Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2510
Peer-review started: July 22, 2021
First decision: October 22, 2021
Revised: October 30, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 16, 2022
Processing time: 231 Days and 17 Hours
Immunoglobulin (Ig) G4-related disease (IgG4-RD) is an autoimmune disease associated with chronic and progressive inflammation and fibrosis. It is difficult to differentiate IgG4-RD involving the kidney from infectious diseases and malignancy on imaging.
We report the case of a 51-year-old Chinese man whose abdominal computed tomography scan showed diffuse bilateral enlargement of the kidneys and perirenal fat, thickening of the renal pelvic walls, and hydronephrosis of the right kidney. Relevant laboratory test results showed a serum creatinine level of 464 μmol/L. The patient was diagnosed with acute renal failure and was started on intermittent hemodialysis. Further tests revealed high serum IgG4 levels (20.8 g/L) and an enlarged right submaxillary lymph node. Biopsy and histopathological examination of the enlarged node led to the diagnosis of IgG4-RD. After corticosteroid therapy, his serum creatinine level quickly decreased to near normal levels.
IgG4-RD affecting the renal pelvis or perirenal fat is rare, with atypical imaging features. Multidisciplinary consultation is critical for accurate diagnosis and treatment of this disease. Suspected cases should undergo biopsy to avoid misdiagnosis.
Core Tip: In this case, computed tomography showed diffuse bilateral enlargement of the kidneys and perirenal fat, thickening of the renal pelvic walls, and hydronephrosis of the right kidney which was similar to those of infectious diseases and malignancy. Histological examination confirmed the diagnosis of IgG4-RD.
- Citation: He JW, Zou QM, Pan J, Wang SS, Xiang ST. Immunoglobulin G4-related kidney disease involving the renal pelvis and perirenal fat: A case report. World J Clin Cases 2022; 10(8): 2510-2515
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2510.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2510
Immunoglobulin (Ig) G4-related disease (IgG4-RD) is an autoimmune disease associated with chronic and progressive inflammation and fibrosis, characterized by heavy IgG4-positive plasma cell infiltration and fibrosis in the affected tissues[1]. It is also known as IgG4-related kidney disease (IgG4-RKD), as it usually involves the renal parenchyma, perirenal fat, renal pelvis, and ureter. The disease manifests on computed tomography (CT) scans as a diffuse enlarged kidney, swollen perirenal fat, and thickened renal pelvis and ureter, which makes it difficult to differentiate from an infectious disease or tumor. Recently, we encountered a 51-year-old Chinese man who was finally diagnosed with IgG4-RD after pathological examination. We report this rare case of IgG4-RD, which is easily misdiagnosed as either a malignant tumor or an infectious disease, along with a summary of relevant literature.
On January 11, 2020, a 51-year-old man was admitted to our hospital with the complaint of chest oppression.
The patient had symptoms of chest oppression for 10 d, with no obvious cause, along with edema of both lower extremities. The patient denied having pain upon urination, low back pain, fever, or any other clinical manifestations.
The patient had no previous medical history.
The patient had no specific personal and family history.
Physical examination of the heart and lungs was unremarkable, but both lower extremities had moderate edema. Blood pressure was normal without significant fluctuations. The patient also had an enlarged right submaxillary lymph node.
Laboratory tests revealed a serum creatinine (Cr) level of 464 μmol/L, potassium concentration of 5.52 mmol/L, brain natriuretic peptide level of 376.2 pg/mL, and high-sensitivity C-reactive protein level of 20.4 mg/L (normal reference range: 0 to 6.0 mg/L). A routine urine test revealed a red blood cell count of 67.3/μL (normal reference range, 0 to 4/μL) and a white blood cell count of 731/μL (normal reference range, 0 to 5/μL). Serum IgG4 level was found to be high at 20.8 g/L (normal reference range, 0.03 to 2 g/L). Hepatitis B surface antigen, antineutrophil cytoplasmic antibody, and rheumatoid factor were negative, and other test results were within the normal limits.
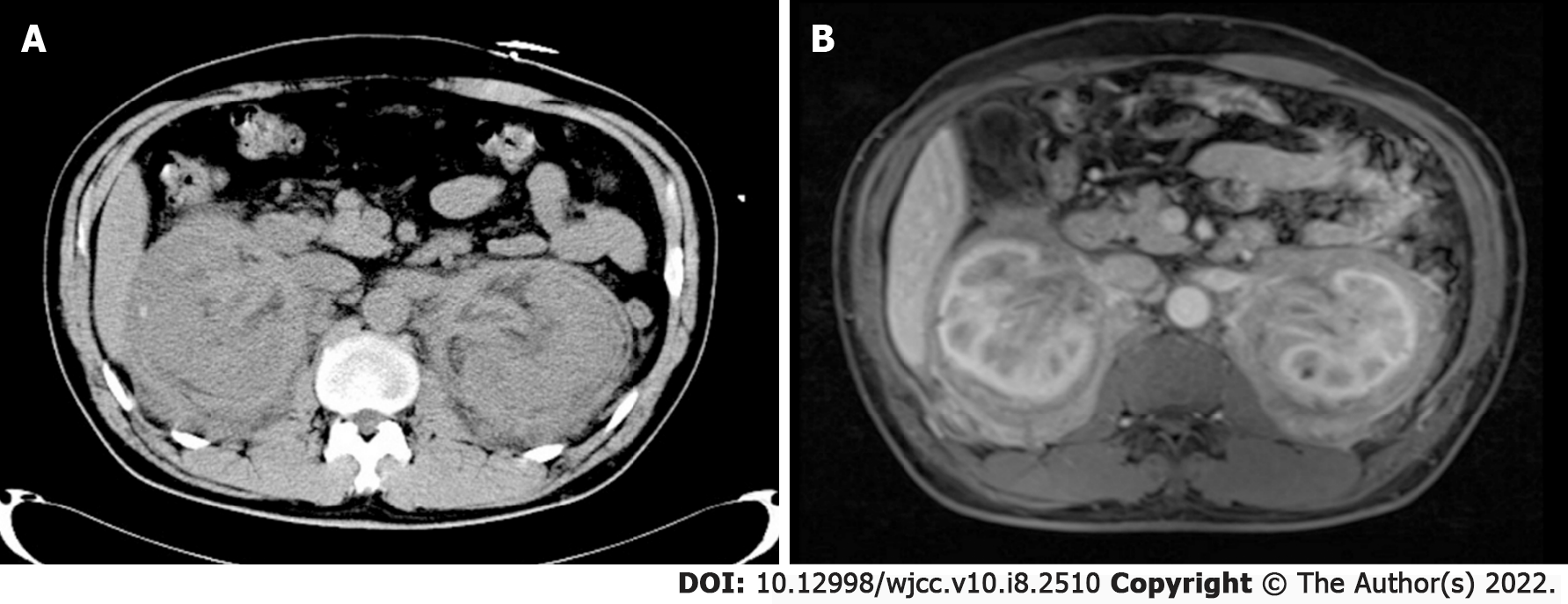
Abdominal CT showed diffuse bilateral enlargement of the kidneys and perirenal fat, thickening of the renal pelvic walls, and hydronephrosis of the right kidney (Figure 1A). On magnetic resonance imaging (MRI) scans, the wall of the renal pelvis and ureter was thickened on contrast-enhanced images (Figure 1B). An ultrasound of the neck revealed an enlarged right submaxillary lymph node measuring approximately 29 mm × 19 mm.
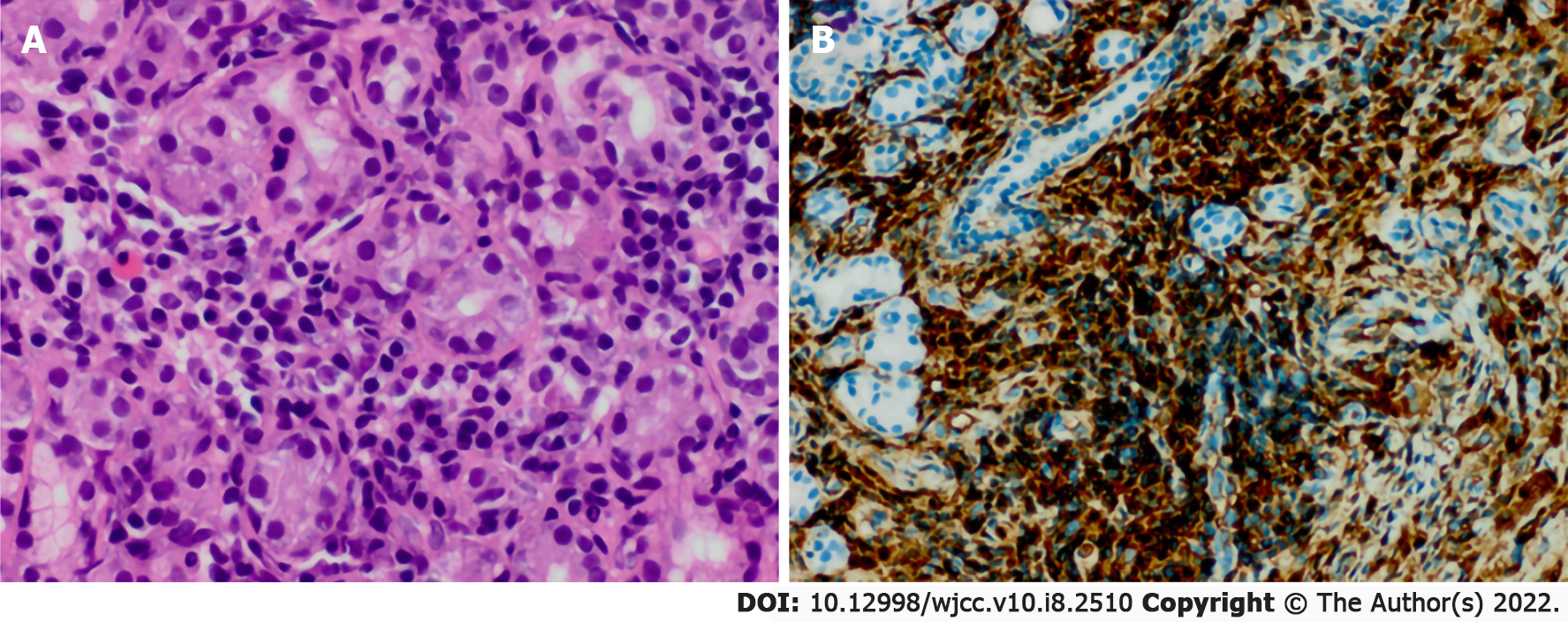
Biopsy of the submaxillary node was performed for histopathological examination, which revealed a dense infiltrate of lymphoplasmacytic cells along with fibrosis. The IgG4+/IgG ratio determined by immunohistochemical staining was 60% in the specimens, and the average IgG4 plasma cell count was 100/high-power field (HPF) (Figure 2).
According to the comprehensive diagnostic criteria for IgG4-RD, the patient was finally diagnosed with IgG4-RD.
The patient first presented with chest oppression to the Cardiovascular Department. However, coronary heart disease was ruled out by the cardiologist according to the laboratory tests. The patient was hospitalized and maintained on intermittent hemodialysis due to acute renal failure. The level of urinary protein excretion was 2129.6 mg/d. Laboratory tests revealed a serum IgG level of 21.15 g/L, and serum complement 4 level of 0.52 g/L. Further tests were conducted to determine the cause of diffuse bilateral enlargement of the kidneys and perirenal and thickening of the renal pelvic walls. Serum IgG4 level was found to be high at 20.8 g/L (normal reference range, 0.03 to 2 g/L), and an enlarged lymph node was found in the right submaxillary area. Ultrasound indicated that the right submaxillary lymph node was enlarged, and was approximately 29 mm × 19 mm in size. Pathological biopsy of the submaxillary mass was performed. Dense lymphoplasmacytic cells with fibrotic changes were found on histopathology. Based on these findings, the patient was diagnosed with IgG4-RD. He was initially started on intravenous methylprednisolone sodium succinate 40 mg/d for 2 wk. Two weeks later, his Cr level decreased to 117 μmol/L; thus, treatment was changed to oral prednisone at 40 mg once a day, and then the dosage was reduced by 10 mg biweekly.
During the follow-up period, urinary tract ultrasound was performed every 6 mo, and the serum Cr level was examined every 2 mo. To date, no recurrence has been identified, and serum Cr level is normal.
IgG4-RD is a new disease recognized in the twenty-first century. Kamisawa proposed the concept of IgG4 systemic disease for the first time in 2003 and named the disease IgG4-RD in 2010[2,3]. IgG4-RD is a systemic disease characterized by diffuse enlargement of single or multiple organs and elevated IgG4 serum levels, with infiltration of lymphocytes and IgG4-positive plasma cells, as well as fibrosis, typically seen on histopathology. Two IgG4-RD study groups, the Umehara and Okazaki teams, organized by the Ministry of Health, Labour and Welfare in Japan, established the definitive diagnostic criteria for IgG4-RD in 2011 as follows: (1) Clinical examination showing characteristic diffuse/localized swelling or masses in a single organ or multiple organs; (2) Hematological examination showing elevated serum IgG4 concentrations (≥ 135 mg/dL); and (3) Histopathologic examination showing: (a) Marked lymphocyte and plasmacyte infiltration and fibrosis; and (b) Infiltration of IgG4+ plasma cells with a ratio of IgG4+/IgG+ cells >40% and >10 IgG4+ plasma cells/HPF[4]. Corticosteroid therapy has been found to be effective for IgG4-RD. All three criteria were met, and a good outcome was achieved after corticosteroid therapy in our case.
IgG4-RD that involves the kidney, also known as IgG4-RKD, is difficult to differentiate from infectious diseases and malignancy on imaging. The characteristic radiological features of IgG4-RKD are as follows: (1) Multiple low-density areas on contrast CT, (2) Diffuse bilateral enlargement of the kidneys in patients with decreased renal function in whom the administration of contrast medium is inadvisable, and (3) Diffuse thickening of the renal pelvic walls with a smooth luminal surface[5]. Kim et al[6] proposed that the characteristic MRI findings of IgG4-RKD were bilateral, multiple, renal parenchymal nodules with T2 hypointensity, diffusion restriction, and a progressive enhancement pattern.
IgG4-RKD usually involves the renal parenchyma. However, renal pelvis and ureter involvement are rare, and perirenal fat involvement is even rarer. Renal pelvis involvement in IgG4-RKD is usually characterized by thickening of the pelvic wall, which is easily misdiagnosed as carcinoma of the renal pelvis and removed surgically. Naoto et al, in 2009, reported the first case of IgG4 systemic disease involving the renal pelvis. The patient was misdiagnosed on imaging with a renal pelvis tumor and underwent nephroureterectomy; however, histopathological examination prompted the diagnosis of IgG4-related disease of the renal pelvis. This was the first case of pathology-confirmed IgG4 systemic disease involving the renal pelvis[7]. We searched the literature for articles published in the English language between January 2000 and June 2020 concerning manifestations of IgG4-RKD affecting the renal pelvis or perirenal fat. We identified and reviewed 13 articles that reported cases of IgG4-RKD affecting the renal pelvis or perirenal fat[8-12]. Among the cases of IgG4-RD affecting the renal pelvis or perirenal fat reported in the literature, 53.8% (7/13) underwent nephrectomy or nephroureterectomy, and the pathological diagnosis was IgG4-RKD[13-17]. From our literature review, we found that the misdiagnosis rate of IgG4-RD involving the renal pelvis was very high, suggesting that currently, urologists generally lack understanding of this disease[18,19]. This causes a certain delay in the diagnosis and treatment stages. Fortunately, renal function was restored after treatment, and further unnecessary surgery was avoided. The manifestations of this disease on imaging are atypical, involving the renal parenchyma, renal pelvis, ureter, and perirenal fat at the same time. The literature review showed that only two cases of IgG4-RD involving the perirenal fat have been reported globally, which are extremely rare cases[17].
There has been little attention to urinary system involvement besides renal parenchyma. According to the study by Zhang et al[12], elevated serum IgG and IgG4 were found in all patients with IgG4-RKD, but no hypocomplementemia was found. Teng et al[20] reported that all patients with IgG4-related urinary disease had marked elevations in serum IgG4 levels, and 76.9% had hyperglobulinemia at diagnosis. The study also showed that 20% of patients with IgG4-related urinary disease had nephritis confirmed by urine tubular injury markers accompanied by acute renal dysfunction and required emergency medical intervention. More than 90% of IgG4-RKD patients have a history of extrarenal lesions, which is an important basis for differential diagnosis. A satisfactory response to corticosteroid therapy is a characteristic feature of IgG4-RD.
IgG4-RD is a recently identified disease, which rarely affects the renal pelvis or perirenal fat and has atypical imaging features that make it difficult to differentiate from infectious diseases and malignancy. Therefore, urological surgeons must improve their understanding of these diseases, so they can identify the nonspecific clinical symptoms and the bilateral, multiple, atypical organ lesions on imaging, and confirm the diagnosis with serological examination. There were several characteristic clinical features of IgG4-RKD in our case, including the involvement of multiple organs, high levels of serum IgG and IgG4, and a rapid initial response to corticosteroids. Multidisciplinary consultation is critical for the accurate diagnosis and treatment of this disease. Suspected cases should undergo biopsy to avoid misdiagnosis.
We thank Xiao-hua Du, Department of Pathology, for his technical assistance with immunohistochemistry staining.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liakina V, Pelaez-Luna M S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 794] [Cited by in RCA: 849] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 2. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 3. | Takahashi H, Yamamoto M, Suzuki C, Naishiro Y, Shinomura Y, Imai K. The birthday of a new syndrome: IgG4-related diseases constitute a clinical entity. Autoimmun Rev. 2010;9:591-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 625] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 5. | Kawano M, Saeki T, Nakashima H, Nishi S, Yamaguchi Y, Hisano S, Yamanaka N, Inoue D, Yamamoto M, Takahashi H, Nomura H, Taguchi T, Umehara H, Makino H, Saito T. Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Kim B, Kim JH, Byun JH, Kim HJ, Lee SS, Kim SY, Lee MG. IgG4-related kidney disease: MRI findings with emphasis on the usefulness of diffusion-weighted imaging. Eur J Radiol. 2014;83:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Kuroda N, Nakamura S, Miyazaki K, Inoue K, Ohara M, Mizuno K, Sato Y, Yoshino T. Chronic sclerosing pyelitis with an increased number of IgG4-positive plasma cells. Med Mol Morphol. 2009;42:236-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Yoshino T, Moriyama H, Fukushima M, Sanda N. A case of IgG4-related retroperitoneal fibrosis mimicking renal pelvic cancer. Urol Int. 2013;90:365-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Inoue S, Takahashi C, Hikita K. A Case of IgG4-Related Retroperitoneal Fibrosis from the Renal Pelvis Mimicking Bilateral Hydronephrosis. Urol Int. 2016;97:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tsuzaka Y, Ookubo K, Sugiyama K, Morimoto H, Amano H, Oota N, Kuriki K, Homma Y. [IgG4-related kidney disease: a long-term follow up case of pseudotumor of the renal pelvis]. Nihon Hinyokika Gakkai Zasshi. 2014;105:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Chen X, Luo R, Wang H, Wang G, Hou Y, Guo J. IgG4-related systemic disease mimicking renal pelvic cancer: a rare case. World J Surg Oncol. 2014;12:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Zhang H, Ren X, Zhang W, Yang D, Feng R. IgG4-related kidney disease from the renal pelvis that mimicked urothelial carcinoma: a case report. BMC Urol. 2015;15:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Inenaga J, Ueno T, Kawada M, Imafuku A, Mise K, Sumida K, Hiramatsu R, Hasegawa E, Hayami N, Suwabe T, Hoshino J, Sawa N, Takaichi K, Fujii T, Ohashi K, Okaneya T, Ubara Y. IgG4-related Disease: A Mass Lesion in the Intrarenal Sinus near the Renal Pelvis. Intern Med. 2015;54:1897-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Park HG, Kim KM. IgG4-related inflammatory pseudotumor of the renal pelvis involving renal parenchyma, mimicking malignancy. Diagn Pathol. 2016;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Li B, Hou J, Zhang Y, Hou X, Shi H. Retroperitoneal IgG4-related sclerosing disease mimics renal pelvic cancer on (18)F-FDG PET/CT. Rev Esp Med Nucl Imagen Mol. 2016;35:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Amakawa R, Akatsuka J, Suzuki Y, Hamasaki T, Kimura G, Ishii H, Kondo Y. [A CASE OF IgG4-RELATED DISEASE WITH THICKENING OF THE RENAL PELVIS AT LEFT RENAL HILUM DIAGNOSED BY OBTURATOR LYMPH NODE DISSECTION]. Nihon Hinyokika Gakkai Zasshi. 2017;108:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Cho YJ, Jung WY, Lee SY, Song JS, Park HJ. Perirenal capsule and scrotal involvement in immunoglobulin G4-related kidney disease: case-based review. Rheumatol Int. 2018;38:1941-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Zhang S, Yang Q. Imaging findings of IgG4-related kidney disease without extrarenal organ involvement: A case report. Medicine (Baltimore). 2019;98:e16934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Jiang Y, Hou G, Cheng W. Renal Pelvis Involvement of Immunoglobulin G4-Related Disease Mimicking Malignancy on 18F-FDG PET/CT. Clin Nucl Med. 2019;44:767-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Teng F, Lu H, Zheng K, Chen G, Wen Y, Liu Z, Peng L, Huo L, Zeng X, Zhang W, Li X. Urinary System Manifestation of IgG4-Related Disease: Clinical, Laboratory, Radiological, and Pathological Spectra of a Chinese Single-Centre Study. J Immunol Res. 2020;2020:5851842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |