Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2529
Peer-review started: August 26, 2021
First decision: October 29, 2021
Revised: November 12, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 196 Days and 19.8 Hours
Due to the rarity of mesenchymal-epithelial transition factor (MET) fusions, the clinical efficacy of crizotinib has only been described in a few patients with MET fusions involving various fusion partners. Herein, we report the clinical response to crizotinib of a patient with advanced poorly differentiated non-small cell carcinoma (NSCLC) having concurrent MET fusions.
A 46-year-old woman was diagnosed with poorly differentiated NSCLC (T4N3M1). With no classic driver mutations, she was treated with two cycles of gemcitabine and cisplatin without clinical benefit. Targeted sequencing revealed the detection of two concurrent MET fusions, KIF5B-MET and novel MET-CDR2. Crizotinib was initiated at a dose of 250 mg twice daily. Within 4 wk of crizotinib therapy, repeat computed chromatography revealed a dramatic reduction in primary and metastatic lesions, assessed as partial response. She continued to benefit from crizotinib for 3 mo until disease progression and died within 1 mo despite receiving nivolumab therapy.
Crizotinib sensitivity was observed in an advanced poorly differentiated NSCLC patient with concurrent MET fusions KIF5B-MET and MET-CDR2. Crizotinib can serve as a therapeutic option for patients with MET fusions. In addition, our case also highlights the importance of comprehensive genomic profiling particularly in patients with no classic driver mutation for guiding alternative therapeutic decisions.
Core Tip: The most common mesenchymal-epithelial transition factor (MET) gene aberrations are gene amplifications and exon 14 splice variants found in approximately 2% to 10% of lung cancer patients. Chromosomal rearrangements resulting in gene fusions involving MET are generally rare but could account for MET-driven oncogenesis. The rarity and diversity of MET fusions in non-small cell lung cancer (NSCLC) limit the volume of evidence documenting the clinical efficacy of crizotinib in treating MET-rearranged NSCLC patients. Herein, we report the clinical response to crizotinib of a patient with advanced poorly differentiated NSCLC harboring concurrent MET-involving rearrangements, including a novel MET-CDR2 gene fusion.
- Citation: Liu LF, Deng JY, Lizaso A, Lin J, Sun S. Effective response to crizotinib of concurrent KIF5B-MET and MET-CDR2-rearranged non-small cell lung cancer: A case report. World J Clin Cases 2022; 10(8): 2529-2536
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2529.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2529
The mesenchymal-epithelial transition (MET) gene, located on chromosome 7q21-31, encodes a receptor tyrosine kinase and is activated by its ligand, hepatocyte growth factor[1,2]. The MET signaling pathway is often upregulated in various human malignancies, including non-small cell lung cancer (NSCLC)[2]. The most common MET gene aberrations are gene amplifications and exon 14 splice variants found de novo in approximately 2% to 10% of lung cancer patients[3]. Chromosomal rearrangements resulting in gene fusions involving MET are generally rare but could account for MET-driven oncogenesis[4]. Currently, a total of five MET fusion partner genes have been reported in NSCLC, including KIF5B[5,6], STARD3NL[5], HLA-DRB1[7,8], UBE2H[9], and ATXN7L1[10] (Table 1). Crizotinib, an FDA-approved tyrosine kinase inhibitor for ALK-rearranged and ROS1-rearranged NSCLC, has been originally designed to target MET amplifications and mutations[11]. Several cases and clinical studies have reported the efficacy of crizotinib and cabozantinib in targeting MET amplification[12,13], exon 14 skipping[14], and certain rearrangements[5-7,10] in NSCLC patients. A recent meta-analysis analyzed six clinical trials (cohort size range: 8-69) on MET-altered NSCLC revealed an objective response rate of 40.6% (95%CI: 28.3%–53.0%) and disease control rate of 78.9% (95%CI: 70.3%–87.4%) for crizotinib, with a median progression-free survival and overall survival of 5.2 and 12.7 mo, respectively[15]. Most of these studies enrolled few MET fusion-positive patients, because they are exceedingly rare. Current knowledge regarding MET fusions is mostly derived from two cohort studies in Chinese lung cancer patients, which identified one (0.04%, 1/2410) fusion[16] and fifteen (0.26%, 15/5695) fusions involving the MET kinase domain[17], respectively.
| Ref. | Age | Sex | Smoker | Stage | Histology | MET fusion | Best overall response | PFS (mo) | Grade ≥ 3 AEs | Notes |
| [5] | 33 | F | Yes | IV | ADC | KIF5B-MET | PR | 8 | NR | |
| [5] | 62 | F | No | IV | ADC | STARD3NL-MET | PR | 14 | NR | |
| [6] | 51 | F | No | IV | ADC | KIF5B-MET | PR | 10 | NR | |
| [7] | 74 | F | No | Recurrent | ADC | HLA-DRB1-MET | Complete resolution of nodules while pleural effusion persisted | 8 | No | |
| [8] | 59 | F | No | Recurrent | ADC | HLA-DRB1-MET | Complete radiographic response | / | No | |
| [9] | 43 | F | No | IV | ADC | MET-UBE2H | PR | 6.5 | NR | MET fusion was acquired on EGFR-targeted therapy |
| [10] | 56 | F | No | IV | ADC | MET–ATXN7L1 | PR | 4 | NR |
Herein, we report the clinical efficacy of crizotinib in a patient with poorly differentiated NSCLC with KIF5B-MET and a concurrent novel MET-CDR2 fusion.
In November 2018, a 46-year-old female never-smoker presented in our clinic with a complaint of persistent dry cough.
The cough had been lasted for over a week.
Past medical history was not remarkable for this patient.
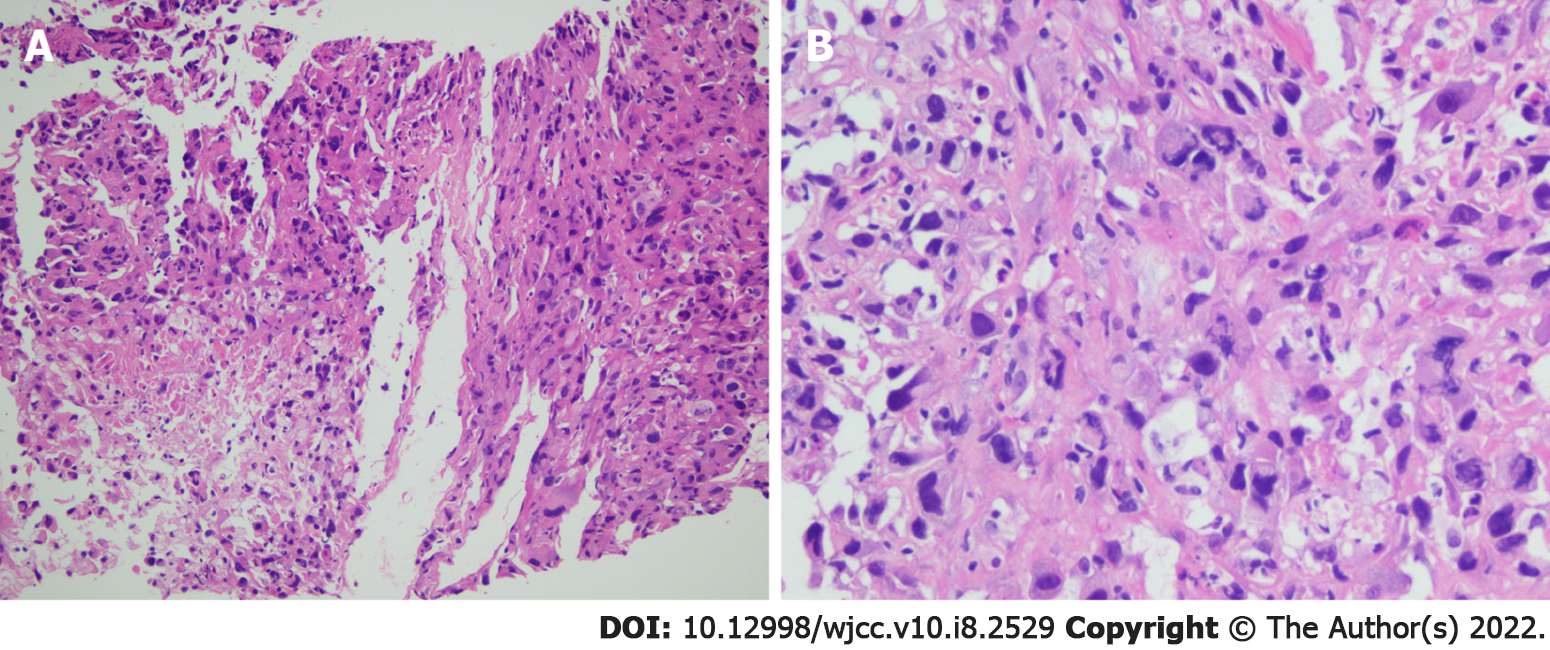
Histopathological analysis of tissue biopsy samples collected from the right lung revealed poorly differentiated NSCLC (Figure 1) with the immunohistochemistry results of AE1/AE3 (+), SMACA4 BRG1 (+), CK18 (+), INI-1 (+), CD56 (-), chromogranin A (-), synaptophysin (-), CK7 (-), ERG (-), GATA3 (-), CD34 (-), CDX2 (-), P40 (-), SALL4 (-), TTF-1 (-), Desmin (-), and S-100 (-). In addition, PD-L1 expression analysis revealed a tumor proportion score of 80%. Molecular analysis of the biopsies detected no driver alterations in EGFR, ALK, or ROS1.
Computed tomography (CT) and magnetic resonance imaging revealed a tumor in the lower lobe of the right lung, right hilar and mediastinal lymph node involvement, and multi-organ metastasis including the left pleura, liver, pericardium, and bone.
The final diagnosis of the patient was NSCLC stage IV (T4N3M1).
Based on the findings presented above, the patient was then treated with two cycles of gemcitabine (1.0 g/m2 on days 1 and 8) plus cisplatin (75 mg/m2 on day 1) with no clinical benefit.
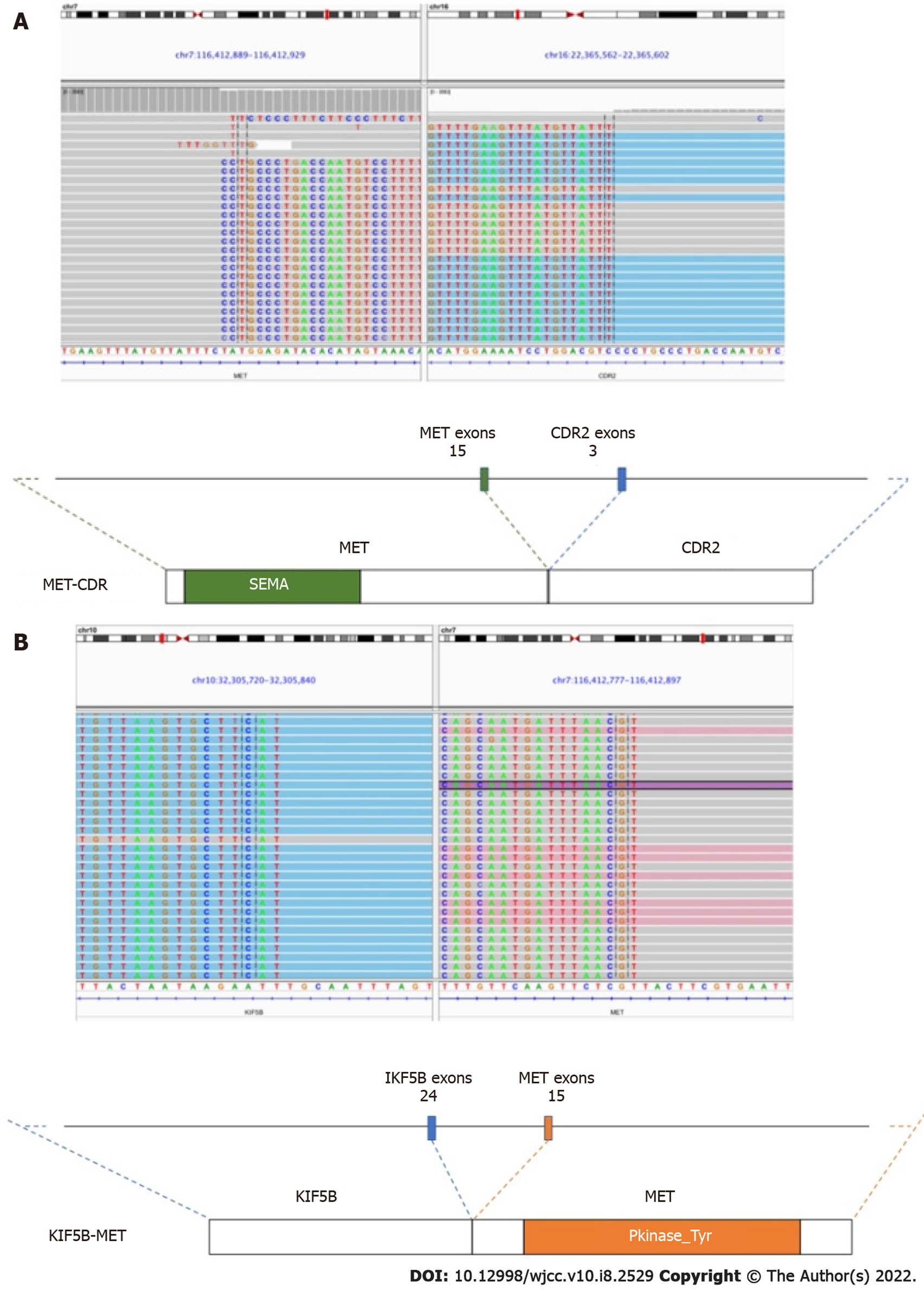
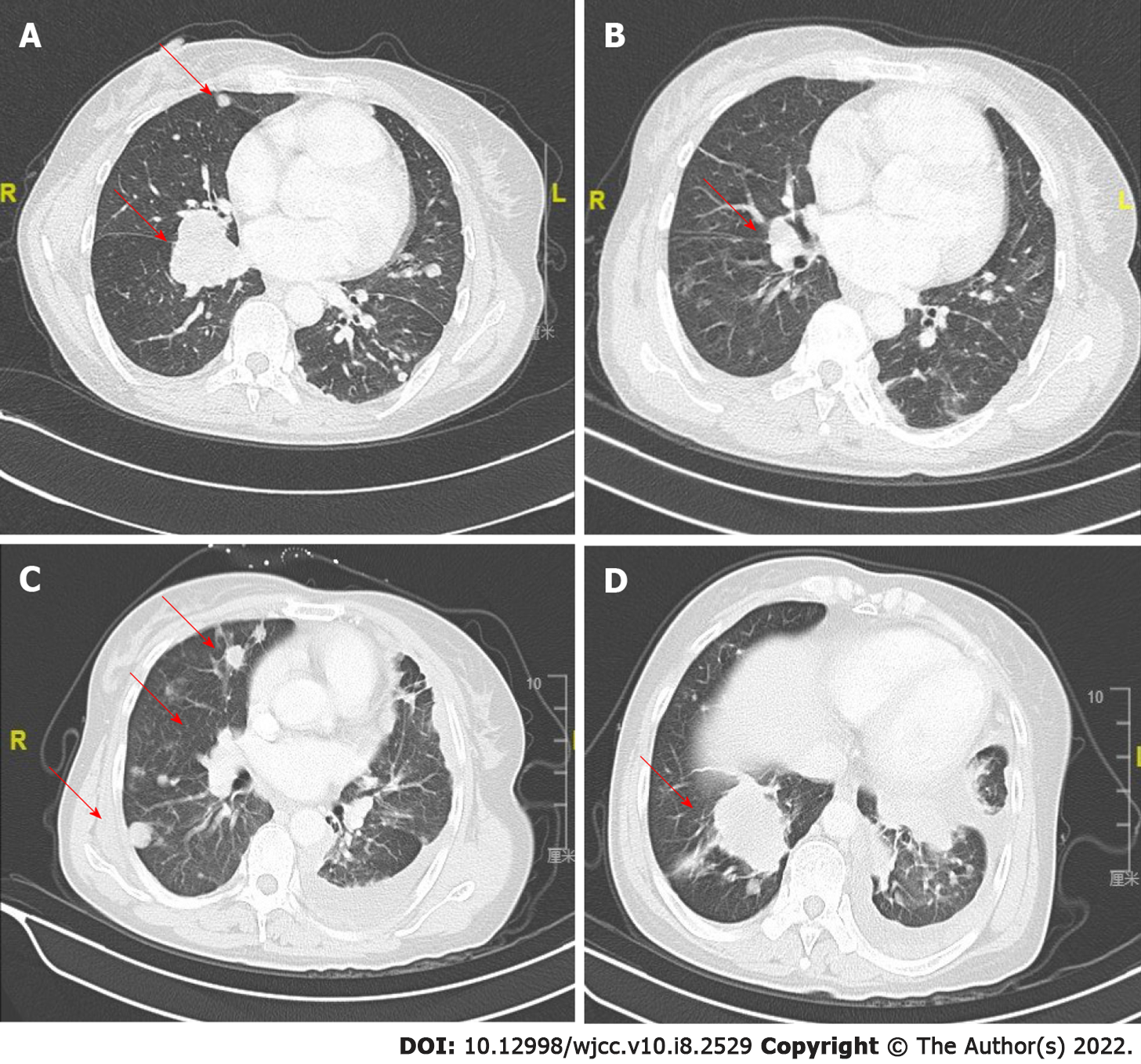
In January 2019, an abdominal CT scan revealed the enlargement of the lung primary and liver metastases. To explore potentially actionable mutations, tumor biopsy samples were submitted for capture-based targeted sequencing using a panel with 520 cancer-related genes (OncoScreen Plus, Burning Rock, China). As shown in Figure 2, the analysis revealed the detection of two concurrent MET fusions with respective partner genes KIF5B (K24:M15) and CDR2 (M15:C3). No other classic lung cancer driver mutations were detected apart from TP53 C277X. Due to economic and insurance conditions and out of concern over evidence suggesting reduced efficacy of immunotherapy in non-small cell lung cancer patients carrying oncogenic driver alterations[18], crizotinib (250 mg, p.o. bid) was started as the second line treatment in February 2019. After 4 wk of therapy, review of chest CT revealed a dramatic reduction of the lesions in the left and right lobes of the lungs with no new lesions, which was evaluated as partial response with Response Evaluation Criteria in Solid Tumors v.1.1 (RECIST 1.1) (Figure 3A and B). At approximately 3 mo from the start of targeted therapy, the patient continued to benefit from crizotinib without side effects. However, the disease progressed afterwards in May, 2019 as per RECIST 1.1. Specifically, compared with the previous evaluation (Figure 3B), new lesions emerged mostly in the right lung, accompanied by growth of the previously reduced tumor (Figure 3C and D).
After crizotinib failure, we chose nivolumab (a human IgG4 PD-1 antibody) as a salvage therapy because of the high PD-L1 expression. However, the patient did not benefit from nivolumab and her condition was declining significantly. She was hospitalized for worsening respiratory function and died shortly thereafter with an overall survival (OS) of 7 mo from diagnosis.
Gene alterations in MET are emerging as clinically relevant biomarker for predicting the response to MET inhibitors[2]. However, due to the rarity of MET fusions, treatment responses have only been clinically evaluated for MET amplification and exon 14 skipping[12-14] and only a few case reports have reported the efficacy of crizotinib in patients with MET fusions with various partners[5-7]. In our report, we describe the detection of KIF5B-MET co-occurring with a novel gene fusion involving MET and CDR2 and provided the clinical evidence of the efficacy of crizotinib in a KIF5B-MET and MET-CDR2-rearranged poorly differentiated NSCLC patient. KIF5B-MET K24:M15 has been reported in 0.5% (1/206) of adenocarcinoma and 4% (2/28) of sarcomatoid lung cancer patients in a recent study in Taiwanese patients[19]. In vitro and in vivo studies consistently demonstrated the oncogenic potential of KIF5B-MET fusion and sensitivity to crizotinib[19]. Consistently, several case reports have observed clinical efficacy of crizotinib in K24:M14[6] and K24:M15[5] KIF5B-MET-rearranged NSCLC[5,6]. The dramatic response to crizotinib observed in our patient highly suggests that the fusions acting either solely or in synergy served as oncogenic driver/s in the patient’s tumor which confers sensitivity to crizotinib. The oncogenic potential and sensitivity to crizotinib or other MET inhibitors of the novel gene fusion MET-CDR2 as well as the presence of two concurrent MET fusions require further investigations.
The negative results for histopathologic markers TTF-1, CK7, P40, and CDX2 and classic driver mutations in EGFR, ALK, and ROS1 provided neither clear indication of the cell differentiation nor any therapeutic targets. With a poor response to the first-line chemotherapy regimen, our patient had a very poor prognosis. Comprehensive genomic profiling allowed us to understand the mutation landscape of the tumor and explore alternative therapeutic targets that provided benefit to our patient. The detection of the potentially targetable MET fusions in our patient with poorly differentiated NSCLC highlights the importance of comprehensive genomic profiling regardless of tumor histology, particularly in patients with no known driver mutations to guide therapeutic decisions.
After the failure of crizotinib, we chose an immune checkpoint inhibitor (ICI) as a salvage therapy. Although with high PD-L1 expression, the patient did not benefit from the ICI. This is similar with the finding of previous studies that ICIs are less effective in NSCLC with EGFR mutation or EML4-ALK fusion[18,20].
Attention should be paid to managing toxicities associated with crizotinib monotherapy. In a study of 2028 Japanese ALK-rearranged patients receiving crizotinib, adverse drug reactions occurred in 91.6% of patients, the most common (incidence ≥ 15%) of which were nausea (32.2%), diarrhea (24.3%), photopsia (18.9%), vomiting (17.5%), and dysgeusia (16.8%). A considerable proportion of patients (623, 30.7%) discontinued treatment within 12 wk after therapy initiation due to adverse events. Only 68.2% of patients remained on crizotinib after 3 mo, 55.2% after 6 mo, and 36.1% after 12 mo, with a median duration of 7.9 mo[10]. Therefore, it is advised to monitor patients for these adverse reactions during the clinical use of crizotinib.
The efficacy of crizotinib in an advanced poorly differentiated NSCLC patient with concurrent KIF5B-MET and MET-CDR2 gene fusions suggests that crizotinib can serve as a therapeutic option in patients with MET fusions. Further clinical studies are required to confirm the clinical value of crizotinib or other MET inhibitors in patients with MET fusion.
The authors thank the patient and her family. We also thank the investigators, study coordinators, operation staff, and the whole project team who worked on this case. We are grateful to Xiao Zou and Lei Lei at Burning Rock Biotech for technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imai Y, Jang HJ, Kung WM S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Matsumoto K, Umitsu M, De Silva DM, Roy A, Bottaro DP. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017;108:296-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 2. | Comoglio PM, Trusolino L, Boccaccio C. Known and novel roles of the MET oncogene in cancer: a coherent approach to targeted therapy. Nat Rev Cancer. 2018;18:341-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 3. | Go H, Jeon YK, Park HJ, Sung SW, Seo JW, Chung DH. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5:305-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 4. | Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nat Commun. 2014;5:4846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 748] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 5. | Plenker D, Bertrand M, de Langen AJ, Riedel R, Lorenz C, Scheel AH, Müller J, Brägelmann J, Daßler-Plenker J, Kobe C, Persigehl T, Kluge A, Wurdinger T, Schellen P, Hartmann G, Zacherle T, Menon R, Thunnissen E, Büttner R, Griesinger F, Wolf J, Heukamp L, Sos ML, Heuckmann JM. Structural Alterations of MET Trigger Response to MET Kinase Inhibition in Lung Adenocarcinoma Patients. Clin Cancer Res. 2018;24:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Cho JH, Ku BM, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ. KIF5B-MET Gene Rearrangement with Robust Antitumor Activity in Response to Crizotinib in Lung Adenocarcinoma. J Thorac Oncol. 2018;13:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Davies KD, Ng TL, Estrada-Bernal A, Le AT, Ennever PR, Camidge DR, Doebele RC, Aisner DL. Dramatic Response to Crizotinib in a Patient with Lung Cancer Positive for an HLA-DRB1-MET Gene Fusion. JCO Precis Oncol. 2017;2017:PO.17.00117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Kunte S, Stevenson J. A Case of HLA-DRB1-MET Rearranged Lung Adenocarcinoma With Rapid Response to Crizotinib. Clin Lung Cancer. 2021;22:e298-e300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Zhu YC, Wang WX, Song ZB, Zhang QX, Xu CW, Chen G, Zhuang W, Lv T, Song Y. MET-UBE2H Fusion as a Novel Mechanism of Acquired EGFR Resistance in Lung Adenocarcinoma. J Thorac Oncol. 2018;13:e202-e204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Zhu YC, Wang WX, Xu CW, Zhang QX, Du KQ, Chen G, Lv TF, Song Y. Identification of a novel crizotinib-sensitive MET-ATXN7L1 gene fusion variant in lung adenocarcinoma by next generation sequencing. Ann Oncol. 2018;29:2392-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tanizaki J, Okamoto I, Okamoto K, Takezawa K, Kuwata K, Yamaguchi H, Nakagawa K. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6:1624-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Caparica R, Yen CT, Coudry R, Ou SI, Varella-Garcia M, Camidge DR, de Castro G Jr. Responses to Crizotinib Can Occur in High-Level MET-Amplified Non-Small Cell Lung Cancer Independent of MET Exon 14 Alterations. J Thorac Oncol. 2017;12:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Camidge DR, Otterson GA, Clark JW, Ou SHI, Weiss J, Ades S, Conte U, Tang YY, Wang SCE, Murphy D, Wilner KD, Villaruz LC. Crizotinib in patients (pts) with MET-amplified non-small cell lung cancer (NSCLC): Updated safety and efficacy findings from a phase 1 trial. J Clin Oncol. 2018;36:9062. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Paik PK, Drilon A, Fan PD, Yu H, Rekhtman N, Ginsberg MS, Borsu L, Schultz N, Berger MF, Rudin CM, Ladanyi M. Response to MET inhibitors in patients with stage IV lung adenocarcinomas harboring MET mutations causing exon 14 skipping. Cancer Discov. 2015;5:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 466] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 15. | Vuong HG, Nguyen TQ, Nguyen HC, Nguyen PT, Ho ATN, Hassell L. Efficacy and Safety of Crizotinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with ROS1 Rearrangement or MET Alteration: A Systematic Review and Meta-Analysis. Target Oncol. 2020;15:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Wang WX, Xu C, Chen Y, Zhu YC, Liu YP, Wang H, Zhuang W, Chen XH, Huang YJ, Lai JH, Fang MY, Zhang ZH, Tao Y, Xu SG, Qian X, Zhao HY, Cai SL, Chen G, Lv TF, Song Y. MET gene fusions in non-small cell lung cancer (NSCLC) in the Chinese population: A multicenter study. J Clin Oncol. 2018;36:e13539. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Zhuo M, Liang Z, Yi Y, Wu N, Yang X, Zhong J, Chen X, Huang Y, Yu Z, Liu C, Zeng X, Gu W, Zhao J. Analysis of MET kinase domain rearrangement in NSCLC. Lung Cancer. 2020;145:140-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Mazieres J, Drilon A, Lusque A, Mhanna L, Cortot AB, Mezquita L, Thai AA, Mascaux C, Couraud S, Veillon R, Van den Heuvel M, Neal J, Peled N, Früh M, Ng TL, Gounant V, Popat S, Diebold J, Sabari J, Zhu VW, Rothschild SI, Bironzo P, Martinez-Marti A, Curioni-Fontecedro A, Rosell R, Lattuca-Truc M, Wiesweg M, Besse B, Solomon B, Barlesi F, Schouten RD, Wakelee H, Camidge DR, Zalcman G, Novello S, Ou SI, Milia J, Gautschi O. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 970] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 19. | Gow CH, Liu YN, Li HY, Hsieh MS, Chang SH, Luo SC, Tsai TH, Chen PL, Tsai MF, Shih JY. Oncogenic Function of a KIF5B-MET Fusion Variant in Non-Small Cell Lung Cancer. Neoplasia. 2018;20:838-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Ueno N, Banno S, Endo Y, Tamura M, Sugaya K, Hashigaki S, Ohki E, Yoshimura A, Gemma A. Treatment status and safety of crizotinib in 2028 Japanese patients with ALK-positive NSCLC in clinical settings. Jpn J Clin Oncol. 2019;49:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |