Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2522
Peer-review started: July 27, 2021
First decision: October 22, 2021
Revised: November 2, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 226 Days and 13 Hours
Type A insulin resistance syndrome (TAIRS) is a rare disorder characterized by severe insulin resistance due to defects in insulin receptor signaling. No specific drugs are available for the treatment of TAIRS. We report a case of TAIRS successfully treated with pioglitazone and flutamide for 5 years.
We present the rare case of a female patient aged 11 years and 9 mo with type A insulin resistance and an INSR heterozygous mutation (c.3614C>T), who was treated with a combination of pioglitazone and flutamide. This treatment regimen reduced hemoglobin A1c, fasting insulin and androgen levels.
Pioglitazone attenuated insulin resistance in this patient with TAIRS, and flutamide ameliorated masculinization.
Core Tip: Type A insulin resistance syndrome (TAIRS) is a rare disorder characterized by severe insulin resistance due to defects in signaling through the insulin receptor. We present the rare case of a female patient aged 11 years and 9 mo who had type A insulin resistance with an INSR heterozygous mutation (c.3614C>T). This is the first case report describing the use of pioglitazone and flutamide used in combination in a child with TAIRS. This protocol for TAIRS is inexpensive, effective, and free of side effects.
- Citation: Chen YH, Chen QQ, Wang CL. Treatment and five-year follow-up of type A insulin resistance syndrome: A case report. World J Clin Cases 2022; 10(8): 2522-2528
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2522.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2522
Insulin resistance (IR) is a condition that cells, tissues or organs can't respond properly to a given dose of insulin.[1]. Type A insulin resistance syndrome (TAIRS) is an autosomal recessive or dominant genetic disease[2]. Insulin receptor gene mutation affects insulin and insulin receptors, leading to insulin dysfunction[3]. Teenage females are more commonly affected and mainly develop severe IR, hyperandrogenism and acanthosis nigricans, which may be accompanied by polycystic ovary syndrome.
In this report, a female patient aged 11 years and 9 mo was diagnosed with severe IR and hyperandrogenism after comprehensive physical examination and related examinations. She was given oral medication, was followed up to observe changes in clinical symptoms and signs, and underwent monitoring of metabolism, IR and androgen levels. This study aimed to observe and analyze the curative effect of drugs in this patient, improve the understanding of the etiology and mechanism of TAIRS and explore effective treatment schemes.
The female patient aged 11 years and 9 mo was admitted to our outpatient clinic due to hairiness and melanosis of the skin and hoarseness.
On October 30, 2015, the girl was admitted to our outpatient clinic due to hairiness and melanosis of the skin and hoarseness. Pigmentation started soon after birth without an obvious cause, and the child had noticeably more hair on her body than children of the same age. In addition, the degree of severity gradually increased with age. Pubic hair appeared 8 mo prior to presentation and was noted to be increasing rapidly; the patient’s voice became hoarse 4 mo prior to presentation. She denied complaints of headache, dizziness, fatigue, abdominal pain and other discomfort.
The patient was born with a birth weight of 2650 g. After birth, she was noted to have excessive hair on her body and excessive skin pigmentation.
The growth and development of the patients are similar to those of their peers, and there is no long-term medication history.
Family history was significant for diabetes in her father, grandfather and aunt.
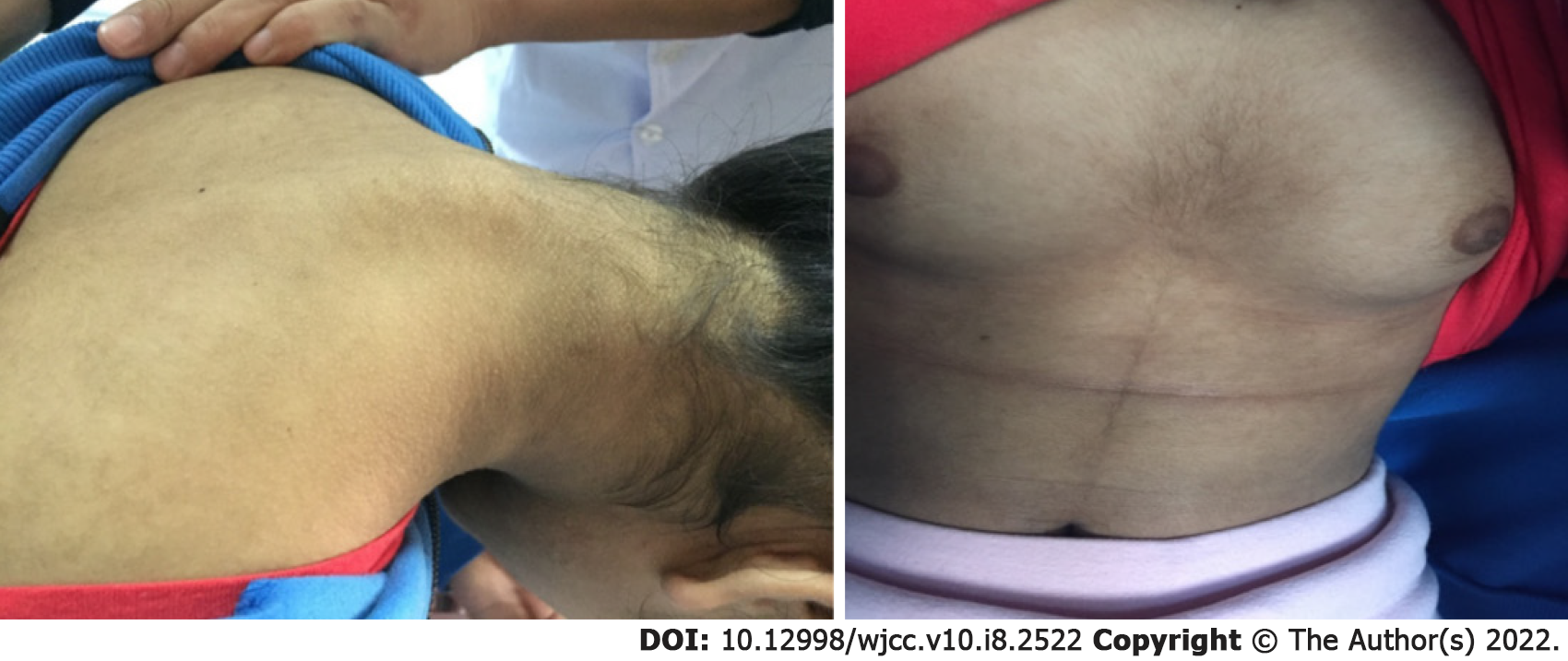
Physical examination showed as follows: height 149 cm, weight 36 kg, BMI 16.21 kg/m2, hirsutism, dense pubic hair, and perineal pubic hair distributed in diamond shape.The skin showed acanthosis-like changes (Figure 1). There was no acne or thyroid enlargement. Her pubertal development was Tanner grade IV.
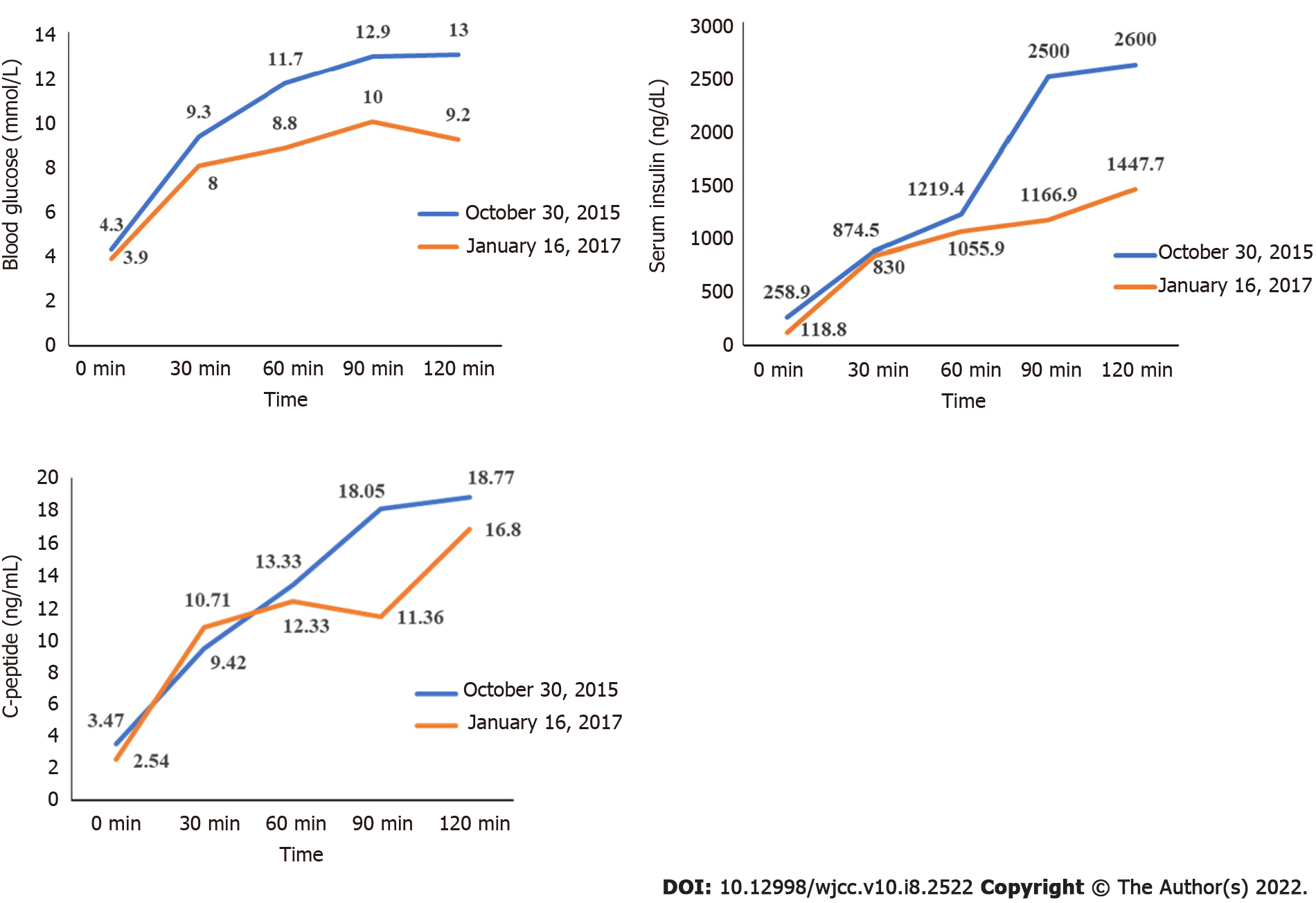
Routine blood tests, blood gas analysis, thyroid function, liver and kidney function tests showed that 17-hydroxyprogesterone, alpha fetoprotein and human chorionic gonadotropin were normal. Cortisol rhythm was also normal (5.48 µg/dL at 8 am; 5.12 µg/dL at 4 pm; and 12.8 pg/mL of adrenocorticotropic hormone at 8 am). At 24 h, urine free cortisol was 95.37 μg (reference range 20.9-292.3 μg). The dehydroepiandrosterone sulfate level was 76.45 μg/mL (reference range 0.3-1.47 μg/mL). The androstenedione level was 5.03 ng/mL (reference range 0.50-4.70 ng/mL). The sex hormone levels were as follows: follicle stimulating hormone was 4.9 mIU/mL, luteinizing hormone was 4.65 mIU/mL, testosterone was 128.7 ng/dL, estradiol was 48.0 pg/mL, and prolactin was 4.5 ng/mL. A 75 g glucose tolerance test was performed, which showed that fasting blood glucose was 4.3 mmol/L and 2-h blood glucose was 13 mmol/L. Hemoglobin A1c (HbA1c) was 6.9%.
Transrectal color Doppler ultrasound of the uterine annex showed no polycystic changes in either ovary. Head MRI, abdominal B-ultrasound, cardiac B-ultrasound and adrenal B-ultrasound were normal.
The clinical manifestations and laboratory diagnosis of this patient indicated TAIRS. Therefore, after obtaining the consent of the patient and her family, genetic testing was performed. The results showed that INSR gene had a mutation. (c.3614C>T).
We recommended that the patient work on strengthening exercises and adopt a controlled diet. Treatment with metformin 1.0 g/d was administered. However, the patient suffered fasting hypoglycemia many times; therefore, metformin was changed to pioglitazone 15 mg/d. In addition, hyperandrogenism was treated with flutamide.
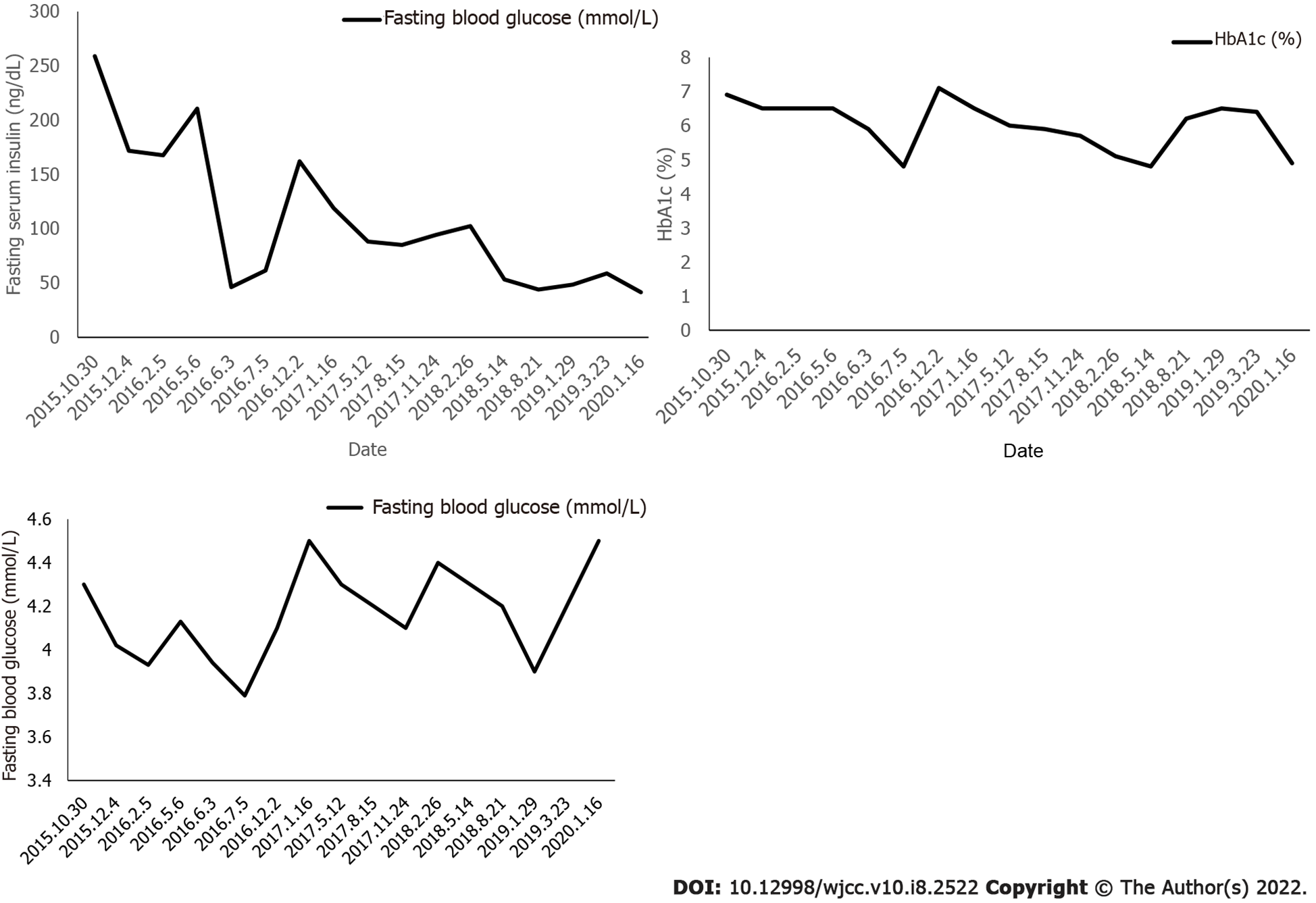
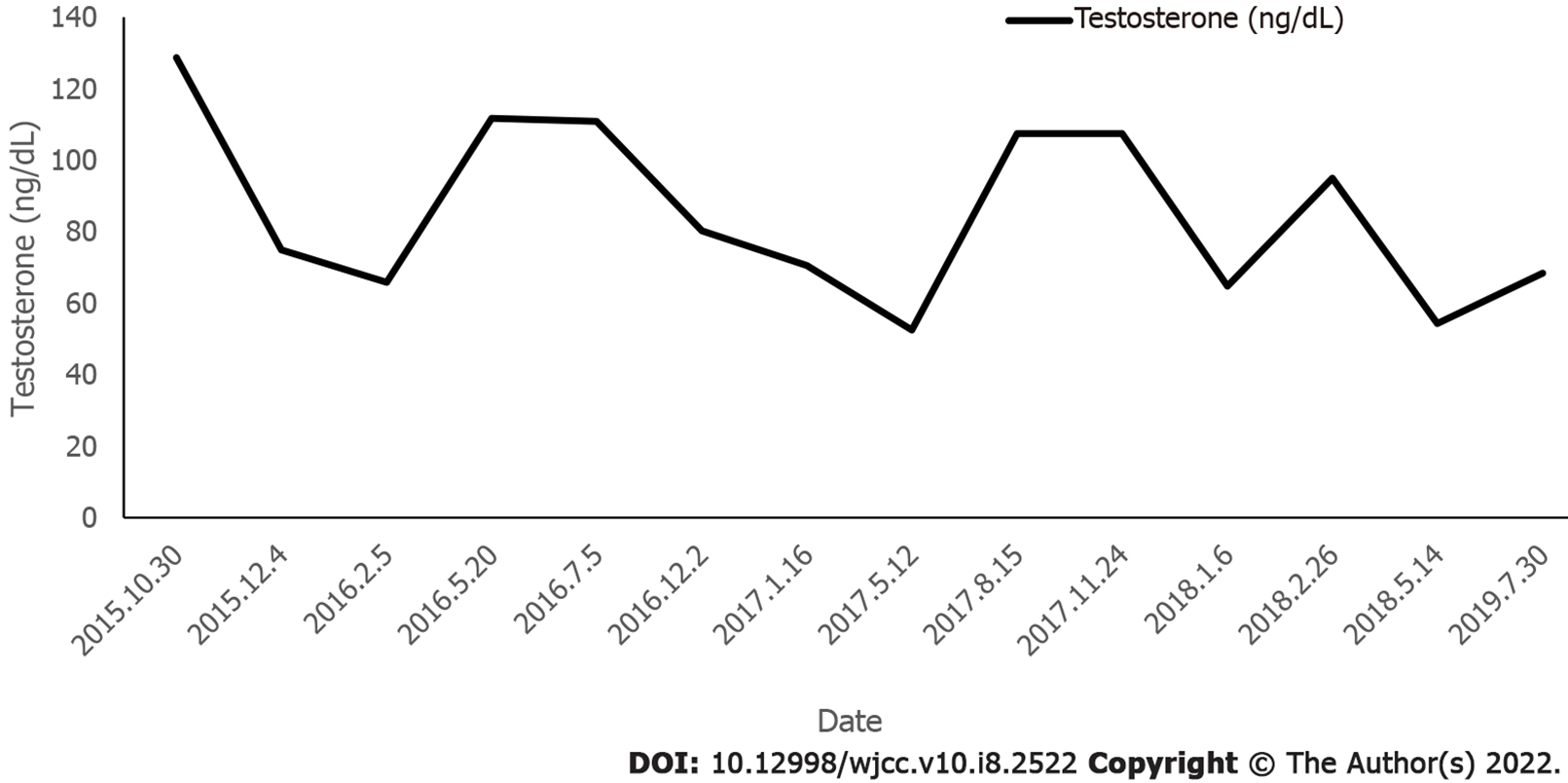
Blood sampling was performed on admission and every 3 mo thereafter to evaluate the patient’s plasma glucose profile, HbA1C, plasma insulin, and sex hormone levels. Height and weight were measured on each admission. A 75 g glucose tolerance test was performed 15 mo after treatment.
Following the administration of pioglitazone, plasma glucose, plasma insulin, and HbA1c normalized, as shown in Figure 2 and Figure 3. During treatment, the patient did not have any hypoglycemic attacks or abnormal routine laboratory data. Acanthosis nigricans also seemed to improve gradually. Hirsutism and serum testosterone concentration slowly improved after the administration of flutamide, as shown in Figure 4. Menstruation started 9 mo after initiation of treatment; however, it was noted to be irregular.
In this report, we described a child with hirsutism and acanthosis nigricans on examination. Blood lipid metabolism abnormalities were not observed on biochemical examination. Hormone level testing suggested the existence of hyperandrogenism and severe IR. Genetic testing showed INSR c.3614C>T; thus, TAIRS was diagnosed.
Insulin is an important regulator of sex hormone metabolism. High insulin leads to a decrease in sex hormone binding protein synthesis in the liver and stimulates androgen synthesis in the ovary and adrenal glands[4]. However, high androgen levels can inhibit the effect of insulin stimulating the uptake of glucose by tissues, thereby causing IR[5]. Therefore, hyperinsulinemia interacts with hyperandrogenism, causing a vicious cycle.
At present, there is no guideline or consensus statement to describe how to best treat patients with severe IR. The treatment of patients mainly aims to prevent long-term complications caused by diabetes and hyperandrogenism[6]. Maintaining body weight and BMI plays an important role in maintaining blood glucose homeostasis[7]. There is no definitive and effective drug treatment for type A IR, and the use of metformin, insulin sensitizer, and insulin-like growth factor-1 has been reported. Metformin, a biguanide derivative, has been demonstrated to have beneficial results in further decreasing BMI. The molecular mechanism of metformin is not completely clear. Many potential mechanisms of action have been proposed, such as inhibiting mitochondrial respiratory chain (complex I), activating AMP-activated protein kinase (AMPK), and inhibiting glucagon-induced increase of cyclic adenosine monophosphate (cAMP), while reducing the activation of protein kinase A (PKA), inhibition of mitochondrial glycerophosphate dehydrogenase, and an effect on gut microbiota[8,9]. Metformin inhibits the secretion of growth hormone, adrenocorticotropic hormone, follicle stimulating hormone and anterior melanocortin from the pituitary basal body, which partly explains its insulin sensitization effect through various actions on tissues, including liver, skeletal muscle, endothelium, adipose tissue and ovaries.[10]. However, in this case, oral metformin was discontinued due to repeated fasting hypoglycemia, and the patient was given pioglitazone. Pioglitazone selectively stimulates nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ) and, to a lesser extent, PPAR-α[11]. Pioglitazone modulates transcription of the genes involved in the control of glucose and lipid metabolism in the muscle, adipose tissue, and liver. As a result, pioglitazone reduces IR in the liver and peripheral tissues, decreases gluconeogenesis in the liver, and reduces the quantities of glucose and glycated hemoglobin in the bloodstream[12]. After treatment, our patient showed a significant decrease in fasting insulin, and her glucose metabolism improved. Acanthosis nigricans also faded significantly. She had menarche when she was 13 years old, but her menstruation was irregular. Up to now, no adverse reactions have occurred.
Treating TAIRS is challenging. Recombinant human IGF-1 can activate IGF-1 receptor, and it can be an effective treatment for TAIRS, because IGF-1 receptor shares structural homology and downstream signal pathway with INSR.[13]. However, recombinant human IGF-1 therapy is expensive, and the drug is not available in China. Glucagon-like peptide 1 (GLP-1) receptor agonists stimulate GLP-1 receptors in the pancreas and thereby increase insulin release and inhibit glucagon secretion, but they are not approved by the FD[14]. SGLT2 inhibitor is a new type of antidiabetic drug, which can reduce blood sugar by inhibiting renal glucose reabsorption, and has nothing to do with insulin.[15]. A recent study showed that a sodium–glucose cotransporter 2 inhibitor had a good therapeutic effect in a patient with TAIRS[16]; however, further research is required. Patients who fail to take oral hypoglycemic drugs usually require a larger dose of insulin.
Patients with hirsutism, acne, and amenorrhea caused by hyperandrogenemia can be treated with anti-androgen drugs such as cyproterone acetate, flutamide, and spironolactone.Flutamide, as a selective antagonist of androgen receptor (AR), competes with androgens such as testosterone and dihydrotestosterone to bind AR in prostate and other tissues. Flutamide prevents their effects and prevents them from stimulating the growth of prostate cancer cells. Studies have shown that flutamide is effective in treating hirsutism[17]. Following flutamide application, hirsutism in this patient was obviously improved.
In conclusion, there is no definite treatment for TAIRS. Based on 5 years of follow-up, our protocol is inexpensive, effective, and was not associated with side effects in this patient with TAIRS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaur M S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Kang S, Tsai LT, Rosen ED. Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol. 2016;26:341-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Yang GQ, Wang BA, Zhao WR, Gu WJ, Lui ZH, Dou JT, Mu YM, Lu JM. Clinical and genetic analysis of the insulin receptor gene in a Chinese patient with extreme insulin resistance. Diabetes Res Clin Pract. 2010;89:e56-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Gehart H, Kumpf S, Ittner A, Ricci R. MAPK signalling in cellular metabolism: stress or wellness? EMBO Rep 2010; 11: 834-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 4. | Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 1163] [Article Influence: 89.5] [Reference Citation Analysis (1)] |
| 5. | Nohara K, Laque A, Allard C, Münzberg H, Mauvais-Jarvis F. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring). 2014;22:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue KC. The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes. 2009;10 Suppl 12:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Spartano NL, Stevenson MD, Xanthakis V, Larson MG, Andersson C, Murabito JM, Vasan RS. Associations of objective physical activity with insulin sensitivity and circulating adipokine profile: the Framingham Heart Study. Clin Obes. 2017;7:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Burcelin R. The antidiabetic gutsy role of metformin uncovered? Gut. 2014;63:706-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 943] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 10. | Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. Metformin in polycystic ovary syndrome. Ann N Y Acad Sci. 2010;1205:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Waugh J, Keating GM, Plosker GL, Easthope S, Robinson DM. Pioglitazone: a review of its use in type 2 diabetes mellitus. Drugs. 2006;66:85-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Colca JR, McDonald WG, Waldon DJ, Leone JW, Lull JM, Bannow CA, Lund ET, Mathews WR. Identification of a novel mitochondrial protein ("mitoNEET") cross-linked specifically by a thiazolidinedione photoprobe. Am J Physiol Endocrinol Metab. 2004;286:E252-E260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | McDonald A, Williams RM, Regan FM, Semple RK, Dunger DW. IGF-1 treatment of insulin resistance. Eur J Endocrinol 2007; 157: 51-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34 Suppl 2:S279-S284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 15. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1917] [Article Influence: 319.5] [Reference Citation Analysis (0)] |
| 16. | Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome – PART 1. Endocr Pract. 2015;21:1291-1300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 17. | Mimoto MS, Oyler JL, Davis AM. Evaluation and Treatment of Hirsutism in Premenopausal Women. JAMA. 2018;319:1613-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |