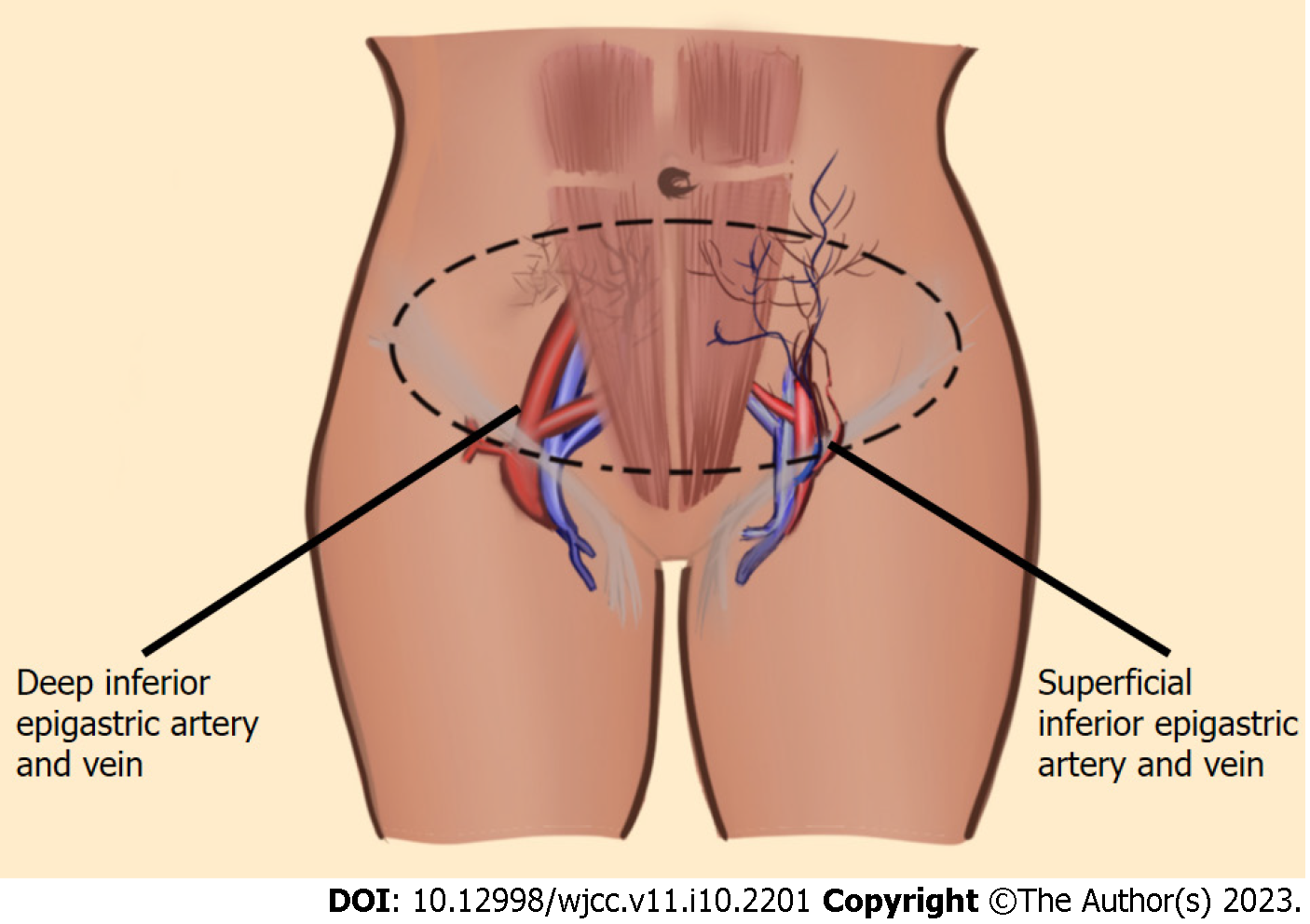
Abdomen-based flaps: Abdomen-based free flaps are the most common free autologous flaps for breast construction. Besides extensive clinical experience, these flaps commonly have reliable vascular anatomy, long pedicles, reduced donor site morbidity, and sufficient soft tissue for breast reconstruction. Free flaps based on the deep inferior epigastric vessels (branches of external iliac artery) include free transverse rectus abdominis myocutaneous (TRAM), free muscle-sparing TRAM (ms-TRAM), and deep inferior epigastric perforator (DIEP). Free flap based on the superficial inferior epigastric vessels (branches of the femoral vessels) is the superficial inferior epigastric artery (SIEA) perforator flap (Figure 4). Less than 10% of abdominal-based flaps are superficially dominant[66].
For patients undergoing immediate breast reconstruction, flap harvest can be performed at the same time of the mastectomy surgery. Flap design is usually inferior to the umbilicus in a lenticular fashion. Superficial inferior epigastric vessels are isolated at the inferior aspect of the flap during flap elevation[67]. Most of the perforators needed for DIEP flap are within 10 cm from the umbilicus. Zones of perforation vary depending on the selected row of perforators[68,69]. For unilateral reconstruction, midline could be crossed to include the zone immediately adjacent to the midline, whereas this option is not available when using SIEA flaps.
The dominant perforator can be identified by temporarily clamping other perforators and assessing the flap while using ICG perfusion imaging. Once the appropriate perforators are selected, anterior rectus facia is opened, and intramuscular dissection of perforators is carried down to the level of deep inferior epigastric vessels. If a segment of muscle between the two rows of the perforators is also harvested, then ms-TRAM flap is created and with complete transaction of the rectus muscle without preserving continuity, a free TRAM flap is created. The vessels, which typically consist of two veins and one artery are ligated distal to takeoff from external iliac vessels.
Robotic-assisted DIEP flap breast reconstruction is drawing attention as a new and alternative way to harvest DIEP flaps due to reduced risk of abdominal wall herniation, bulging, motor weakness and chronic pain[70]. It is associated with improved visualization, dexterity, ergonomics, and more importantly decreased size of facial incision[71]. If the length of the intra-muscular section of the pedicle is more than 5 cm, then it is unclear if robotic-assisted DIEP would reduce the rate of complications compared to the conventional DIEP approach[72]. This method requires pre-operative vascular imaging for appropriate planning and the microsurgeon needs to be additionally trained for robotic surgery.
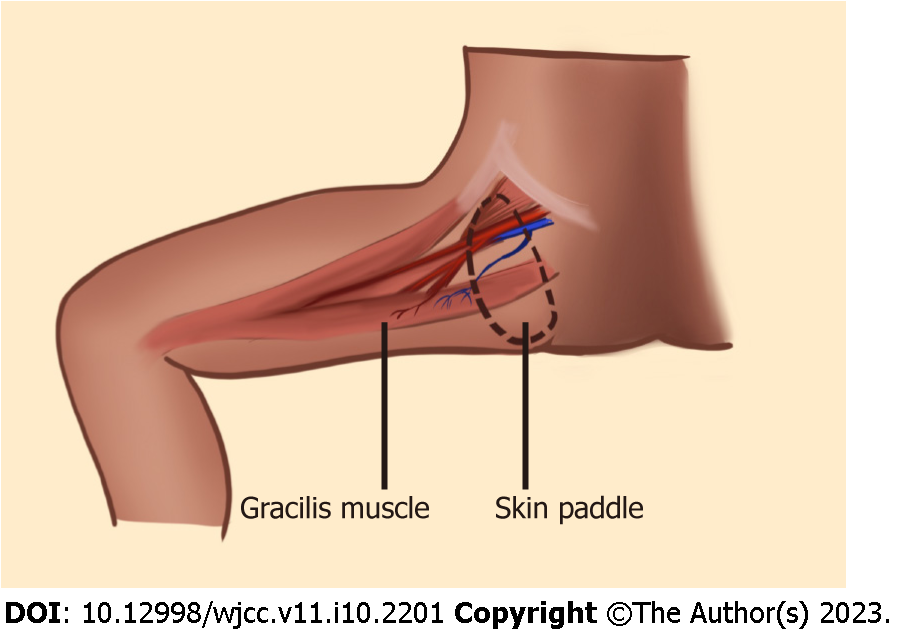
Medial thigh flaps: Medial thigh flaps incorporate gracilis muscle and is an option for construction in patients with prior abdominoplasty or those with insufficient abdominal tissue[73]. This flap is based on the medial circumflex femoral artery which is a branch of the profunda femoris artery (Figure 5). The skin paddle can be oriented transversely in transverse upper gracilis flap, vertically in vertical upper gracilis flap or as a combination of both. This flap is better for patients with small- to moderate-sized breasts and the flap artery is usually smaller than the recipient artery.
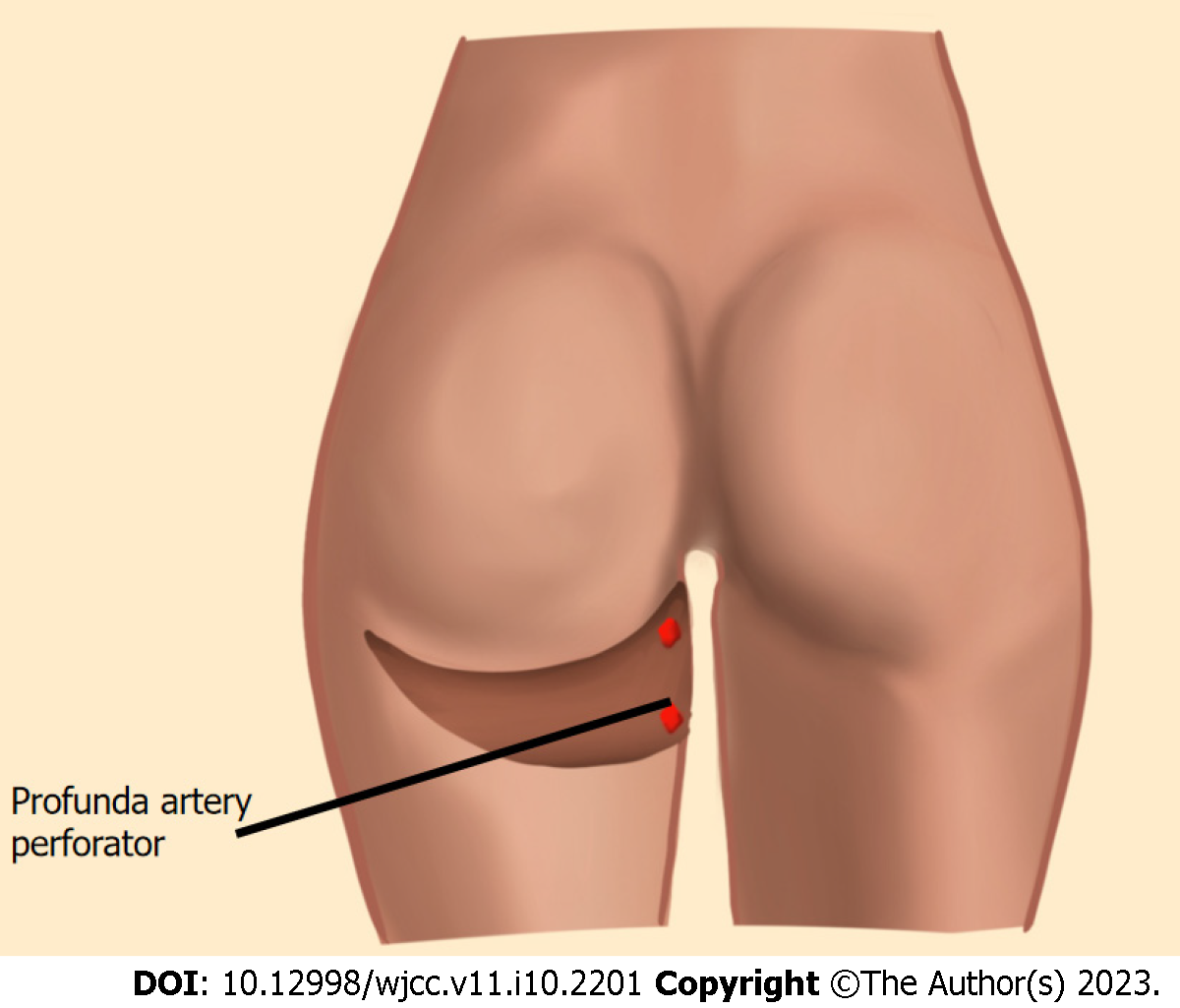
Posterior thigh flaps: Posterior thigh skin and adipose tissue can be used for breast reconstruction based on the profunda artery perforator (PAP) flap (Figure 6). PAP flaps provide longer pedicles compared to medial thigh flaps[74]. This flap is designed as a horizontal ellipse with the superior incision below the gluteal crease. PAP flap can be harvested in prone position, though, to facilitate two-team approach, PAP flap is elevated in the lithotomy position.
Gluteal artery perforator flaps: Another option for patients who cannot have abdomen-based flaps is superior or inferior gluteal artery perforator flaps (SGAP or IGAP). The superior gluteal artery exists the pelvis above the piriformis muscle and inferior gluteal artery exits the pelvis below the piriformis muscle[75]. Patients are either positioned laterally or prone which requires positional change intraoperatively to inset the flap. Although gluteal artery perforator flaps provide adequate amount of adipose tissue for breast reconstruction, they often have relatively short pedicle lengths.
The decision making about the choice of the autologous flap is based on several factors. First and foremost is the patient’s choice after providing adequate information so that the patient can make an informed decision[76]. Anatomical considerations are critical in the decision making about the choice of the flap. DIEP flaps are harvested in the same position and can allow two teams to work at the same time[77]. It also provides a reliable pedicle of enough length closely matching the recipient vessels[78]. Donor site comorbidities are acceptable for DIEP flaps. Other choices of free flaps, that are discussed above, have shortcomings in one or more of these factors such as providing short length of pedicle, need for repositioning in the OR and increased donor site comorbidities[79]. This is why currently DIEP flaps are the most favorable free flaps for breast reconstruction[80].
Post operative protocol can differ from surgeon-to-surgeon and institution-to-institution. Typically, postoperatively, flaps are kept warm and are evaluated every hour for at least the first 24-48 h. Examination consists of clinical exam, capillary refill, warmth, color, and pencil Doppler exam if no implantable Doppler is used. It is common practice to prescribe aspirin as an anti-platelet agent and to place the patients on venous thromboembolism prophylaxis. During the first 48 h, vascular compromise due to vessel positioning, hematoma, thrombus formation and compression can be devastating leading to flap loss. Early vascular compromise equals reoperation for flap salvage.
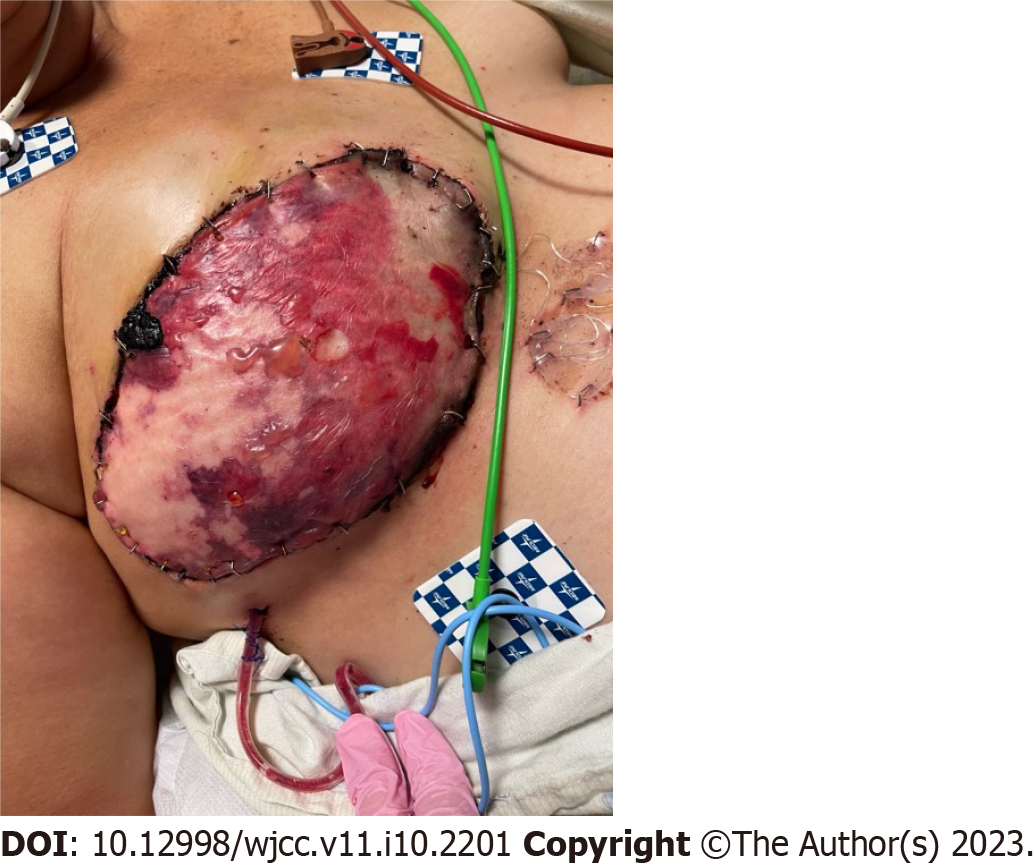
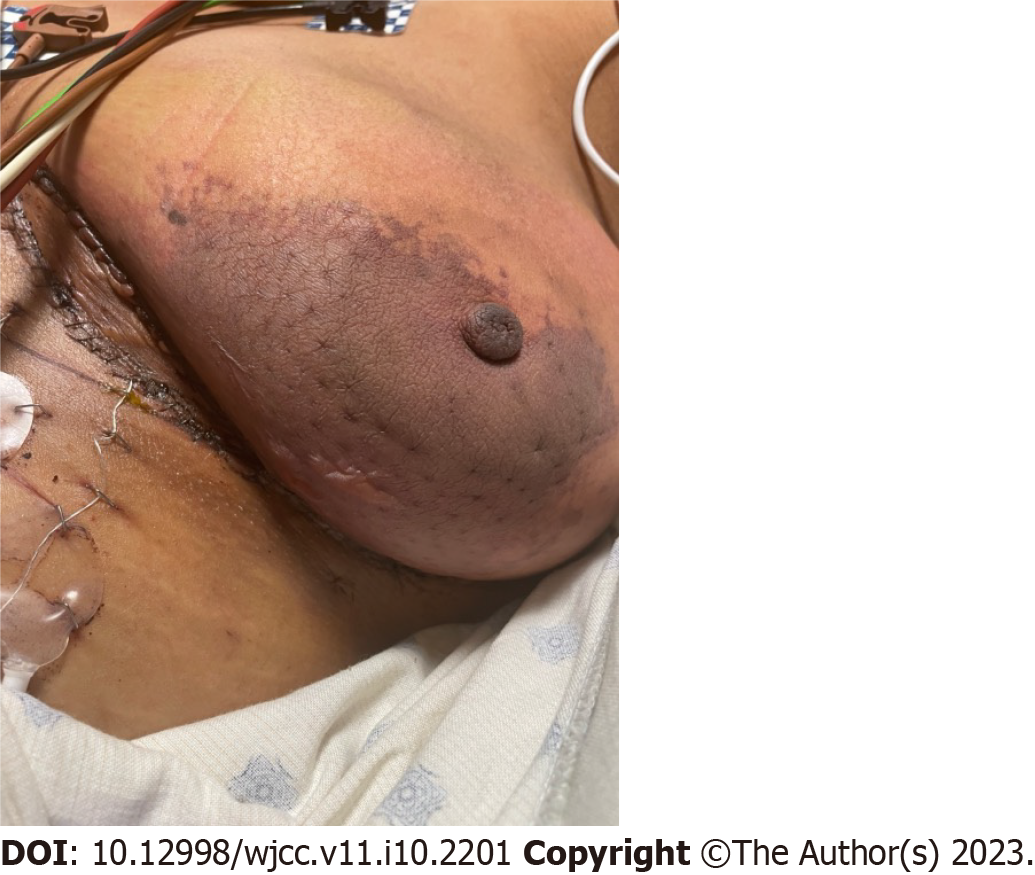
Other postoperative complications include partial flap loss (Figure 7), fat necrosis, infections (Figure 8), wound dehiscence, hematoma (Figure 9), and seroma formation. Donor site complications include infections, seroma, hematoma, wound dehiscence, necrosis, and hernia formation. It is shown that frailty is a reliable predictor of postoperative complications in autologous reconstruction[81].
Boyed et al[43] performed a 10-year follow-up study on a cohort of patients of whom about 25% had autologous breast reconstruction and found no association between increased recurrence and operation[43]. No significant difference is found in terms of locoregional recurrence of cancer between implant-based and autologous reconstruction[82,83]. Low locoregional recurrence rate (3.5%) after immediate autologous breast reconstruction is shown in another study by Wu et al[46] with a median follow-up of 7 years[46].
Plastic surgeons have an armamentarium of implant-based and autologous reconstructive options to manage patients with breast cancer which should be tailormade for each individual patient. Both options have favorable long-term oncologic outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang Z, China; Wang SY, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Albornoz CR, Bach PB, Mehrara BJ, Disa JJ, Pusic AL, McCarthy CM, Cordeiro PG, Matros E. A paradigm shift in U.S. Breast reconstruction: increasing implant rates. Plast Reconstr Surg. 2013;131:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 707] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 2. | Fischer JP, Nelson JA, Serletti JM, Wu LC. Peri-operative risk factors associated with early tissue expander (TE) loss following immediate breast reconstruction (IBR): a review of 9305 patients from the 2005-2010 ACS-NSQIP datasets. J Plast Reconstr Aesthet Surg. 2013;66:1504-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Fischer JP, Nelson JA, Kovach SJ, Serletti JM, Wu LC, Kanchwala S. Impact of obesity on outcomes in breast reconstruction: analysis of 15,937 patients from the ACS-NSQIP datasets. J Am Coll Surg. 2013;217:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Hart A, Funderburk CD, Chu CK, Pinell-White X, Halgopian T, Manning-Geist B, Carlson G, Losken A. The Impact of Diabetes Mellitus on Wound Healing in Breast Reconstruction. Ann Plast Surg. 2017;78:260-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Drucker-Zertuche M, Robles-Vidal C. A 7 year experience with immediate breast reconstruction after skin sparing mastectomy for cancer. Eur J Surg Oncol. 2007;33:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Nahabedian MY. Implant-based breast reconstruction: Strategies to achieve optimal outcomes and minimize complications. J Surg Oncol. 2016;113:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Gdalevitch P, Ho A, Genoway K, Alvrtsyan H, Bovill E, Lennox P, Van Laeken N, Macadam S. Direct-to-implant single-stage immediate breast reconstruction with acellular dermal matrix: predictors of failure. Plast Reconstr Surg. 2014;133:738e-747e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Basta MN, Gerety PA, Serletti JM, Kovach SJ, Fischer JP. A Systematic Review and Head-to-Head Meta-Analysis of Outcomes following Direct-to-Implant versus Conventional Two-Stage Implant Reconstruction. Plast Reconstr Surg. 2015;136:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Colwell AS, Damjanovic B, Zahedi B, Medford-Davis L, Hertl C, Austen WG Jr. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Ganesh Kumar N, Berlin NL, Kim HM, Hamill JB, Kozlow JH, Wilkins EG. Development of an evidence-based approach to the use of acellular dermal matrix in immediate expander-implant-based breast reconstruction. J Plast Reconstr Aesthet Surg. 2021;74:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Forsberg CG, Kelly DA, Wood BC, Mastrangelo SL, DeFranzo AJ, Thompson JT, David LR, Marks MW. Aesthetic outcomes of acellular dermal matrix in tissue expander/implant-based breast reconstruction. Ann Plast Surg. 2014;72:S116-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Salzberg CA, Ashikari AY, Berry C, Hunsicker LM. Acellular Dermal Matrix-Assisted Direct-to-Implant Breast Reconstruction and Capsular Contracture: A 13-Year Experience. Plast Reconstr Surg. 2016;138:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 13. | Lanier ST, Wang ED, Chen JJ, Arora BP, Katz SM, Gelfand MA, Khan SU, Dagum AB, Bui DT. The effect of acellular dermal matrix use on complication rates in tissue expander/implant breast reconstruction. Ann Plast Surg. 2010;64:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Rohrich RJ, Reece EM. Breast augmentation today: saline versus silicone--what are the facts? Plast Reconstr Surg. 2008;121:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Macadam SA, Ho AL, Cook EF Jr, Lennox PA, Pusic AL. Patient satisfaction and health-related quality of life following breast reconstruction: patient-reported outcomes among saline and silicone implant recipients. Plast Reconstr Surg. 2010;125:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Gahm J, Edsander-Nord Å, Jurell G, Wickman M. No differences in aesthetic outcome or patient satisfaction between anatomically shaped and round expandable implants in bilateral breast reconstructions: a randomized study. Plast Reconstr Surg. 2010;126:1419-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Ostapenko E, Nixdorf L, Devyatko Y, Exner R, Wimmer K, Fitzal F. Prepectoral Versus Subpectoral Implant-Based Breast Reconstruction: A Systemic Review and Meta-analysis. Ann Surg Oncol. 2023;30:126-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (1)] |
| 18. | Woo A, Harless C, Jacobson SR. Revisiting an Old Place: Single-Surgeon Experience on Post-Mastectomy Subcutaneous Implant-Based Breast Reconstruction. Breast J. 2017;23:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Escandón JM, Sweitzer K, Christiano JG, Gooch JC, Olzinski AT, Prieto PA, Skinner KA, Langstein HN, Manrique OJ. Subpectoral versus prepectoral two-stage breast reconstruction: A propensity score-matched analysis of 30-day morbidity and long-term outcomes. J Plast Reconstr Aesthet Surg. 2023;76:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 20. | Frey JD, Salibian AA, Choi M, Karp NS. Mastectomy Flap Thickness and Complications in Nipple-Sparing Mastectomy: Objective Evaluation using Magnetic Resonance Imaging. Plast Reconstr Surg Glob Open. 2017;5:e1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Highton L, Johnson R, Kirwan C, Murphy J. Prepectoral Implant-Based Breast Reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Sbitany H. Important Considerations for Performing Prepectoral Breast Reconstruction. Plast Reconstr Surg. 2017;140:7S-13S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Sbitany H, Piper M, Lentz R. Prepectoral Breast Reconstruction: A Safe Alternative to Submuscular Prosthetic Reconstruction following Nipple-Sparing Mastectomy. Plast Reconstr Surg. 2017;140:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 24. | Nahabedian MY, Cocilovo C. Two-Stage Prosthetic Breast Reconstruction: A Comparison Between Prepectoral and Partial Subpectoral Techniques. Plast Reconstr Surg. 2017;140:22S-30S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 25. | Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg. 2011;127:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013;132:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat. 2011;127:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Santosa KB, Chen X, Qi J, Ballard TNS, Kim HM, Hamill JB, Bensenhaver JM, Pusic AL, Wilkins EG. Postmastectomy Radiation Therapy and Two-Stage Implant-Based Breast Reconstruction: Is There a Better Time to Irradiate? Plast Reconstr Surg. 2016;138:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Cordeiro PG, Albornoz CR, McCormick B, Hudis CA, Hu Q, Heerdt A, Matros E. What Is the Optimum Timing of Postmastectomy Radiotherapy in Two-Stage Prosthetic Reconstruction: Radiation to the Tissue Expander or Permanent Implant? Plast Reconstr Surg. 2015;135:1509-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 160] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Lentz R, Ng R, Higgins SA, Fusi S, Matthew M, Kwei SL. Radiation therapy and expander-implant breast reconstruction: an analysis of timing and comparison of complications. Ann Plast Surg. 2013;71:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Ricci JA, Epstein S, Momoh AO, Lin SJ, Singhal D, Lee BT. A meta-analysis of implant-based breast reconstruction and timing of adjuvant radiation therapy. J Surg Res. 2017;218:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Kronowitz SJ, Lam C, Terefe W, Hunt KK, Kuerer HM, Valero V, Lance S, Robb GL, Feng L, Buchholz TA. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plast Reconstr Surg. 2011;127:2154-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Nava MB, Pennati AE, Lozza L, Spano A, Zambetti M, Catanuto G. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plast Reconstr Surg. 2011;128:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Sbitany H, Wang F, Peled AW, Lentz R, Alvarado M, Ewing CA, Esserman LJ, Fowble B, Foster RD. Immediate implant-based breast reconstruction following total skin-sparing mastectomy: defining the risk of preoperative and postoperative radiation therapy for surgical outcomes. Plast Reconstr Surg. 2014;134:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Momoh AO, Ahmed R, Kelley BP, Aliu O, Kidwell KM, Kozlow JH, Chung KC. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 36. | Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: A meta-analysis. J Surg Oncol. 2015;112:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Wang F, Peled AW, Chin R, Fowble B, Alvarado M, Ewing C, Esserman L, Foster R, Sbitany H. The Impact of Radiation Therapy, Lymph Node Dissection, and Hormonal Therapy on Outcomes of Tissue Expander-Implant Exchange in Prosthetic Breast Reconstruction. Plast Reconstr Surg. 2016;137:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Donker M, Hage JJ, Woerdeman LA, Rutgers EJ, Sonke GS, Vrancken Peeters MJ. Surgical complications of skin sparing mastectomy and immediate prosthetic reconstruction after neoadjuvant chemotherapy for invasive breast cancer. Eur J Surg Oncol. 2012;38:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Vardanian AJ, Clayton JL, Roostaeian J, Shirvanian V, Da Lio A, Lipa JE, Crisera C, Festekjian JH. Comparison of implant-based immediate breast reconstruction with and without acellular dermal matrix. Plast Reconstr Surg. 2011;128:403e-410e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 40. | Zhao X, Wu X, Dong J, Liu Y, Zheng L, Zhang L. A Meta-analysis of Postoperative Complications of Tissue Expander/Implant Breast Reconstruction Using Acellular Dermal Matrix. Aesthetic Plast Surg. 2015;39:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Wan D, Rohrich RJ. Revisiting the Management of Capsular Contracture in Breast Augmentation: A Systematic Review. Plast Reconstr Surg. 2016;137:826-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Phillips BT, Halvorson EG. Antibiotic Prophylaxis following Implant-Based Breast Reconstruction: What Is the Evidence? Plast Reconstr Surg. 2016;138:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Boyd CJ, Salibian AA, Bekisz JM, Axelrod DM, Guth AA, Shapiro RL, Schnabel FR, Karp NS, Choi M. Long-Term Cancer Recurrence Rates following Nipple-Sparing Mastectomy: A 10-Year Follow-Up Study. Plast Reconstr Surg. 2022;150:13S-19S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Blanckaert M, Vranckx J. Oncological safety of therapeutic 'nipple-sparing mastectomy' followed by reconstruction: a systematic review. Acta Chir Belg. 2021;121:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Wu ZY, Kim HJ, Lee JW, Chung IY, Kim JS, Lee SB, Son BH, Eom JS, Kim SB, Gong GY, Kim HH, Ahn SH, Ko B. Breast Cancer Recurrence in the Nipple-Areola Complex After Nipple-Sparing Mastectomy With Immediate Breast Reconstruction for Invasive Breast Cancer. JAMA Surg. 2019;154:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 47. | Wu ZY, Han HH, Han J, Son BH, Eom JS, Kim SB, Gong G, Kim HH, Ahn SH, Ko B. Breast Cancer Recurrence after Smooth versus Textured Implant-Based Breast Reconstruction: A Matched Cohort Study. Plast Reconstr Surg. 2022;150:30S-37S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Cordeiro PG, Ghione P, Ni A, Hu Q, Ganesan N, Galasso N, Dogan A, Horwitz SM. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J Plast Reconstr Aesthet Surg. 2020;73:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 49. | Collett DJ, Rakhorst H, Lennox P, Magnusson M, Cooter R, Deva AK. Current Risk Estimate of Breast Implant-Associated Anaplastic Large Cell Lymphoma in Textured Breast Implants. Plast Reconstr Surg. 2019;143:30S-40S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 50. | Calobrace MB, Schwartz MR, Zeidler KR, Pittman TA, Cohen R, Stevens WG. Long-Term Safety of Textured and Smooth Breast Implants. Aesthet Surg J. 2017;38:38-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | McGuire PA, Deva AK, Glicksman CA, Adams WP Jr, Haws MJ. Management of Asymptomatic Patients With Textured Surface Breast Implants. Aesthet Surg J Open Forum. 2019;1:ojz025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Karp N, McGuire P, Adams WP, Jewell ML. US FDA Patient Decision Checklist for Breast Implants: Results of a Survey to Members of The Aesthetic Society, April 2022. Aesthet Surg J. 2023;43:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Goldberg MT, Llaneras J, Willson TD, Boyd JB, Venegas RJ, Dauphine C, Kalantari BN. Squamous Cell Carcinoma Arising in Breast Implant Capsules. Ann Plast Surg. 2021;86:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 54. | Soni SE, Laun JC, Beard AS, Kuykendall LV. Breast Implant Capsule-Associated Squamous Cell Carcinoma during Pregnancy: A Mimicker of Breast Implant-Associated Anaplastic Large-Cell Lymphoma. Plast Reconstr Surg. 2022;150:926e-928e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 55. | Nichter LS, Hardesty RA, Anigian GM. IDEAL IMPLANT Structured Breast Implants: Core Study Results at 6 Years. Plast Reconstr Surg. 2018;142:66-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Gabriel A, Maxwell GP. The Science of Cohesivity and Elements of Form Stability. Plast Reconstr Surg. 2019;144:7S-12S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Barnow A, Canfield T, Liao R, Yadalam S, Kalsekar I, Khanna R. Breast Reconstruction Among Commercially Insured Women With Breast Cancer in the United States. Ann Plast Surg. 2018;81:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Sheckter CC, Panchal HJ, Razdan SN, Rubin D, Yi D, Disa JJ, Mehrara B, Matros E. The Influence of Physician Payments on the Method of Breast Reconstruction: A National Claims Analysis. Plast Reconstr Surg. 2018;142:434e-442e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Sheckter CC, Yi D, Panchal HJ, Razdan SN, Pusic AL, McCarthy CM, Cordeiro PG, Disa JJ, Mehrara B, Matros E. Trends in Physician Payments for Breast Reconstruction. Plast Reconstr Surg. 2018;141:493e-499e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Jagsi R, Momoh AO, Qi J, Hamill JB, Billig J, Kim HM, Pusic AL, Wilkins EG. Impact of Radiotherapy on Complications and Patient-Reported Outcomes After Breast Reconstruction. J Natl Cancer Inst. 2018;110:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 182] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 61. | Chetta MD, Aliu O, Zhong L, Sears ED, Waljee JF, Chung KC, Momoh AO. Reconstruction of the Irradiated Breast: A National Claims-Based Assessment of Postoperative Morbidity. Plast Reconstr Surg. 2017;139:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Agarwal S, Talia J, Liu PS, Momoh AO, Kozlow JH. Determining the Cost of Incidental Findings for Patients Undergoing Preoperative Planning for Abdominally Based Perforator Free Flap Breast Reconstruction with Computed Tomographic Angiography. Plast Reconstr Surg. 2016;138:804e-810e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Teunis T, Heerma van Voss MR, Kon M, van Maurik JF. CT-angiography prior to DIEP flap breast reconstruction: a systematic review and meta-analysis. Microsurgery. 2013;33:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 64. | Atisha D, Alderman AK, Janiga T, Singal B, Wilkins EG. The efficacy of the surgical delay procedure in pedicle TRAM breast reconstruction. Ann Plast Surg. 2009;63:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Vyas RM, Dickinson BP, Fastekjian JH, Watson JP, DaLio AL, Crisera CA. Risk factors for abdominal donor-site morbidity in free flap breast reconstruction. Plast Reconstr Surg. 2008;121:1519-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 66. | Sbitany H, Mirzabeigi MN, Kovach SJ, Wu LC, Serletti JM. Strategies for recognizing and managing intraoperative venous congestion in abdominally based autologous breast reconstruction. Plast Reconstr Surg. 2012;129:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Spiegel AJ, Khan FN. An Intraoperative algorithm for use of the SIEA flap for breast reconstruction. Plast Reconstr Surg. 2007;120:1450-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Schaverien M, Saint-Cyr M, Arbique G, Brown SA. Arterial and venous anatomies of the deep inferior epigastric perforator and superficial inferior epigastric artery flaps. Plast Reconstr Surg. 2008;121:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 69. | Holm C, Mayr M, Höfter E, Ninkovic M. Perfusion zones of the DIEP flap revisited: a clinical study. Plast Reconstr Surg. 2006;117:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Chang EI, Chang EI, Soto-Miranda MA, Zhang H, Nosrati N, Robb GL, Chang DW. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg. 2013;132:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 71. | Selber JC. The Robotic DIEP Flap. Plast Reconstr Surg. 2020;145:340-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 72. | Choi JH, Song SY, Park HS, Kim CH, Kim JY, Lew DH, Roh TS, Lee DW. Robotic DIEP Flap Harvest through a Totally Extraperitoneal Approach Using a Single-Port Surgical Robotic System. Plast Reconstr Surg. 2021;148:304-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Park JE, Alkureishi LWT, Song DH. TUGs into VUGs and Friendly BUGs: Transforming the Gracilis Territory into the Best Secondary Breast Reconstructive Option. Plast Reconstr Surg. 2015;136:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 74. | DeLong MR, Hughes DB, Bond JE, Thomas SM, Boll DT, Zenn MR. A detailed evaluation of the anatomical variations of the profunda artery perforator flap using computed tomographic angiograms. Plast Reconstr Surg. 2014;134:186e-192e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | LoTempio MM, Allen RJ. Breast reconstruction with SGAP and IGAP flaps. Plast Reconstr Surg. 2010;126:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Nahabedian MY, Momen B, Galdino G, Manson PN. Breast Reconstruction with the free TRAM or DIEP flap: patient selection, choice of flap, and outcome. Plast Reconstr Surg. 2002;110:466-475; discussion 476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 77. | Patel NG, Ramakrishnan V. Microsurgical Tissue Transfer in Breast Reconstruction. Clin Plast Surg. 2017;44:345-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 78. | Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. J Reconstr Microsurg. 2014;30:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Allen RJ Jr, Lee ZH, Mayo JL, Levine J, Ahn C, Allen RJ Sr. The Profunda Artery Perforator Flap Experience for Breast Reconstruction. Plast Reconstr Surg. 2016;138:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Leyngold MM. Is Unipedicled Transverse Rectus Abdominis Myocutaneous Flap Obsolete Owing to Superiority of DIEP Flap? Ann Plast Surg. 2018;80:S418-S420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Joo A, Giatsidis G. In Autologous Breast Reconstruction, Frailty Is a More Accurate Predictor of Postoperative Complications: A Retrospective Cohort Analysis. Plast Reconstr Surg. 2022;150:82S-94S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Kim S, Lee S, Bae Y. Nipple-sparing mastectomy for breast cancer close to the nipple: a single institution's 11-year experience. Breast Cancer. 2020;27:999-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 83. | Stanec Z, Žic R, Budi S, Stanec S, Milanović R, Vlajčić Z, Roje Z, Rudman F, Martić K, Held R, Božo G. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |