Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2591
Peer-review started: September 15, 2021
First decision: November 11, 2021
Revised: November 12, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Processing time: 176 Days and 10.7 Hours
Radiotherapy for hepatocellular carcinoma (HCC) is considered to have limited efficacy because of treatment intensity considering that the irradiated area includes the liver, which is highly radiosensitive. In this report, we present two cases in which tumor control by surgical resection, radiofrequency ablation, transcatheter arterial chemoembolization (TACE), and lenvatinib administration was difficult, but stereotactic body radiotherapy (SBRT) using the Synchrony system by Radixact™ and Gold Anchor® (GA) was effective.
A 60-year-old man had a single 10-cm HCC in the right lobe. Viable lesions remained after TACE, and levels of alpha-fetoprotein and protein induced by vitamin K antagonists II (PIVKA-II) decreased and quickly re-elevated. We performed SBRT with GA. Three weeks after implantation, localized radiotherapy (SBRT; 40 Gy/5 fractions) was performed using the Synchrony system by Radixact™. Four weeks later, the viable lesion had disappeared, and the PIVKA-II levels decreased. A 77-year-old man had a single 12-cm HCC in the right lobe. The patient experienced recurrence after hepatectomy. Further recurrence occurred after TACE, and we performed SBRT with GA. Because of the proximity of the HCC to the gastrointestinal tract, localized radiotherapy (SBRT; 39 Gy/13 fractions) to the HCC was performed 3 wk after implantation using the Synchrony system by Radixact™. Four weeks later, the viable lesion had disappeared on computed tomography, and the PIVKA-Ⅱ levels decreased.
SBRT using the Synchrony system and GA can deliver a large dose accurately and safely, and could have a high therapeutic effect.
Core Tip: Radiotherapy using fiducial markers has been performed for hepatocellular carcinoma (HCC) for several years. However, the Gold Anchor® (GA) used in this report is a new fiducial marker with a small diameter, which is expected to reduce the incidence of complications. The Synchrony system by Radixact™ is also a new radiation device, which is useful in respiratory motion management and allows complete tracking of the HCC in which the GA is implanted. Radiotherapy using these devices was highly effective for HCC that could not be controlled by surgery, transcatheter arterial chemoembolization, or molecular targeted drugs.
- Citation: Masuda S, Tsukiyama T, Minagawa Y, Koizumi K, Kako M, Kinbara T, Haruki U. Hepatocellular carcinoma effective stereotactic body radiotherapy using Gold Anchor and the Synchrony system: Two case reports and review of literature. World J Clin Cases 2022; 10(8): 2591-2603
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2591.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2591
Hepatocellular carcinoma (HCC) ranks as the sixth cause of cancer incidence and the fourth cause of cancer-related deaths worldwide[1]. There are various treatment methods for HCC, including surgical resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), systemic chemotherapy, and radiotherapy[2]. Since the major risk factors for HCC include chronic hepatitis and liver cirrhosis, many patients have an underlying liver disease at the time of treatment. In addition to tumor and technical factors, the patient's liver function has a significant impact on determining the optimal treatment for HCC. Delis et al[3] and Bruix et al[4] reported that only 10%-30% of HCC cases are indicative of curative surgery because most HCC is diagnosed at intermediate or advanced stages. Radiotherapy is not included in the Barcelona Clinic Liver Cancer staging treatment allocation scheme; however, evidence showing that radiotherapy may be a potential tool for primary treatment or for bridging/downsizing purposes is growing[5]. A variety of technological progress has led to more accurate and dose-escalated radiotherapy, particularly stereotactic body radiation therapy (SBRT)[6]. SBRT sufficiently reduces the dose to adjacent organs due to its sharp dose fall-down outside of the target, while enhancing the biological effectiveness of a large single dose[7]. However, with SBRT it can be difficult to track tumors in the liver due to respiratory motion management (RMM)[8]. SBRT with RMM is becoming more common; however, RMM is often performed with breath-holding or fixation of the trunk. For a more focused and accurate delivery of SBRT, fiducial markers are used to locate tumors in the liver[9]. Using Gold Anchor® (GA) (Naslund Medical AB, Huddinge, Sweden), a new marker of fine diameter, we performed SBRT in two cases and thereby confirmed its efficacy. The GA used in these cases is characterized by a low risk of complications and high visibility on computed tomography (CT) and magnetic resonance imaging (MRI)[9].
Case 1: For treatment of HCC.
Case 2: For further management of HCC.
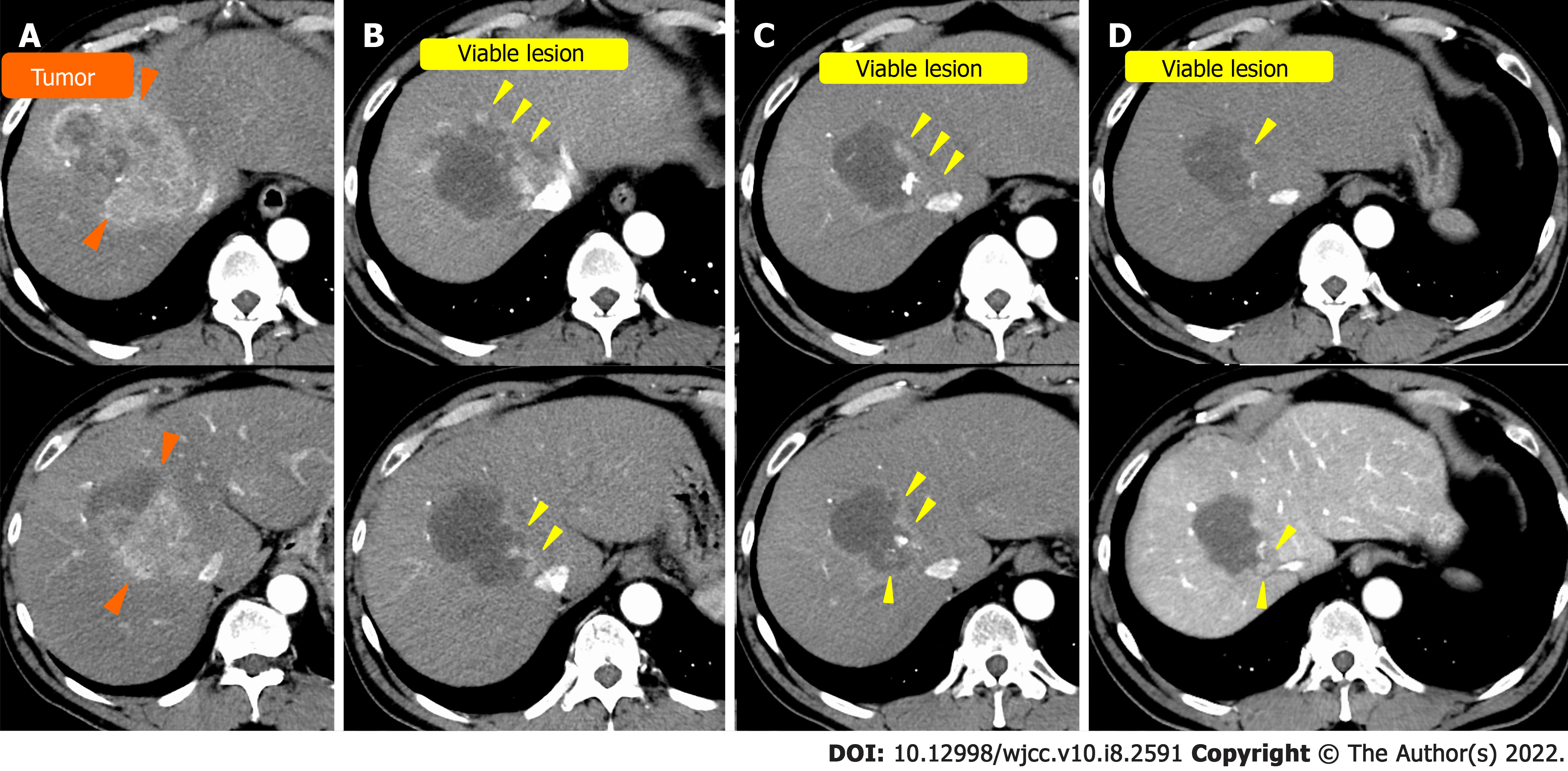
Case 1: The patient was a 60-year-old man with alcoholic cirrhosis. For three years, he underwent regular CT examinations annually, and CT revealed a single 10-cm HCC in the right lobe (Figure 1A).
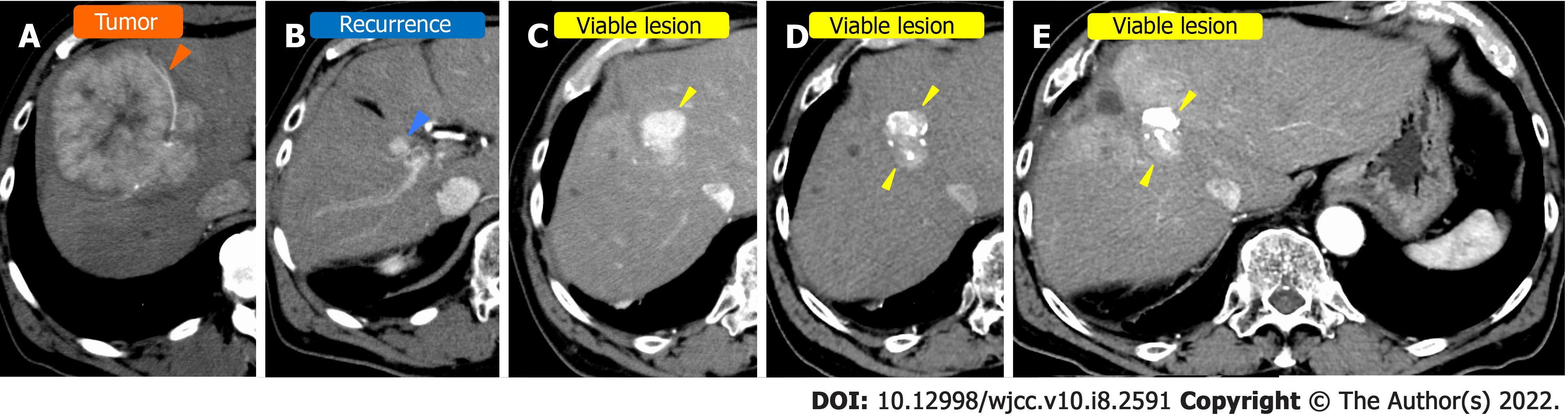
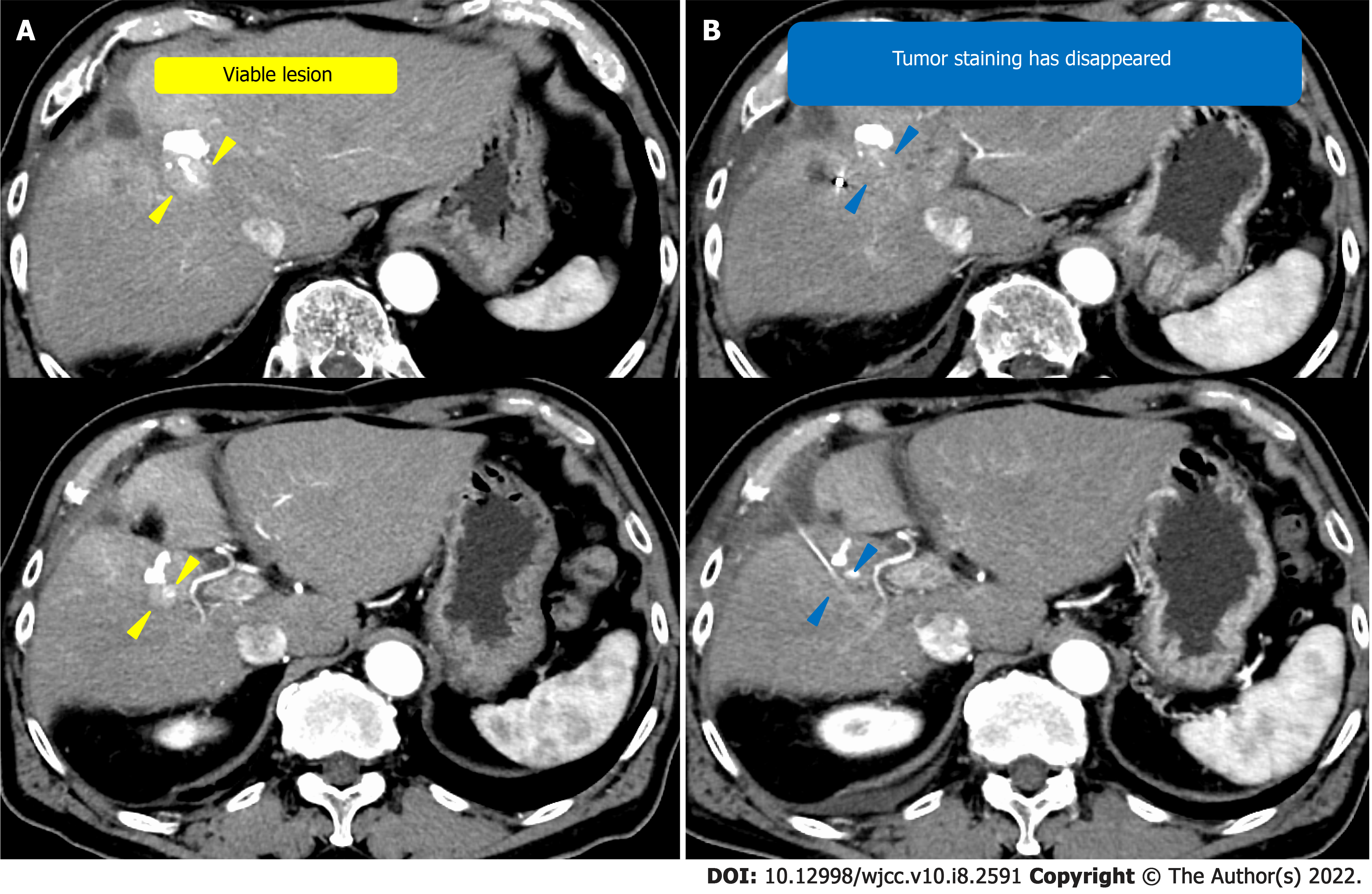
Case 2: The patient was a 77-year-old man with hepatitis B infection. He underwent regular CT examinations annually at a nearby hospital. Four years ago, CT revealed a single HCC measuring 12-cm in the right lobe (Figure 2A). The patient underwent hepatectomy at the same institution, but recurrence occurred (Figure 2B). TACE was then performed to treat the recurrence; however, there was further recurrence (Figure 2C). The patient was subsequently admitted to our hospital for further management of the HCC.
Case 1: The patient was diagnosed with hypertension, diabetes, and gout 10 years ago, and with alcoholic cirrhosis and angina 3 years ago. He had been taking the following medications: Telmisartan, amlodipine basilate, febuxostat, empagliflozin linagliptin, aspirin, pitavastatin calcium hydrate, and diltiazem hydrochloride.
Case 2: The patient was diagnosed with hepatitis B seven years ago at a nearby hospital. He was previously diagnosed with benign prostatic hyperplasia. He was not on any medication.
Case 1: He had been drinking 50-100 g of alcohol daily for 40 years.
Case 2: He rarely drank. He was exposed to the atomic bomb in World War II.
Cases 1 and 2: There were no findings of anemia or jaundice. The abdominal findings were also unremarkable.
Case 1: Blood tests showed negative hepatitis virus markers (Table 1), and Child-Pugh status was A.
| Laboratory examination | ||
| White blood cells | 8100/μL | 4200/μL |
| Hb | 15.6 g/dL | 15.1 g/dL |
| Ht | 45.90% | 43.50% |
| MCV | 96.2 fl | 94.2 fl |
| PLT | 162000/μL | 141000/μL |
| PT-INR | 0.96 | 1 |
| PT% | 107% | 100.50% |
| T-BIL | 0.6 mg/dL | 1.3 mg/dL |
| AST | 62 U/L | 18 U/L |
| ALT | 60 U/L | 14 U/L |
| γGTP | 689 U/L | 68 U/L |
| ALP | 423 U/L | 249 U/L |
| TP | 8 g/dL | 7.1 g/dL |
| ALB | 3.8 g/dL | 4 g/dL |
| HbA1c | 7.10% | 5.50% |
| BUN | 16.4 mg/dL | 21.7 mg/dL |
| CRE | 0.68 mg/dL | 1.05 mg/dL |
| CRP | 0.524 mg/dL | 0.149 mg/dL |
| AFP | 276.8 ng/mL | 3.6 ng/mL |
| PIVKA-II | 33224 mAU/mL | 44 mAU/mL |
| CEA | 5.9 ng/mL | 4.5 ng/mL |
| HBs antigens | (-) | (-) |
| HBc antibody | (-) | (+) |
| HCV antibody | (-) | (-) |
Case 2: Blood tests were positive for hepatitis B core antibody (Table 1), and Child-Pugh status was A.
Case 1: Contrast-enhanced CT revealed a typical 10-cm HCC in the right lobe. The contrast enhanced the HCC in the early phase and washed out in the late phase (Figure 1A).
Case 2: Contrast-enhanced CT revealed a typical 12-cm HCC in the right lobe. The contrast stained the HCC in the early phase and washed out in late phase (Figure 2A).
The final diagnosis was HCC due to alcoholic liver cirrhosis.
The final diagnosis was HCC due to hepatitis B infection.
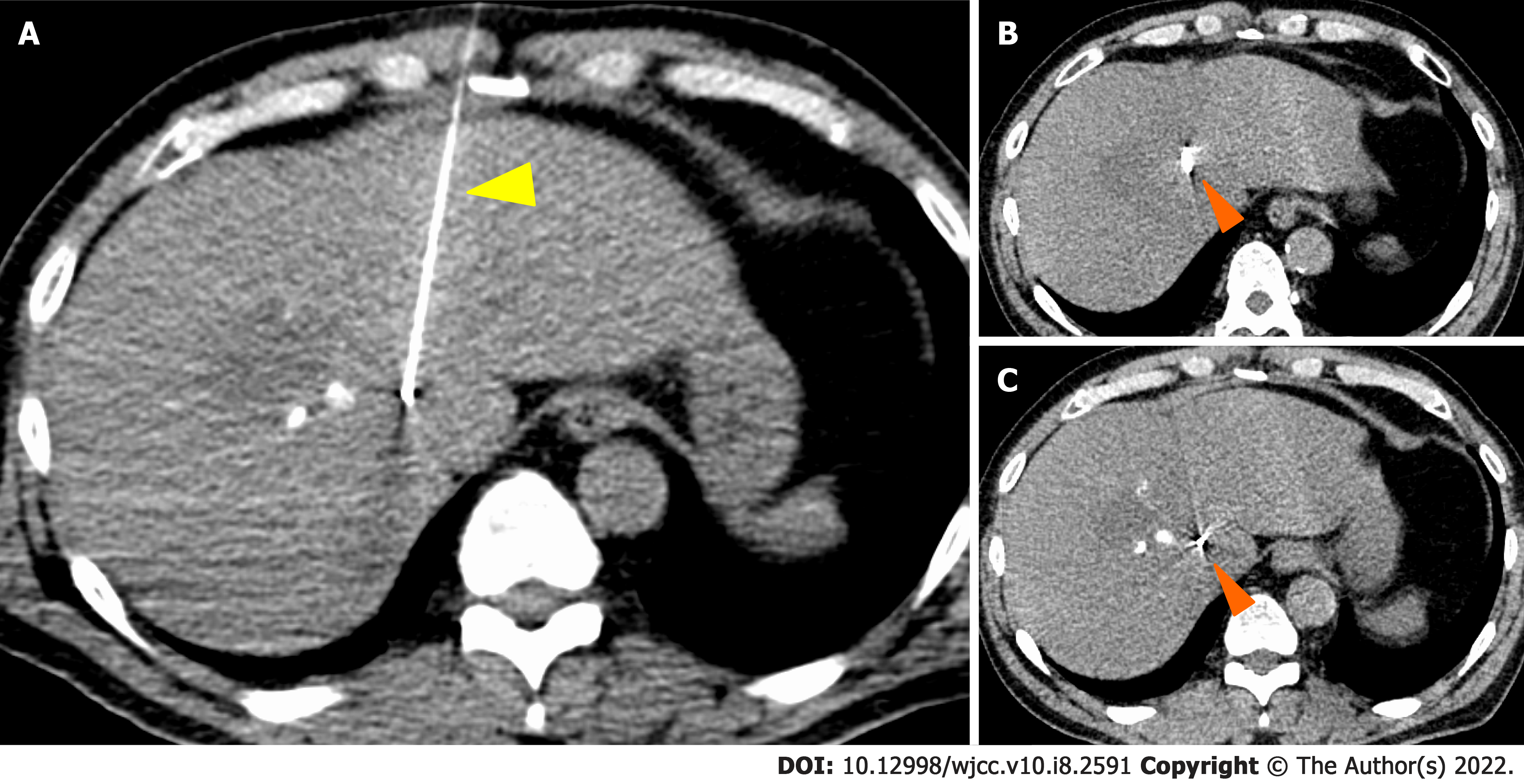
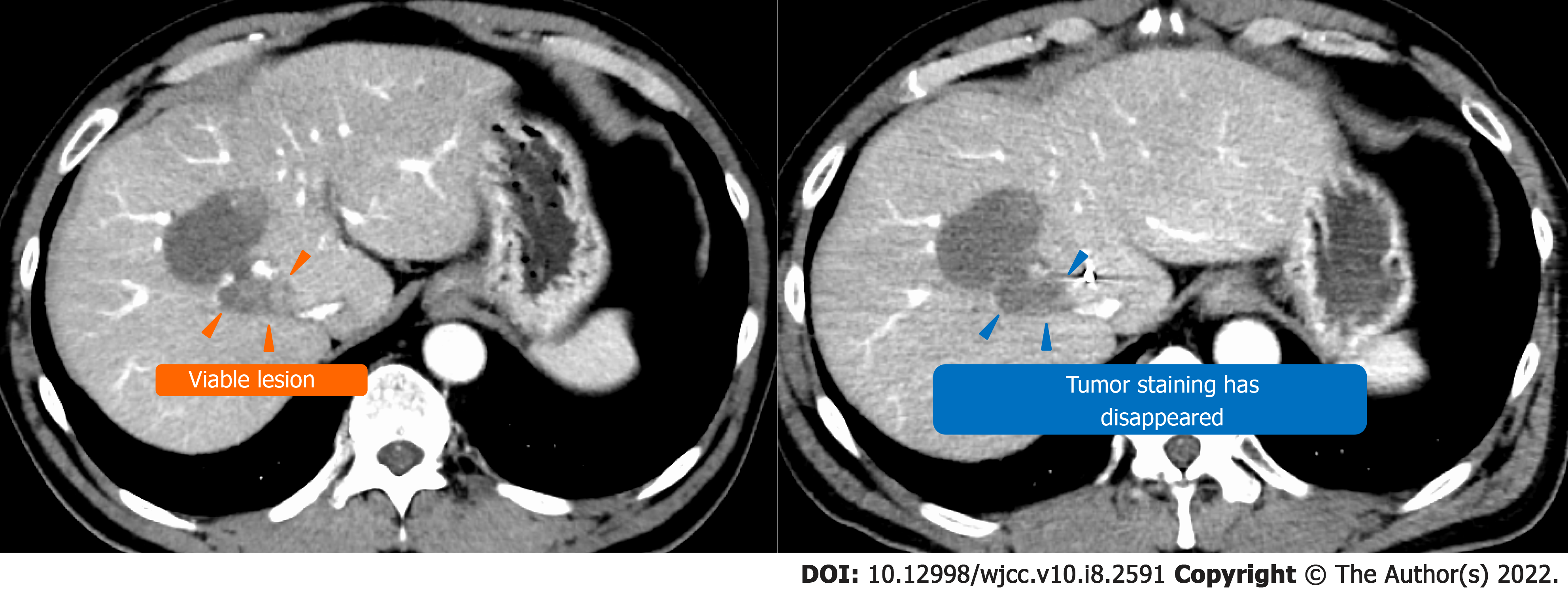
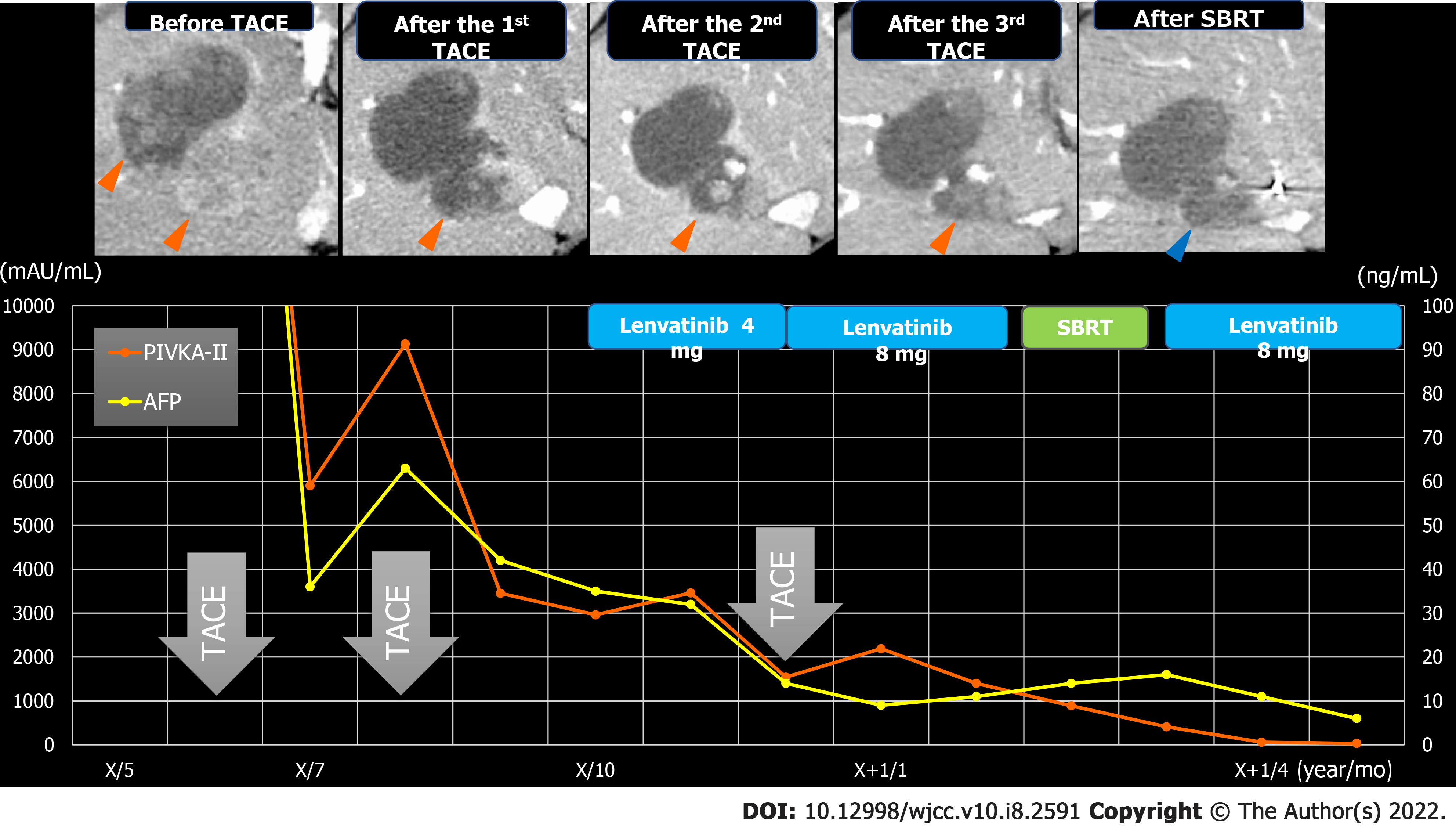
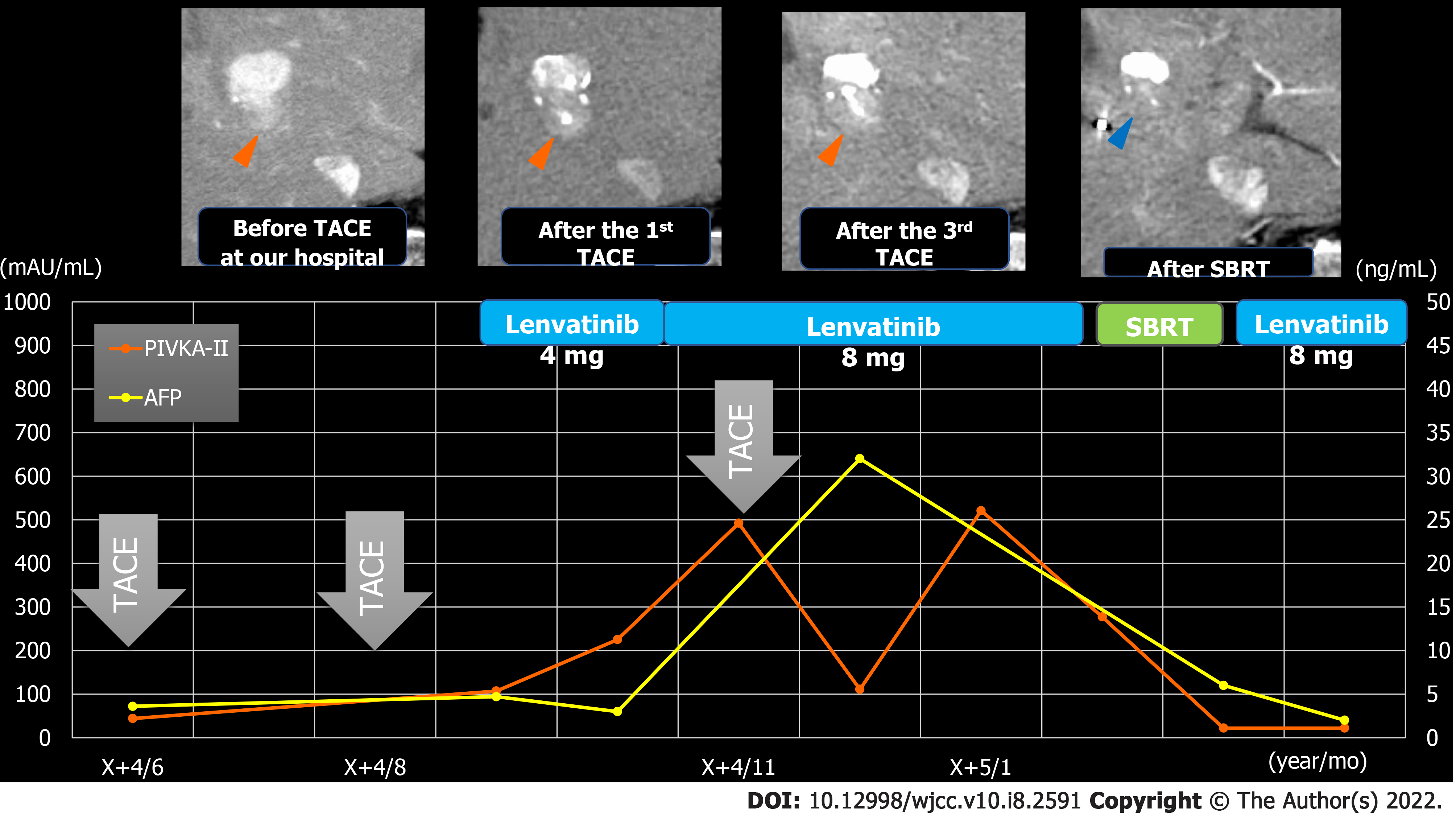
TACE was performed twice for HCC, but in both instances, viable lesions remained, and levels of alpha-fetoprotein and protein induced by vitamin K antagonists-Ⅱ (PIVKA-Ⅱ) decreased then quickly re-elevated. A third TACE was performed; however, the results were similar to the previous TACEs: 2.5 cm of viable lesions remained, and the once-decreased PIVKA-Ⅱ level immediately increased again (Figure 1B-D). Therefore, we decided to perform SBRT with GA; an interventional radiology specialist with 30 years of experience selected a 22G needle and implanted two GAs within 2 cm of the HCC (Figure 3). One week after implantation, CT registration was performed, and radiotherapy was planned. Three weeks after implantation, localized radiotherapy (SBRT; 40 Gy/5 fractions) was performed for HCC using the Synchrony system by Radixact™ (Accuray Japan K.K., Tokyo, Japan).
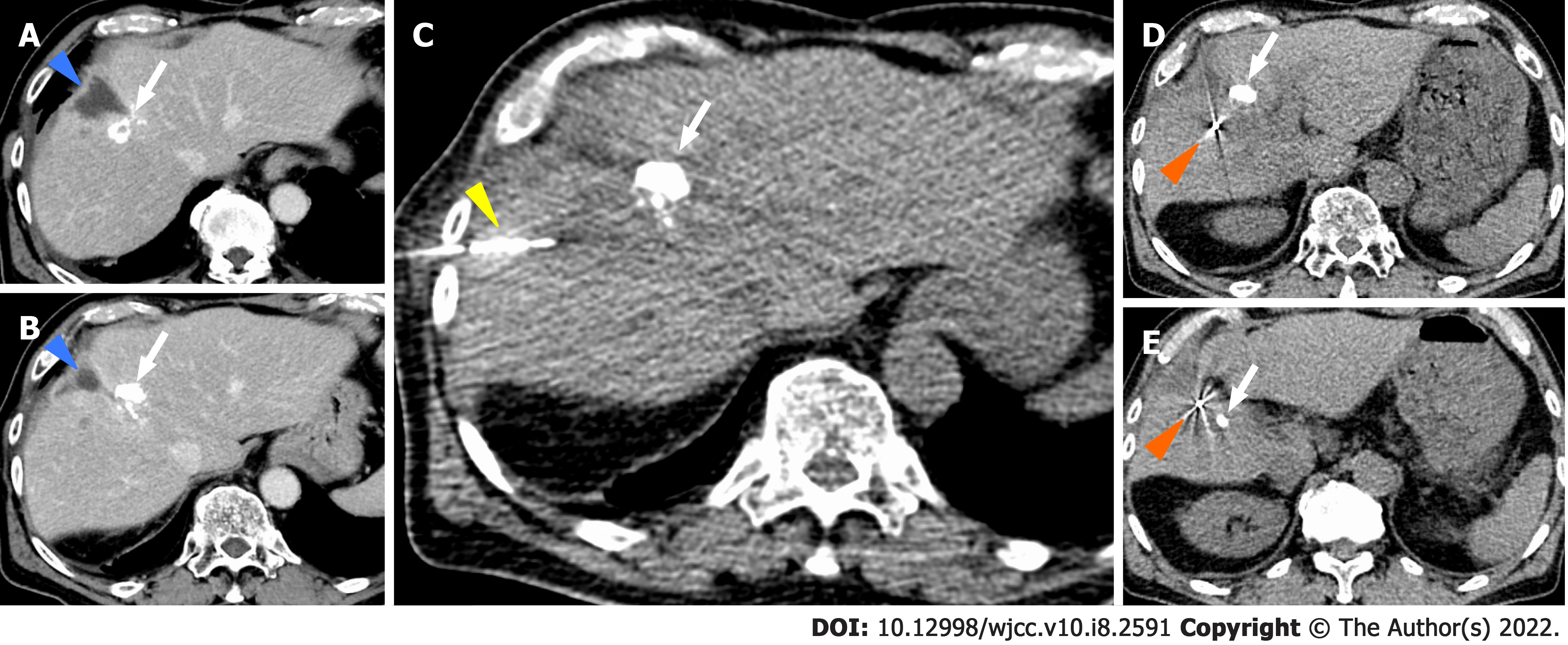
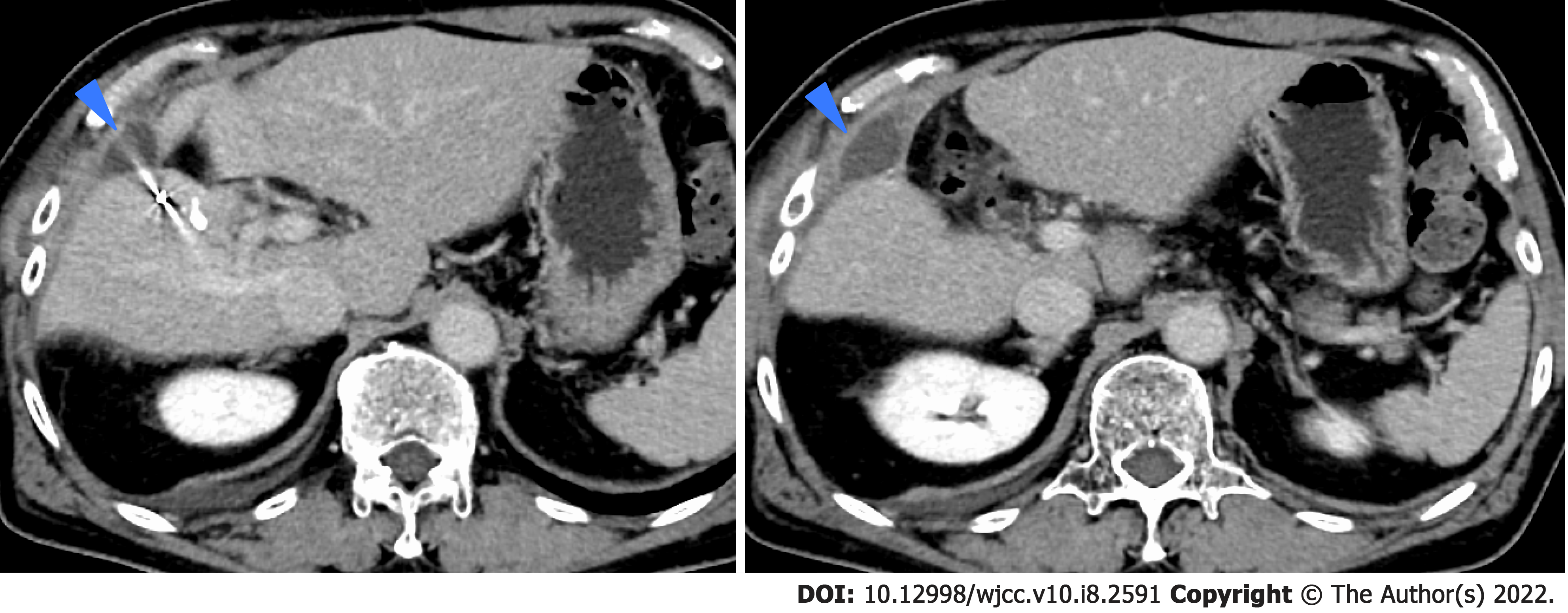
We performed TACE twice for HCC in our hospital, but both times viable lesions remained. The PIVKA-Ⅱ level decreased but quickly re-elevated. A third TACE was performed; however, the results were similar to the previous TACEs: 2 cm of viable lesions remained, and the decreased PIVKA-Ⅱ level increased again (Figure 2D and E). Therefore, we decided to perform SBRT with GA, and two GAs were implanted within 2 cm of the HCC with a 22G needle. In this case, the biloma was complicated after hepatectomy at the previous hospital, and the puncture route for GA implantation passed near the site (Figure 4). One week after implantation, CT registration was performed, and radiotherapy was planned. Because of the proximity of the HCC to the gastrointestinal tract, localized radiotherapy (SBRT; 39 Gy/13 fractions) to the HCC was performed 3 wk after implantation using the Synchrony system by Radixact™.
Four weeks later, CT showed that the viable lesion had disappeared and the PIVKA-Ⅱ levels decreased (Figure 5). There were no complications of grade 3 or higher during the clinical course (Figure 6). No recurrence has been observed at 9 mo after SBRT with GA.
Four weeks later, the viable lesion had disappeared on CT and the PIVKA-Ⅱ levels decreased (Figure 7). In this case, the puncture route for GA implantation passed near the biloma. As a result, the biloma developed complications from infection (Figure 8), which improved through conservative treatment. There were no other complications of grade 3 or higher during the clinical course (Figure 9). Recurrence occurred 7 mo after SBRT with GA; however, without the short-term recurrence seen after TACE, progression-free survival was longer with this treatment.
Regarding early-stage HCC, it is standard to undergo curative treatments, such as surgical resection (partial liver resection or liver transplantation) and percutaneous ablation, most commonly RFA. Regarding intermediate-stage HCC, many patients undergo TACE as their first local regional treatment[5,8]. However, such treatments have limitations in locally advanced cases. For example, TACE is contraindicated in cases with portal vein thrombosis, malignant portal vein thrombosis, and untreatable arteriovenous fistula. RFA is contraindicated in cases with bleeding disorders and can be difficult in tumors near the diaphragm, digestive tract, pancreas, hepatic hilum, and major bile ducts or vessels[10]. Furthermore, Lin et al[11] reported that RFA was less effective for HCCs larger than 4 cm. Patients who do not satisfy the Milan criteria or San Francisco criteria are not commonly indicated for surgical resection[5]. It has also been reported that patients with intermediate- to advanced-stage HCC have a high recurrence rates. Five-year tumor recurrence rates after surgical resection and RFA may be more than 50% and up to 80%, respectively. These high recurrence rates include patients who undergo TACE[8,12]. In our case, one patient had a recurrence after surgery and again after TACE. In the other case, recurrence occurred after TACE. Bearing in mind the aforementioned reasons, as well as the fact that radiotherapy is constantly evolving due to technological innovation, radiotherapy is expected to be the fourth local therapy available for HCC[5,8,10].
Currently, there are useful treatment guidelines for managing HCC from America[13,14], Europe[15], Japan[16], Korea[17], China[18], and Taiwan[19]. There is a variety of evidence on the efficacy of radiotherapy for HCC, but phase III randomized trials are lacking. Therefore, based on the current guidelines, radiation therapy is not mentioned or is listed as having a limited role. However, modern radiotherapy has become increasingly important in the management of patients with HCC due to two changes. First, advances in radiotherapy techniques, for example SBRT and proton therapy, have allowed for more accurate delivery of radiation to enhance tumor control and reduce complications in organ close to the HCC. Second, the development of molecular targeted drugs and checkpoint-blockade immunotherapy has prolonged the overall survival of patients with HCC, reaffirming the importance of local tumor control. In this regard, several clinical trials of SBRT for HCC are underway[8,10]. The reason for choosing radiotherapy in the present cases was that they showed local recurrence and were refractory to surgery, TACE, or chemotherapy; in addition, RFA was difficult because, in the first case, the recurrence was close to the inferior vena cava, whereas in the second case, it was close to the left branch of the portal vein.
SBRT builds on the principle of delivering high doses per fraction using steep dose gradients and smaller margins of uncertainty. A large dose of radiation is typically defined as one over 2 Gy. SBRT delivers extremely precise high doses in a limited number of treatment fractions (usually 3-6 fractions at > 5 Gy per fraction) over a treatment course of 1-2 wk[8]. High doses of radiation in a few fractions allows for a higher proportion of cancer cell death while reducing the chance of tumor DNA repair or repopulation[5]. Kim et al[20] reported that patients in the high-dose group achieved higher relative objective responses. To deliver high doses safely, image guidance and RMM are required. However, it is difficult to track a tumor in the liver during radiotherapy. Therefore, fiducial markers are used to track tumors in the liver. RMM using the Synchrony system with Radixact™ in combination with fiducial markers has higher accuracy and shorter irradiation time than other RMMs. The irradiation area to normal liver tissue is narrow, the treatment time is short, and posture maintenance is easy since it tracks HCC under free breathing. This makes it easier to deal with patients with impaired liver function and older patients who have difficulty maintaining a posture for a long time. SBRT with GA and Radixact™ is advantageous for HCC, which is more dose-dependent than several other organ cancers, and for normal liver cells, which are more radiosensitive than the lung[21,22].
There are no complications specific to Radixact™, but specific complications associated with SBRT and GA should be considered. Commonly used fiducial markers are 0.35-1.1 mm in diameter. Larger markers are relatively easy to identify on CT or MRI. However, larger needle diameters increase the frequency of implantation metastases, bleeding, and the occurrence of serious complications[23,24]. In addition, the risk of bleeding is particularly high in patients with cirrhosis. Major complications after fiducial marker placement include pain, migration of fiducial markers, implantation of metastases, bleeding, and infection. Although the rate of complications is 12%-14%, complications other than pain are rare[25,26]. However, the fiducial marker is a relatively new method for HCC, and the accumulation of data is still limited. Referring to the data of percutaneous image-guided liver biopsy with comparable puncture needle thickness, the rate of implantation metastases was 3%, of bleeding was 2%, and of infection was 0.35%[23,24,27]. In fiducial markers, the tumor itself is not punctured; therefore, the risk of implantation metastases is expected to be lower than that of percutaneous image-guided liver biopsy. In one of our cases, the puncture route was in the vicinity of a biloma complicated by surgical resection which led to infection of the biloma. Although complications of infection due to fiducial marker placement are very rare, care should be taken when there is a biloma on the puncture route. In this case, we believe that infection could have been avoided by changing the puncture route.
The GA used in this study is characterized by its small diameter which can reduce the risk of complications. Another feature is that the GA can be implanted in a zigzag or spherical shape owing to its concavo-convex shape, and pure gold contains 0.5% pure iron, which results in low migration and highly visibility on CT and MRI[9].
Ultrasound-guided placement, CT-guided placement, and endoscopic ultrasound-guided placement have been performed, and each method has the same success and complication rates[25,28,29]. Because of the risk of fiducial marker migration, multiple implantations are recommended. Placing the fiducial markers in close proximity to each other or on the same plane of the CT may cause the irradiator to misidentify them during radiation therapy. Therefore, we performed CT-guided placement to improve the accuracy of GA placement.
One of the advantages of SBRT over conventional radiotherapy is that the total dose to adjacent organs is low owing to the rapid dose fall-off inherent in SBRT. Nonetheless, even with SBRT, potential liver-related toxicities include sequelae associated with radiation-induced liver disease (RILD), including fatigue, abdominal pain, ectopic hepatomegaly, ascites, and elevated liver enzymes, which can usually develop within 4 months of radiotherapy[10]. In addition to these findings, patients may also experience jaundice, thrombocytopenia, and changes in coagulation factors. A review of the prospective literature shows that adverse events of grade 3 or higher are rare, with most studies reporting less than 12%[5]. It should be noted that patients with a Child-Pugh classification of B7 or lower have a lower risk of RILD compared to patients with a Child-Pugh classification of B8 or higher[30]. In our cases, no RILD was observed even 4 mo after SBRT. Another report stated that the concurrent use of SBRT with sorafenib should be avoided because it is likely to cause grade 3 gastrointestinal disorders and tumor rupture[31]. Therefore, lenvatinib, a molecular targeted drug similar to sorafenib, was discontinued during the SBRT treatment period in our cases.
As described above, SBRT with GA and the Synchrony system can be considered a useful fourth local therapy for HCC. However, the irradiation range is limited by the patients’ liver function; in the literature, most patients treated with SBRT have Child-Pugh classification of A and have a few tumors (often < 3 tumors)[32].
SBRT combined with GA and the Synchrony system by Radixact™ is minimally invasive, highly accurate, and can deliver a large dose of radiation to HCC.
In our cases, tumor control was difficult with surgery, TACE, or molecular targeted drugs; however, SBRT combined with GA and the Synchrony system by Radixact™ was highly effective for HCC. The GA used in this report is a new fiducial marker with a small diameter which is expected to reduce the incidence of complications. The Synchrony system by Radixact™ is also a new radiation device that is useful in RMM and allows complete tracking of the HCC in which the GA is implanted.
The method and systems used in this study are considered safe and effective, and we would like to use them in future work for the treatment of HCC in which tumor control by surgical resection, RFA, TACE, and molecular targeted drug administration is difficult. However, this is a case report and further research, such as phase III trials, is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R, Li Y, Sahin TT S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, Abdulle ASM, Abebe ND, Abraha HN, Abu-Raddad LJ, Abualhasan A, Adedeji IA, Advani SM, Afarideh M, Afshari M, Aghaali M, Agius D, Agrawal S, Ahmadi A, Ahmadian E, Ahmadpour E, Ahmed MB, Akbari ME, Akinyemiju T, Al-Aly Z, AlAbdulKader AM, Alahdab F, Alam T, Alamene GM, Alemnew BTT, Alene KA, Alinia C, Alipour V, Aljunid SM, Bakeshei FA, Almadi MAH, Almasi-Hashiani A, Alsharif U, Alsowaidi S, Alvis-Guzman N, Amini E, Amini S, Amoako YA, Anbari Z, Anber NH, Andrei CL, Anjomshoa M, Ansari F, Ansariadi A, Appiah SCY, Arab-Zozani M, Arabloo J, Arefi Z, Aremu O, Areri HA, Artaman A, Asayesh H, Asfaw ET, Ashagre AF, Assadi R, Ataeinia B, Atalay HT, Ataro Z, Atique S, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Awoke N, Ayala Quintanilla BP, Ayanore MA, Ayele HT, Babaee E, Bacha U, Badawi A, Bagherzadeh M, Bagli E, Balakrishnan S, Balouchi A, Bärnighausen TW, Battista RJ, Behzadifar M, Bekele BB, Belay YB, Belayneh YM, Berfield KKS, Berhane A, Bernabe E, Beuran M, Bhakta N, Bhattacharyya K, Biadgo B, Bijani A, Bin Sayeed MS, Birungi C, Bisignano C, Bitew H, Bjørge T, Bleyer A, Bogale KA, Bojia HA, Borzì AM, Bosetti C, Bou-Orm IR, Brenner H, Brewer JD, Briko AN, Briko NI, Bustamante-Teixeira MT, Butt ZA, Carreras G, Carrero JJ, Carvalho F, Castro C, Castro F, Catalá-López F, Cerin E, Chaiah Y, Chanie WF, Chattu VK, Chaturvedi P, Chauhan NS, Chehrazi M, Chiang PP, Chichiabellu TY, Chido-Amajuoyi OG, Chimed-Ochir O, Choi JJ, Christopher DJ, Chu DT, Constantin MM, Costa VM, Crocetti E, Crowe CS, Curado MP, Dahlawi SMA, Damiani G, Darwish AH, Daryani A, das Neves J, Demeke FM, Demis AB, Demissie BW, Demoz GT, Denova-Gutiérrez E, Derakhshani A, Deribe KS, Desai R, Desalegn BB, Desta M, Dey S, Dharmaratne SD, Dhimal M, Diaz D, Dinberu MTT, Djalalinia S, Doku DT, Drake TM, Dubey M, Dubljanin E, Duken EE, Ebrahimi H, Effiong A, Eftekhari A, El Sayed I, Zaki MES, El-Jaafary SI, El-Khatib Z, Elemineh DA, Elkout H, Ellenbogen RG, Elsharkawy A, Emamian MH, Endalew DA, Endries AY, Eshrati B, Fadhil I, Fallah Omrani V, Faramarzi M, Farhangi MA, Farioli A, Farzadfar F, Fentahun N, Fernandes E, Feyissa GT, Filip I, Fischer F, Fisher JL, Force LM, Foroutan M, Freitas M, Fukumoto T, Futran ND, Gallus S, Gankpe FG, Gayesa RT, Gebrehiwot TT, Gebremeskel GG, Gedefaw GA, Gelaw BK, Geta B, Getachew S, Gezae KE, Ghafourifard M, Ghajar A, Ghashghaee A, Gholamian A, Gill PS, Ginindza TTG, Girmay A, Gizaw M, Gomez RS, Gopalani SV, Gorini G, Goulart BNG, Grada A, Ribeiro Guerra M, Guimaraes ALS, Gupta PC, Gupta R, Hadkhale K, Haj-Mirzaian A, Hamadeh RR, Hamidi S, Hanfore LK, Haro JM, Hasankhani M, Hasanzadeh A, Hassen HY, Hay RJ, Hay SI, Henok A, Henry NJ, Herteliu C, Hidru HD, Hoang CL, Hole MK, Hoogar P, Horita N, Hosgood HD, Hosseini M, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hussen MM, Ileanu B, Ilic MD, Innos K, Irvani SSN, Iseh KR, Islam SMS, Islami F, Jafari Balalami N, Jafarinia M, Jahangiry L, Jahani MA, Jahanmehr N, Jakovljevic M, James SL, Javanbakht M, Jayaraman S, Jee SH, Jenabi E, Jha RP, Jonas JB, Jonnagaddala J, Joo T, Jungari SB, Jürisson M, Kabir A, Kamangar F, Karch A, Karimi N, Karimian A, Kasaeian A, Kasahun GG, Kassa B, Kassa TD, Kassaw MW, Kaul A, Keiyoro PN, Kelbore AG, Kerbo AA, Khader YS, Khalilarjmandi M, Khan EA, Khan G, Khang YH, Khatab K, Khater A, Khayamzadeh M, Khazaee-Pool M, Khazaei S, Khoja AT, Khosravi MH, Khubchandani J, Kianipour N, Kim D, Kim YJ, Kisa A, Kisa S, Kissimova-Skarbek K, Komaki H, Koyanagi A, Krohn KJ, Bicer BK, Kugbey N, Kumar V, Kuupiel D, La Vecchia C, Lad DP, Lake EA, Lakew AM, Lal DK, Lami FH, Lan Q, Lasrado S, Lauriola P, Lazarus JV, Leigh J, Leshargie CT, Liao Y, Limenih MA, Listl S, Lopez AD, Lopukhov PD, Lunevicius R, Madadin M, Magdeldin S, El Razek HMA, Majeed A, Maleki A, Malekzadeh R, Manafi A, Manafi N, Manamo WA, Mansourian M, Mansournia MA, Mantovani LG, Maroufizadeh S, Martini SMS, Mashamba-Thompson TP, Massenburg BB, Maswabi MT, Mathur MR, McAlinden C, McKee M, Meheretu HAA, Mehrotra R, Mehta V, Meier T, Melaku YA, Meles GG, Meles HG, Melese A, Melku M, Memiah PTN, Mendoza W, Menezes RG, Merat S, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Mihretie KMM, Miller TR, Mills EJ, Mir SM, Mirzaei H, Mirzaei HR, Mishra R, Moazen B, Mohammad DK, Mohammad KA, Mohammad Y, Darwesh AM, Mohammadbeigi A, Mohammadi H, Mohammadi M, Mohammadian M, Mohammadian-Hafshejani A, Mohammadoo-Khorasani M, Mohammadpourhodki R, Mohammed AS, Mohammed JA, Mohammed S, Mohebi F, Mokdad AH, Monasta L, Moodley Y, Moosazadeh M, Moossavi M, Moradi G, Moradi-Joo M, Moradi-Lakeh M, Moradpour F, Morawska L, Morgado-da-Costa J, Morisaki N, Morrison SD, Mosapour A, Mousavi SM, Muche AA, Muhammed OSS, Musa J, Nabhan AF, Naderi M, Nagarajan AJ, Nagel G, Nahvijou A, Naik G, Najafi F, Naldi L, Nam HS, Nasiri N, Nazari J, Negoi I, Neupane S, Newcomb PA, Nggada HA, Ngunjiri JW, Nguyen CT, Nikniaz L, Ningrum DNA, Nirayo YL, Nixon MR, Nnaji CA, Nojomi M, Nosratnejad S, Shiadeh MN, Obsa MS, Ofori-Asenso R, Ogbo FA, Oh IH, Olagunju AT, Olagunju TO, Oluwasanu MM, Omonisi AE, Onwujekwe OE, Oommen AM, Oren E, Ortega-Altamirano DDV, Ota E, Otstavnov SS, Owolabi MO, P A M, Padubidri JR, Pakhale S, Pakpour AH, Pana A, Park EK, Parsian H, Pashaei T, Patel S, Patil ST, Pennini A, Pereira DM, Piccinelli C, Pillay JD, Pirestani M, Pishgar F, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Prakash S, Prasad N, Qorbani M, Rabiee M, Rabiee N, Radfar A, Rafiei A, Rahim F, Rahimi M, Rahman MA, Rajati F, Rana SM, Raoofi S, Rath GK, Rawaf DL, Rawaf S, Reiner RC, Renzaho AMN, Rezaei N, Rezapour A, Ribeiro AI, Ribeiro D, Ronfani L, Roro EM, Roshandel G, Rostami A, Saad RS, Sabbagh P, Sabour S, Saddik B, Safiri S, Sahebkar A, Salahshoor MR, Salehi F, Salem H, Salem MR, Salimzadeh H, Salomon JA, Samy AM, Sanabria J, Santric Milicevic MM, Sartorius B, Sarveazad A, Sathian B, Satpathy M, Savic M, Sawhney M, Sayyah M, Schneider IJC, Schöttker B, Sekerija M, Sepanlou SG, Sepehrimanesh M, Seyedmousavi S, Shaahmadi F, Shabaninejad H, Shahbaz M, Shaikh MA, Shamshirian A, Shamsizadeh M, Sharafi H, Sharafi Z, Sharif M, Sharifi A, Sharifi H, Sharma R, Sheikh A, Shirkoohi R, Shukla SR, Si S, Siabani S, Silva DAS, Silveira DGA, Singh A, Singh JA, Sisay S, Sitas F, Sobngwi E, Soofi M, Soriano JB, Stathopoulou V, Sufiyan MB, Tabarés-Seisdedos R, Tabuchi T, Takahashi K, Tamtaji OR, Tarawneh MR, Tassew SG, Taymoori P, Tehrani-Banihashemi A, Temsah MH, Temsah O, Tesfay BE, Tesfay FH, Teshale MY, Tessema GA, Thapa S, Tlaye KG, Topor-Madry R, Tovani-Palone MR, Traini E, Tran BX, Tran KB, Tsadik AG, Ullah I, Uthman OA, Vacante M, Vaezi M, Varona Pérez P, Veisani Y, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vosoughi K, Vu GT, Vujcic IS, Wabinga H, Wachamo TM, Wagnew FS, Waheed Y, Weldegebreal F, Weldesamuel GT, Wijeratne T, Wondafrash DZ, Wonde TE, Wondmieneh AB, Workie HM, Yadav R, Yadegar A, Yadollahpour A, Yaseri M, Yazdi-Feyzabadi V, Yeshaneh A, Yimam MA, Yimer EM, Yisma E, Yonemoto N, Younis MZ, Yousefi B, Yousefifard M, Yu C, Zabeh E, Zadnik V, Moghadam TZ, Zaidi Z, Zamani M, Zandian H, Zangeneh A, Zaki L, Zendehdel K, Zenebe ZM, Zewale TA, Ziapour A, Zodpey S, Murray CJL. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1723] [Cited by in RCA: 1750] [Article Influence: 291.7] [Reference Citation Analysis (0)] |
| 2. | Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, Navarria P, Fogliata A, Tomatis S, D'Agostino G, Iftode C, Mancosu P, Ceriani R, Torzilli G. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol. 2015;141:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 3. | Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. 2008;14:3452-3460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 4. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 5. | Bang A, Dawson LA. Radiotherapy for HCC: Ready for prime time? JHEP Rep. 2019;1:131-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Guckenberger M, Andratschke N, Alheit H, Holy R, Moustakis C, Nestle U, Sauer O; Deutschen Gesellschaft für Radioonkologie (DEGRO). Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol. 2014;190:26-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Gerum S, Heinz C, Belka C, Walter F, Paprottka P, De Toni EN, Roeder F. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol. 2018;13:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Chen LC, Lin HY, Hung SK, Chiou WY, Lee MS. Role of modern radiotherapy in managing patients with hepatocellular carcinoma. World J Gastroenterol. 2021;27:2434-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 9. | Tanaka O, Nishigaki Y, Hayashi H, Iida T, Yokoyama T, Takenaka E, Yama E, Tomita E. The advantage of iron-containing fiducial markers placed with a thin needle for radiotherapy of liver cancer in terms of visualization on MRI: an initial experience of Gold Anchor. Radiol Case Rep. 2017;12:416-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Chen CP. Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol. 2019;7:183-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or = 4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 12. | Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, Fan ST, Lo CM. Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 2013;19:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Benson AB, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, Anders R, Are C, Brown D, Chang DT, Cloyd J, Covey AM, Hawkins W, Iyer R, Jacob R, Karachristos A, Kelley RK, Kim R, Palta M, Park JO, Sahai V, Schefter T, Sicklick JK, Singh G, Sohal D, Stein S, Tian GG, Vauthey JN, Venook AP, Hammond LJ, Darlow SD. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw. 2019;17:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (1)] |
| 14. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3238] [Article Influence: 462.6] [Reference Citation Analysis (1)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6052] [Article Influence: 864.6] [Reference Citation Analysis (3)] |
| 16. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 17. | Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019;13:227-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 18. | Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer. 2018;7:235-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 444] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 19. | Surveillance group; Diagnosis group; Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc. 2018;117:381-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, Kim SS, Lee WJ, Kim DY, Kim CM. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol. 2014;190:882-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Pan CC, Kavanagh BD, Dawson LA, Li XA, Das SK, Miften M, Ten Haken RK. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 23. | Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Midia M, Odedra D, Shuster A, Midia R, Muir J. Predictors of bleeding complications following percutaneous image-guided liver biopsy: a scoping review. Diagn Interv Radiol. 2019;25:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 25. | Dutta D, Kataki KJ, George S, Reddy SK, Sashidharan A, Kannan R, Madhavan R, Nair H, Tatineni T, Holla R. Prospective evaluation of fiducial marker placement quality and toxicity in liver CyberKnife stereotactic body radiotherapy. Radiat Oncol J. 2020;38:253-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Park SH, Won HJ, Kim SY, Shin YM, Kim PN, Yoon SM, Park JH, Kim JH. Efficacy and safety of ultrasound-guided implantation of fiducial markers in the liver for stereotactic body radiation therapy. PLoS One. 2017;12:e0179676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Cervini P, Hesley GK, Thompson RL, Sampathkumar P, Knudsen JM. Incidence of infectious complications after an ultrasound-guided intervention. AJR Am J Roentgenol. 2010;195:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Oldrini G, Taste-George H, Renard-Oldrini S, Baumann AS, Marchesi V, Troufléau P, Peiffert D, Didot-Moisei A, Boyer B, Grignon B, Henrot P. Implantation of fiducial markers in the liver for stereotactic body radiation therapy: Feasibility and results. Diagn Interv Imaging. 2015;96:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Choi JH, Seo DW, Park DH, Lee SK, Kim MH. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014;8:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, Kwo P, Cárdenes H. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e443-e449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Brade AM, Ng S, Brierley J, Kim J, Dinniwell R, Ringash J, Wong RR, Cho C, Knox J, Dawson LA. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2016;94:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Culleton S, Jiang H, Haddad CR, Kim J, Brierley J, Brade A, Ringash J, Dawson LA. Outcomes following definitive stereotactic body radiotherapy for patients with Child-Pugh B or C hepatocellular carcinoma. Radiother Oncol. 2014;111:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |