Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2569
Peer-review started: September 1, 2021
First decision: November 7, 2021
Revised: November 21, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 190 Days and 7.9 Hours
Little is known about the safety and efficacy of using two or more biologics for the treatment of immune-mediated diseases, including Crohn’s disease (CD).
This case report and narrative review demonstrate the potential safety of dual biologic therapy (DBT) in a 45-year-old female with two separate immune-mediated diseases. She had a history of multiple sclerosis for which she was receiving treatment with ocrelizumab, and she had been recently diagnosed with CD after presenting with diarrhoea. The CD diagnosis was confirmed radiologically, endoscopically, histologically, and biochemically. The patient received treatment with vedolizumab, a gut-specific inhibitor of the α4β7 integrin on leukocytes. No adverse reactions were observed for the duration of treatment. The safety of ocrelizumab and vedolizumab for the treatment of different immune-mediated diseases was demonstrated.
DBT may be a safe and effective option for the treatment of refractory disease or multiple immune-mediated diseases. Newer biologics, which have improved safety profiles and gut specificity, may provide promising avenues for treatment. However, caution must be exercised in the appropriate selection of biologics given their inherent immunosuppressive properties, side effects, and efficacy profiles. Current evidence suggests that biologic therapy is not associated with a worse prognosis in patients with coronavirus disease 2019, but treatment decisions should be made in a multidisciplinary setting. Further research from controlled trials is needed to better understand the safety profile of DBT in CD. The immunopathological mechanisms underlying DBT also remain to be clarified.
Core Tip: This paper describes the use of two biologics for the treatment of Crohn's disease and multiple sclerosis. Only a few papers have reported the safety and efficacy of these treatments due to inherent concerns regarding immunosuppression, infection, and malignancy. We present the case of a patient who was safely treated with vedolizumab and ocrelizumab. The combination of biologics may be a safe and effective treatment for immune-mediated diseases.
- Citation: Au M, Mitrev N, Leong RW, Kariyawasam V. Dual biologic therapy with ocrelizumab for multiple sclerosis and vedolizumab for Crohn’s disease: A case report and review of literature. World J Clin Cases 2022; 10(8): 2569-2576
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2569.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2569
Biologics play an important role in treating the full spectrum of Crohn’s disease (CD). Biologics are a group of drugs derived from living biological sources that undergo complex processes, such as recombinant DNA processes, genetic isolation, or protein purification[1]. Biologics can be divided into monoclonal antibodies (mAbs), receptor modulators, and enzyme modulators[2]. In CD, biologics are incorporated into the treatment regimen when a response to thiopurines and glucocorticoids cannot be achieved[3]. Biologics used for the treatment of CD include inhibitors of tumour necrosis factor-α (TNF-α) (infliximab, adalimumab, and certolizumab), inhibitors of α4β7 integrins on leukocytes (vedolizumab and natalizumab), and inhibitors of the p40 subunit of interleukins (IL)-12 and IL-23 (ustekinumab)[4]. Patients who respond to biologic therapy show improved clinical outcomes, can avoid surgery, and have a reduced hospitalisation rate, fewer complications, and improved quality of life[5,6].
In the setting of CD, dual biologic therapy (DBT) refers to the use of two different biologic agents to achieve remission. Typically, biologics have been used alone or in combination with other immunomodulators to enhance the therapeutic response and prevent the formation of human anti-chimeric antibodies (HACAs). The use of DBT has been cautioned due to side effects, including immunosuppression, infections, and malignancy[7,8]. Newer biologics such as vedolizumab and ustekinumab offer improved safety profiles that do not cause systemic immunosuppression and lower the risk of HACA formation; no cases of progressive multifocal leukoencephalopathy (PML) from John Cunningham virus (JCV) have been reported; and theoretically, there is a lower risk of malignancy due to the gut-specific activity of these biologics[9,10].
Few studies have explored the safety and efficacy of using DBT for CD, and further details regarding the safety of concurrent use of multiple biologics as indicated for different immune-mediated disorders remains to be explored[11,12]. Whether a cumulative immunosuppressive effect exists with DBT remains to be clarified, resulting in hesitancy in the widespread use of DBT. In a systematic review with a pooled analysis of patients with inflammatory bowel diseases (IBDs) receiving DBT with TNF-α inhibitors, vedolizumab, or ustekinumab, clinical improvement was observed in all patients, with seven out of 18 patients experiencing mild side effects but no serious adverse events[13]. The current evidence is promising, and DBT appears safe, but further controlled trials are required. Furthermore, the coronavirus disease 2019 (COVID-19) pandemic presents new challenges to the clinical setting and raises concerns over the safety of biologic therapy[14]. The following case report demonstrates the safety of DBT in a patient receiving two biologics for separate immune-mediated diseases and provides an update on recent developments from research into the use of DBT for CD.
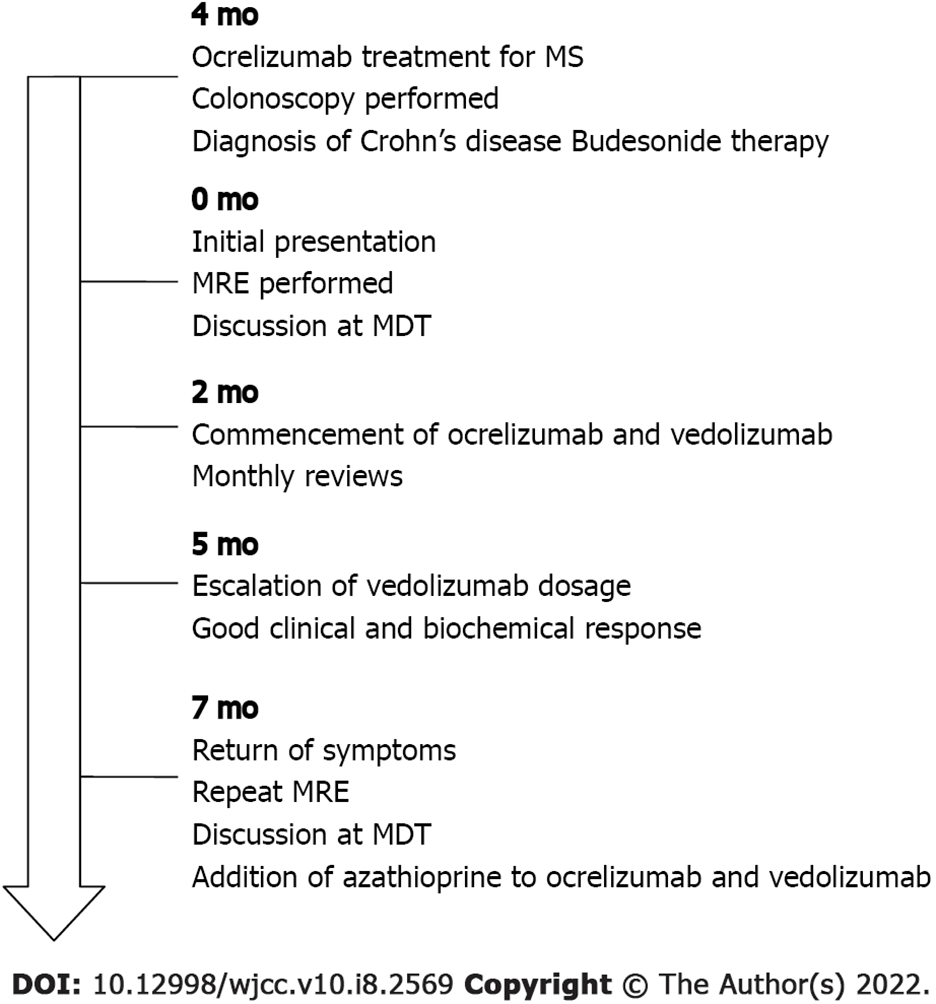
A 45-year-old female presented with diarrhoea associated with ileal CD, which was diagnosed four months prior to presentation, without strictures or fistulising complications.
Colonoscopy demonstrated active ileitis with a Simple Endoscopic Score for Crohn’s disease of 5 (2, 1, 2, 0).
After a brief good clinical response to budesonide therapy and the resolution of diarrhoea, recru
The patient had a history of multiple sclerosis (MS) and had been receiving treatment for the past five years with ocrelizumab, a humanised anti-CD20 B cell depletory drug with similar properties to rituximab, with which it shares a similar epitope. Anti-CD20 therapy has been associated with immune-mediated colitis; however, it is not a recognised cause of ileitis. At the time of review, the patient was not taking any other medication or nonsteroidal anti-inflammatory drugs. The patient had been previously treated with fingolimod and natalizumab for her MS. However, natalizumab was subsequently withdrawn, as she was positive for JCV.
The clinical examination findings were unremarkable.
Her faecal calprotectin was elevated at presentation (> 1000 μg/g). Other pathogenic causes of diarrhoea, such as bacteria, parasites, and viruses, were excluded on the basis of blood test and stool culture results. Histopathological analysis of a terminal ileum biopsy sample demonstrated patchy mild active inflammation.
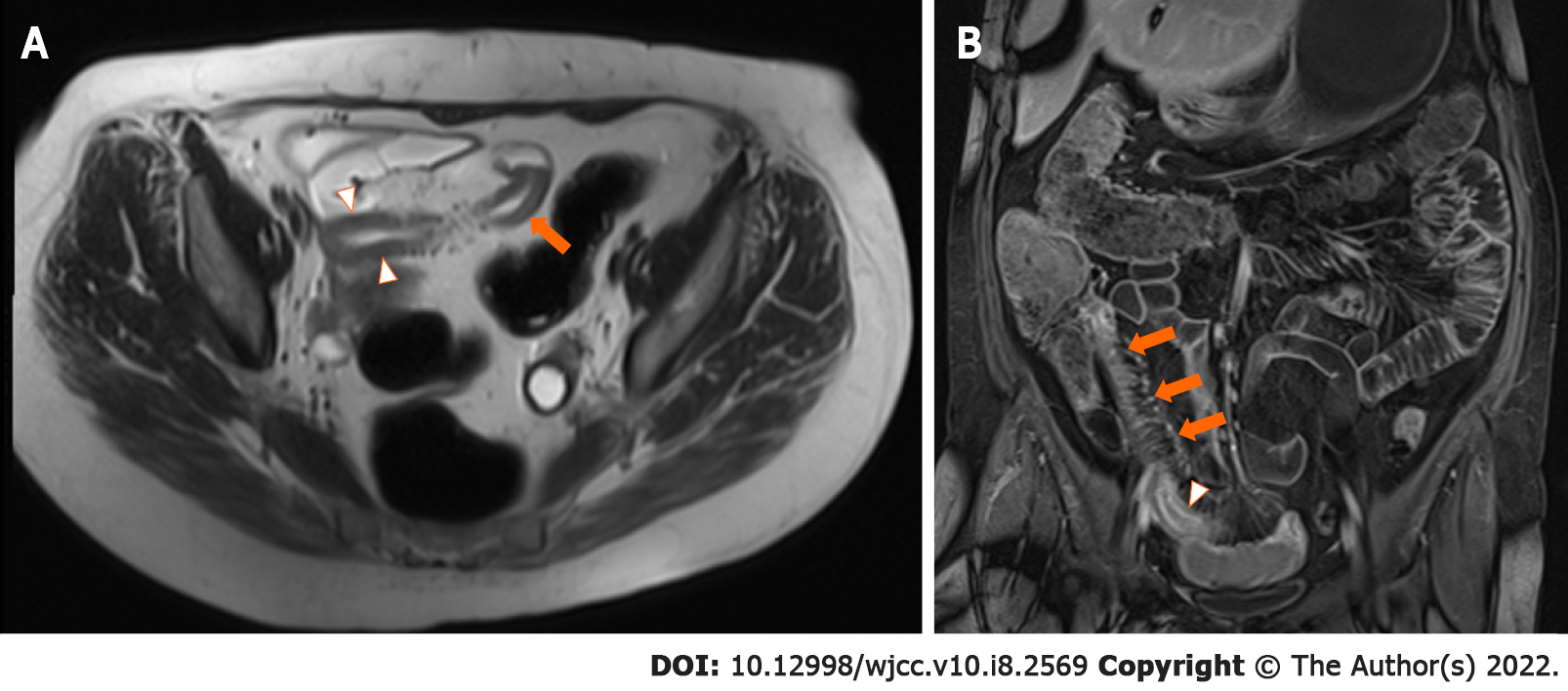
Active ileitis, with the involvement of 30 cm from the ileal-caecal junction and an increased bowel wall thickness of 5 mm without upstream small bowel dilatation, was confirmed on magnetic resonance enterography.
The patient was referred for multidisciplinary care given her complex medical history. She was counselled on the potential need for surgical management. In conjunction with the patient, the decision was made to commence vedolizumab with induction and maintenance therapy.
The patient was diagnosed with CD with active terminal ileitis, and she safely commenced DBT given her concurrent MS.
The patient received treatment with vedolizumab in conjunction with her usual treatment with ocrelizumab for her MS. Because the biochemical response after the first three months was inadequate, the dose of vedolizumab was escalated to 300 mg every 4 wk, which resulted in a good clinical and biochemical response. The patient’s diarrhoea and abdominal pain resolved, her C-reactive protein (CRP) decreased from 47 mg/L prior to dose escalation to 28 mg/L following dose escalation, and her erythrocyte sedimentation rate decreased from 17 mm/h pre-treatment to 5 mm/h post-treatment. During maintenance therapy, the patient completed monthly follow-ups, and she did not develop any adverse reactions.
Unfortunately, after 5 mo of therapy, her diarrhoea returned, despite achieving therapeutic levels of vedolizumab at 33 μg/mL. Repeat magnetic resonance enterography demonstrated terminal ileitis with some worsening interval changes, including a bowel wall thickness increase to 7 mm in the terminal ileum but no upstream dilation or complications (Figure 1). The Simplified Modified Magnetic Resonance Index of Activity (MaRIA) score of the terminal ileum was 3, suggesting severe disease. Repeat CRP analysis showed an increase to 52 mg/L. Following multidisciplinary discussion, azathioprine was added to her DBT, as the patient wished to avoid surgery. To date, the patient has safely completed five months of DBT without any adverse side effects noted (Figure 2).
Significant gaps still exist in the understanding of the use of multiple biologic therapies in patients with CD. This case report demonstrated the short-term safety of the use of two biologics, ocrelizumab and vedolizumab, in a patient with different immune-mediated conditions. Only a few studies have attempted to elucidate the safety and efficacy of DBT for refractory CD, but some of the results were promising. In a retrospective study by Yang et al[15], 22 patients who had CD refractory to a single biologic underwent 24 trials of DBT (consisting of either infliximab, adalimumab, vedolizumab, ustekinumab, certolizumab, or golimumab), with 50% achieving a clinical response and 41% achieving clinical remission. Adverse events occurred in three trials due to infection, malignancy, or drug-induced lupus[15]. Additionally, Kwapisz et al[16] described 14 patients with CD and one patient with ulcerative colitis (UC) treated with combination biologics for refractory disease. Eleven patients had symptomatic improvement, with 10 patients achieving a reduction in their corticosteroid dose requirements and only three patients requiring surgery. Adverse events in this case series included three hospitalisations and four infections treated with antibiotics[16]. In the only randomised controlled trial investigating DBT in CD, Sands et al[17] investigated 79 patients with active CD despite infliximab treatment in a multicentre double-blind, placebo-controlled trial. A similar overall incidence of adverse events between patients who were administered natalizumab and infliximab and those who were administered placebo with infliximab was observed, and positive trends towards greater efficacy were seen in the patients who received natalizumab and infliximab[17]. Further evidence is needed to investigate the safety and efficacy of DBT in controlled settings. A current trial is underway to examine the use of DBT (vedolizumab and adalimumab) with methotrexate for patients with newly diagnosed CD who are at higher risk for complications[18]. Additional studies are needed to gain further insight into the immunopathological mechanisms underlying DBT and to clarify whether an additive or synergistic effect occurs.
In this case report, DBT was used for the treatment of two different immune-mediated disease entities. Limited evidence is available on the use of multiple biologics for different immune-mediated disease entities, with most studies examining the role of DBT in refractory disease. To the best of our knowledge, only one other case series, by Fumery et al[19], reported the use of DBT with ocrelizumab and vedolizumab, which was administered to one patient. No adverse events were observed for a period of 6 mo in this patient, who had been diagnosed with UC and MS[19].
One differential diagnosis that needs to be made for the patient presented here is ocrelizumab-induced colitis, which has been reported in case reports[20]. The presentation of ocrelizumab-induced colitis may be similar to that of new-onset CD[21]. Rituximab, another CD20-depleting drug, has also been reported to be possibly associated with colitis[22]. This condition cannot be excluded for the patient in this case report. However, disease activity restriction to the terminal ileum, as well as the prolonged duration of ocrelizumab use in this patient prior to the onset of diarrhoea, favoured the diagnosis of CD rather than medication-induced colitis. Consideration should be given to medication-induced colitis in patients receiving biologics who have symptoms of colitis, although the differentiation of medication-induced colitis from IBD can be difficult[23].
In the era of the COVID-19 pandemic, the safety of DBT needs to be considered, and COVID-19 concerns were a part of this patient’s pre-immunosuppression workup. In one case report, a patient receiving DBT with adalimumab and ustekinumab for CD had a positive severe acute respiratory system coronavirus 2 (SARS-CoV-2) result, but the patient was asymptomatic and the infection did not affect the course of treatment, with the patient safely continuing DBT[14]. To date, there is no evidence to suggest a worse prognosis in patients with SARS-CoV-2 infection receiving biologics[24]. The SECURE-IBD registry demonstrated that TNF-α antagonist treatment was not associated with severe COVID-19[25]. However, further evidence is required to determine whether DBT confers increased immunosuppression and increased severity of SARS-CoV-2 infection.
The choice of biologic agent in this case report was made in a multidisciplinary setting. Consideration was given to natalizumab, but the risk of PML from JCV reactivation has been reported[26]. Furthermore, TNF-α inhibitors are contraindicated for MS due to case reports of demyelination[27]. The gut-specific integrin inhibitor vedolizumab has been given preference, and in this case scenario, vedolizumab initially led to a favourable response. In the treatment of IBD, combination therapy incorporating vedolizumab is theoretically a safe approach given its specificity[9]. The specificity and safety of vedolizumab provide new opportunities for its use in DBT, particularly with other systemic immunosuppressants such as ocrelizumab, which can cause profound B cell depletion.
However, in patients with extraintestinal manifestations, gut-specific vedolizumab may not be as effective as TNF-α inhibitors. In fact, in a case report by Hirten et al[28], a patient who had a brief overlap in infliximab and vedolizumab treatment experienced a flare of an extraintestinal manifestation of CD when infliximab was withdrawn and a flare of mucosal symptoms when vedolizumab was withdrawn, with improvements in symptoms when biologics were restarted in both instances[28]. Another case report of a patient with UC and spondylarthritis described the successful control of both diseases with vedolizumab and etanercept[29]. Privitera et al[30] also retrospectively examined sixteen patients receiving dual biologics or one biologic and one small molecule, either for refractory IBD or for IBD with extraintestinal manifestations. A clinical response was reported by all patients, with only three patients experiencing an adverse event, which included a perianal abscess, a cutaneous reaction, and drug-induced liver injury[30]. Indeed, if the indications for each combination therapeutic are carefully considered, dual ‘targeted’ therapies may be an avenue of treatment in certain patients. Considering the current evidence, DBT can be considered safe in some limited circumstances. Careful selection of DBT, discussion with a multidisciplinary team, and an understanding of the patient’s history and the emerging literature are all needed prior to the commencement of treatment.
Several limitations of this case report should be noted. Although the short-term safety of DBT has been demonstrated, the efficacy of DBT remains to be clarified. Vedolizumab is a promising and excellent therapeutic choice for many patients, as demonstrated in the GEMINI studies[31]. Further research, particularly in the form of randomised controlled trials examining the efficacy of DBT with vedolizumab, is needed.
DBT offers a new treatment strategy to patients with CD and those with different immune-mediated conditions. In addition, the combination of biologics may be an avenue of treatment for patients who have been refractory to existing treatment regimens or those with multiple immune-mediated diseases. Vedolizumab and ocrelizumab in combination may be safe for the treatment of patients with different immune-mediated diseases, such as CD and MS. However, careful selection of biologics with respect to patient characteristics in multidisciplinary settings is required. Further evidence is needed to guide clinical decision-making and the selection of biologics, particularly in the form of randomised controlled trials.
Images courtesy of Dr. Jessica Yang, Radiologist, Macquarie University Hospital and Concord Repatriation General Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen LJ, Liu C S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Chan JCN, Chan ATC. Biologics and biosimilars: what, why and how? ESMO Open. 2017;2: e000180. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Kinch MS. An overview of FDA-approved biologics medicines. Drug Discov Today. 2015;20:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63:629-642. [RCA] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 264] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 4. | Paramsothy S, Rosenstein AK, Mehandru S, Colombel JF. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal Immunol. 2018;11:1558-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Vogelaar L, Spijker AV, van der Woude CJ. The impact of biologics on health-related quality of life in patients with inflammatory bowel disease. Clin Exp Gastroenterol. 2009;2:101-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Feagan BG, Panaccione R, Sandborn WJ, D'Haens GR, Schreiber S, Rutgeerts PJ, Loftus EV Jr, Lomax KG, Yu AP, Wu EQ, Chao J, Mulani P. Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn's disease: results from the CHARM study. Gastroenterology. 2008;135:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2374] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 8. | Lemaitre M, Kirchgesner J, Rudnichi A, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Association Between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA. 2017;318:1679-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 445] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 9. | Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Engel T, Yung DE, Ma C, Pariente B, WIls P, Eliakim R, Ungar B, Ben-Horin S, Kopylov U. Effectiveness and safety of Ustekinumab for Crohn's disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis. 2019;51:1232-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Mao EJ, Lewin S, Terdiman JP, Beck K. Safety of dual biological therapy in Crohn's disease: a case series of vedolizumab in combination with other biologics. BMJ Open Gastroenterol. 2018;5:e000243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Biscaglia G, Piazzolla M, Cocomazzi F, Melchionda G, De Cata A, Bossa F, Palmieri O, Andriulli A. Landmarks for dual biological therapy in inflammatory bowel disease: lesson from two case reports of vedolizumab in combination with ustekinumab. Eur J Gastroenterol Hepatol. 2020;32:1579-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Ribaldone DG, Pellicano R, Vernero M, Caviglia GP, Saracco GM, Morino M, Astegiano M. Dual biological therapy with anti-TNF, vedolizumab or ustekinumab in inflammatory bowel disease: a systematic review with pool analysis. Scand J Gastroenterol. 2019;54:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 14. | Dimopoulos C, Al-Bawardy B. SARS-CoV-2 Infection and Dual-Biologic Therapy for Crohn's Disease. Inflamm Bowel Dis. 2020;26:e153-e154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Yang E, Panaccione N, Whitmire N, Dulai PS, Vande Casteele N, Singh S, Boland BS, Collins A, Sandborn WJ, Panaccione R, Battat R. Efficacy and safety of simultaneous treatment with two biologic medications in refractory Crohn's disease. Aliment Pharmacol Ther. 2020;51:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 16. | Kwapisz L, Raffals LE, Bruining DH, Pardi DS, Tremaine WJ, Kane SV, Papadakis KA, Coelho-Prabhu N, Kisiel JB, Heron V, Faubion WA, Loftus EV Jr. Combination Biologic Therapy in Inflammatory Bowel Disease: Experience From a Tertiary Care Center. Clin Gastroenterol Hepatol. 2021;19:616-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Sands BE, Kozarek R, Spainhour J, Barish CF, Becker S, Goldberg L, Katz S, Goldblum R, Harrigan R, Hilton D, Hanauer SB. Safety and tolerability of concurrent natalizumab treatment for patients with Crohn's disease not in remission while receiving infliximab. Inflamm Bowel Dis. 2007;13:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 18. | NIH. Triple Combination Therapy in High Risk Crohn's Disease (CD). [cited 10 August 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT02764762. |
| 19. | Fumery M, Yzet C, Brazier F. Letter: combination of biologics in inflammatory bowel diseases. Aliment Pharmacol Ther. 2020;52:566-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Sunjaya DB, Taborda C, Obeng R, Dhere T. First Case of Refractory Colitis Caused by Ocrelizumab. Inflamm Bowel Dis. 2020;26:e49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Barnes A, Hofmann D, Hall LA, Klebe S, Mountifield R. Ocrelizumab-induced inflammatory bowel disease-like illness characterized by esophagitis and colitis. Ann Gastroenterol. 2021;34:447-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Eckmann JD, Chedid V, Quinn KP, Bonthu N, Nehra V, Raffals LE. De Novo Colitis Associated With Rituximab in 21 Patients at a Tertiary Center. Clin Gastroenterol Hepatol. 2020;18:252-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Freeman HJ. Colitis associated with biological agents. World J Gastroenterol. 2012;18:1871-1874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Macaluso FS, Orlando A. COVID-19 in patients with inflammatory bowel disease: A systematic review of clinical data. Dig Liver Dis. 2020;52:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Ruemmele FM, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481-491.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 580] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 26. | Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol. 2017;16:925-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 27. | Kemanetzoglou E, Andreadou E. CNS Demyelination with TNF-α Blockers. Curr Neurol Neurosci Rep. 2017;17:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 28. | Hirten R, Longman RS, Bosworth BP, Steinlauf A, Scherl E. Vedolizumab and Infliximab Combination Therapy in the Treatment of Crohn's Disease. Am J Gastroenterol. 2015;110:1737-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Bethge J, Meffert S, Ellrichmann M, Conrad C, Nikolaus S, Schreiber S. Combination therapy with vedolizumab and etanercept in a patient with pouchitis and spondylarthritis. BMJ Open Gastroenterol. 2017;4:e000127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Privitera G, Onali S, Pugliese D, Renna S, Savarino E, Viola A, Ribaldone DG, Buda A, Bezzio C, Fiorino G, Fantini MC, Scaldaferri F, Guidi L, Danese S, Gasbarrini A, Orlando A, Armuzzi A. Dual Targeted Therapy: a possible option for the management of refractory Inflammatory Bowel Disease. J Crohns Colitis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1567] [Article Influence: 130.6] [Reference Citation Analysis (1)] |