Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2537
Peer-review started: August 15, 2021
First decision: October 20, 2021
Revised: October 30, 2021
Accepted: January 27, 2022
Article in press: January 27, 2022
Published online: March 16, 2022
Processing time: 207 Days and 22.4 Hours
The drug instructions for dabigatran recommend adjusting the dosage to 110 mg twice daily for patients with bleeding risk, and performing at least one renal function test per year for patients with moderate renal impairment. However, owing to chronic insidiously worsening renal insufficiency, dabigatran can still accumulate abnormally, necessitating therapy with idarucizumab to reverse the anticoagulation due to severe erosive gastritis with widespread stomach mucosal bleeding.
A 76-year-old woman with a history of atrial fibrillation who took dabigatran 110 mg twice daily as directed to lessen the chance of stroke, was transported to the hospital with hematemesis and melena. Laboratory findings revealed severe life-threatening, blood-loss-induced anemia with a hemoglobin (Hb) level of 41.0 g/L and marked coagulation abnormalities with thrombin time (TT) > 180 s, most likely caused by dabigatran-induced metabolic disorder. Aggressive acid sup
Renal function, coagulation function, and dabigatran concentration should be regularly monitored in older patients. Proton pump inhibitor and dabigatran coadministration is still controversial in preventing upper gastrointestinal tract bleeding.
Core Tip: The anticoagulatory effect of dabigatran resolves completely after five half-lives, which is approximately 2.5-3.5 d after the last dose for patients with normal renal function. Thrombin time (TT) is sensitive to the effects of dabigatran and can be prolonged even with trivial amounts of the drug. This patient exhibited persistent bleeding in the normal coagulation test (except for TT), possibly due to the anticoagulatory effects of the drug administered 4 days after the last dose for her renal insufficiency. Therefore, idarucizumab was administered for hemostasis, thus stopping the bleeding. This case highlights the importance of regular monitoring of renal function in older patients.
- Citation: Jia Y, Wang SH, Cui NJ, Liu QX, Wang W, Li X, Gu YM, Zhu Y. Idarucizumab reverses dabigatran-induced anticoagulation in treatment of gastric bleeding: A case report. World J Clin Cases 2022; 10(8): 2537-2542
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2537.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2537
Dabigatran is an oral direct-acting thrombin inhibitor that was initially approved by the US Food and Drug Administration for the prevention of stroke and systemic embolism caused by nonvalvular atrial fibrillation (AF)[1,2]. It is considered safer and more effective than warfarin and does not require regular coagulation monitoring or dose adjustment, except for those with renal insufficiency (RI), advanced age, and low body weight[3].
However, even long-term dose-adjusted dabigatran therapy in older patients may also increase the risk of major bleeding such as the gastrointestinal (GI) hemorrhage described in this report or cerebral hemorrhage. Idarucizumab was introduced as a dabigatran antidote in December 2015, and its safety and efficacy have been proven in various studies[4]; however, clinical data are still limited, especially in Asians. Here, we report a case of an older Asian woman whose coagulation function was timely and successfully restored by idarucizumab to rescue her from this life-threatening GI bleeding.
On January 26, 2021, a 76-year-old Asian woman was admitted to our hospital with hematemesis and melena, which she had never experienced before and began the previous day.
Four days prior to this reported incident, the patient experienced upper abdominal discomfort and appetite loss without any recognizable precipitating factors.
The patient had a history of AF since 2019, and had been taking dabigatran (110 mg twice daily) to reduce her stroke risk. She had stopped taking dabigatran for at least 4 d before presenting to the hospital. In addition, she had a history of hypertension and coronary atherosclerotic heart disease for > 20 years, type 2 diabetes for > 5 years, and chronic RI (creatinine clearance 30-50 mL/min per 1.73 m2) for 1 year. The present event occurred > 12 years after she underwent surgery for bladder cancer and 7 years after thyroid nodule surgery.
The patient had no other disease history and relevant family disease history.
On arrival at the ward, the temperature, heart rate, respiratory rate, and blood pressure of the patient were 36.3 °C, 90 bpm, 18 breaths/min, and 105/80 mmHg, respectively. Her palpebral conjunctiva and complexion were pale, abdomen was soft, and middle and upper abdomen showed slight tenderness. In addition, the bowel sounds of the patient were 6/min.
The routine blood tests of the patient showed a white blood cell count of 6890/μL and hemoglobin (Hb) level of 41 g/dL. The coagulation function test showed the following results: thrombin time (TT) > 180 s; activated partial thromboplastin time, 36.2 s; and international normalized ratio (INR), 1.20. The biochemical parameters of the patient were as follows: albumin, 34.6 g/L; blood urea nitrogen, 26.96 mmol/L; and serum creatinine, 251.0 μmol/L (Table 1). The tumor markers -fetoprotein, carcinoembryonic antigen, cancer antigen (CA)199, and CA125 were all within the normal range. The 13C urea breath test for detection of Helicobacter pylori (H. pylori) was negative.
| Hospital day | Hb (g/L) | TT (s) | PT (s) | APTT (s) | INR | SCr (μmol/L) |
| Day 1 | 41 | > 180 | 13.7 | 36.2 | 1.20 | 251 |
| Day 2 | 67 | N/A | N/A | N/A | N/A | N/A |
| Day 3 | 44 | 121.20 | 14.2 | 36.3 | 1.25 | 229 |
| Day 4 | 56 | 17.40 | 13.1 | 25.9 | 1.15 | 213 |
| Day 5 | 57 | 18 | 12.6 | 26.0 | 1.10 | 202 |
| Day 6 | 76 | N/A | N/A | N/A | N/A | 182 |
| Day 8 | 78 | 20.90 | 12.0 | 28.0 | 1.04 | N/A |
| Day 10 | 85 | 17.70 | 12.7 | 29.6 | 1.11 | N/A |
| Day 14 | 104 | N/A | N/A | N/A | N/A | 216 |
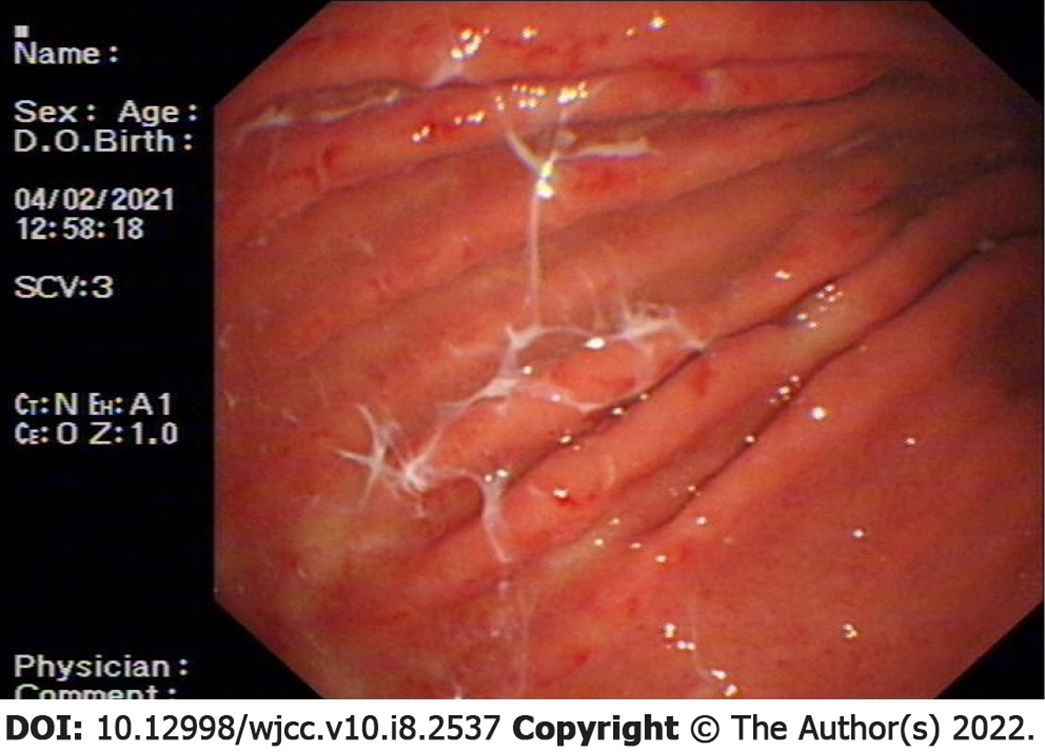
Computed tomography of the entire abdomen showed no obvious abnormalities and electrocardiography showed normal sinus rhythm and abnormal ST-T changes. The electronic gastroscopy showed acute erosive gastritis with extensive gastric mucosal bleeding (Figure 1).
Acute erosive gastritis with extensive gastric mucosal bleeding was diagnosed using an electronic gastroscope.
The patient was administered 2 U 400 mL packed red blood cells (PRBCs), a proton-pump inhibitor (PPI), and octreotide intravenously. On day 2, Hb level increased to 67 g/L and the chief complaints were nausea and retching, which appeared to be well controlled; the remaining concern was abnormal coagulation. On the next day, the patient defecated approximately 400 mL black stools with an Hb level, TT, PT and INR of 44 g/L, 121.20 s, 14.2 s, and 1.25, respectively and was immediately administered 2 U PRBCs.
Single doses of idarucizumab (2.5 g) were administered twice via intravenous infusion to reverse the effect of dabigatran, and the related commonly encountered adverse reactions such as fever, headache, hypokalemia, and delirium were not observed. Twelve hours later, the TT of the patient was 17.4 s, which was within the normal range. On day 4, she was administered an additional 2 U PRBCs for the third time, without symptoms of hematemesis and melena on the following days.
The patient had no recurrence of AF during hospitalization and her routine stool and occult blood test results were normal. Finally, she was discharged on hospitalization day 14, with Hb level of 104 g/L and TT of 17.7 s.
In this study, we presented the case of an older Asian woman whose coagulation function was effectively restored using idarucizumab to reverse the life-threatening GI bleeding experienced following administration of dabigatran. The prodrug of dabigatran, dabigatran etexilate, is rapidly converted to its active form following oral administration. It is an oral non-vitamin K antagonist anticoagulant that acts as a direct reversible and competitive inhibitor of both free and platelet-bound thrombin, thereby affecting the final step of blood clotting[5]. Because of properties such as a short half-life, rapid onset of action, fewer effects on food and drugs, and no INR monitoring requirement[6], dabigatran is deemed a safer and more effective medicine for preventing stroke than some other available agents.
Nevertheless, the elimination of dabigatran is highly dependent on the kidney, through which approximately 85% of plasma dabigatran is excreted, and the process can be prolonged with RI[7]. The RE-LY study demonstrated that dabigatran could reduce all-cause mortality and intracranial hemorrhage, but increased GI bleeding compared with warfarin. The risk of dabigatran-related GI bleeding seems to be evenly distributed between the upper and lower canals (53% vs 47%), whereas warfarin-related upper canal bleeding dominated (75% vs 25%).
The mechanism by which bleeding is induced remains unclear. One possible theory suggests that the local metabolism of dabigatran etexilate increases the concentration of active dabigatran during transit through the GI tract8,9. Dabigatran-induced GI hemorrhage is also related to age and primarily occurs in patients aged ≥ 75 years.[10]
H. pylori infection, liver cirrhosis, malignant tumors, genetic factors, history of major bleeding, peptic ulcers, and GI injury such as diverticulosis and intestinal vascular dysplasia can also increase the risk of bleeding11,12. A study showed that coadministration of a PPI and dabigatran not only markedly reduced the risk of upper GI hemorrhage, but also the dabigatran plasma levels in patients with AF[13].
In this case report, the patient was a 76-year-old Asian woman with a history of AF and concealed progressive RI. She had undergone long-term dabigatran therapy with dose adjustments for 1 year, regular blood coagulation function monitoring, and oral administration of a PPI. The massive hemorrhage from the gastric mucosa was likely induced by prolonged dabigatran excretion because of RI.
Idarucizumab is a humanized monoclonal antibody that specifically and efficiently inhibits the biological activity of dabigatran etexilate. After antibody-antigen binding, it irreversibly neutralizes the anticoagulant effect. The binding affinity of idarucizumab to dabigatran is 350 times higher than that of dabigatran to thrombin, and the reversal effect shows rapid onset and lasts 12 h, which is suitable for life-threatening bleeding, uncontrolled hemorrhage, or emergency surgery in patients administered dabigatran[14,15]. A single dose of 5 g idarucizumab is reported to be sufficient to reverse the effect of dabigatran etexilate in 98% of patients, and the effect is maintained in most patients for 24 h[16].
Considering the extensive gastric mucosal bleeding experienced by this patient, endoscopic hemostasis was less efficient. The conventional therapeutic regimen of acid suppression, hemostasis, and blood transfusion did not achieve hemostasis in this patient and idarucizumab was administered to reverse the effect of dabigatran to rescue her from the second episode of life-threatening bleeding. Subsequently, the patient, whose coagulation function was normalized during hospitalization, was relieved of the symptoms of hematemesis and melena, and her Hb level increased to 104 g/L on day 14. Finally, the patient was discharged in stable conditions.
This study had the following limitations and shortcomings that are worth mentioning. (1) The serum level of dabigatran was not measured because of restricted laboratory conditions; (2) Colonoscopy was not performed because we could not obtain informed consent from the patient; and (3) We were unable to detect any possible intracardiac thrombus caused by AF because the transesophageal echocardiography technique was unavailable.
We report a case of safe and successful reversal of dabigatran-induced abnormal coagulation function by idarucizumab. In addition, we provide evidence to support recommendations for regular renal and coagulation function tests and dabigatran concentration monitoring for older patients where clinical conditions permit. This is to ensure that proper dose adjustments of dabigatran are instituted or the drug discontinuation is timely if unpredictable blood loss occurs. As mentioned in the discussion regarding dabigatran-induced GI-bleeding-related factors, especially H. pylori infection, there is currently no consensus on the benefits of coadministration of PPIs with dabigatran, which warrants further investigation.
We would like to thank the attending physician Zhu Y and deputy chief physician Wang SH for their strong support for the publication of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Geriatrics and gerontology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trifan A S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 2. | Greig SL, McKeage K. Dabigatran etexilate: a review of its use in the treatment of acute venous thromboembolism and prevention of venous thromboembolism recurrence. Drugs. 2014;74:1785-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Cardiology Branch of Chinese Medical Association; Electrophysiology and Pacing Branch of Chinese Medical Association; Professional Committee of Cardiology of Chinese Medical Association. Application of new oral anticoagulants in patients with non-valvular atrial fibrillation-consensus and recommendations from Chinese experts. Chin J Cardiac Arryth 2014; 18: 321-329. [DOI] [Full Text] |
| 4. | Das A, Liu D. Novel antidotes for target specific oral anticoagulants. Exp Hematol Oncol. 2015;4:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 6. | Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, Xavier D, Kim SS, Omar R, Dans AL, Tan RS, Chen JH, Tanomsup S, Watanabe M, Koyanagi M, Ezekowitz MD, Reilly PA, Wallentin L, Yusuf S; RE-LY Investigators. Dabigatran vs warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. 2013;44:1891-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 7. | Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 549] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 8. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3181] [Cited by in RCA: 3656] [Article Influence: 332.4] [Reference Citation Analysis (0)] |
| 9. | Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, Diener HC, Franzosi MG, Huber K, Reilly P, Varrone J, Yusuf S. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 810] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 10. | Romanelli RJ, Nolting L, Dolginsky M, Kym E, Orrico KB. Dabigatran Versus Warfarin for Atrial Fibrillation in Real-World Clinical Practice: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2016;9:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1351] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 12. | Cheung KS, Leung WK. Gastrointestinal bleeding in patients on novel oral anticoagulants: Risk, prevention and management. World J Gastroenterol. 2017;23:1954-1963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (4)] |
| 13. | Bolek T, Samoš M, Škorňová I, Galajda P, Staško J, Kubisz P, Mokáň M. Proton Pump Inhibitors and Dabigatran Therapy: Impact on Gastric Bleeding and Dabigatran Plasma Levels. Semin Thromb Hemost. 2019;45:846-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, Nar H, Litzenburger T. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554-3562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 435] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 15. | Pollack CV Jr, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1093] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 16. | Pollack CV Jr, Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kam CW, Kamphuisen PW, Kreuzer J, Levy JH, Royle G, Sellke FW, Stangier J, Steiner T, Verhamme P, Wang B, Young L, Weitz JI. Idarucizumab for Dabigatran Reversal - Full Cohort Analysis. N Engl J Med. 2017;377:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 731] [Article Influence: 91.4] [Reference Citation Analysis (0)] |