Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2491
Peer-review started: July 13, 2021
First decision: September 28, 2021
Revised: October 9, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Processing time: 240 Days and 20.3 Hours
Trastuzumab is a generally safe agent prescribed in the systemic treatment of breast cancer. Tinnitus is not a currently known adverse event related to trastuzumab. Here, we describe a rare case of severe tinnitus and a migraine headache induced by trastuzumab used for adjuvant therapy.
A 37-year-old woman was diagnosed with hormone receptor-positive and human epidermal growth factor receptor 2-positive breast cancer. After surgery, she was treated with four cycles of epirubicin and cyclophosphamide; she then received docetaxel and a loading dose of trastuzumab plus pertuzumab. Less than half an hour after trastuzumab infusion, the patient complained of severe tinnitus and left-sided migraine headache. Trastuzumab monotherapy was discontinued immediately, and symptoms disappeared after 10 min. Trastuzumab was readministered, and severe tinnitus and migraine headache recurred. Trastu
Although trastuzumab is well-tolerated in most patients, we should pay attention to the risk of severe tinnitus and migraine.
Core Tip: Trastuzumab is an important treatment for human epidermal growth factor receptor 2-positive breast cancer and is generally well-tolerated, although both acute and subacute adverse events have been reported. Here, we report a rare case of severe tinnitus and migraine induced by trastuzumab used for adjuvant therapy which may help guide future clinical treatment.
- Citation: Liu YZ, Jiang H, Zhao YH, Zhang Q, Hao SC, Bao LP, Wu W, Jia ZB, Jiang HC. Severe tinnitus and migraine headache in a 37-year-old woman treated with trastuzumab for breast cancer: A case report. World J Clin Cases 2022; 10(8): 2491-2496
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2491.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2491
Trastuzumab (Herceptin®) is a humanized monoclonal antibody against the extracellular domain of human epidermal growth factor receptor (HER2) protein and is the first HER2-targeted therapy approved for the treatment of HER2-positive breast cancer[1]. Trastuzumab is generally a well-tolerated drug, although both acute and subacute adverse events such as flu-like syndrome and cardiotoxicity have been observed[2]. Tinnitus has not previously been reported as an adverse event related to trastuzumab. Here, we report a rare case of severe tinnitus and migraine induced by trastuzumab during the first cycle of adjuvant therapy.
A 37-year-old Chinese woman was diagnosed as hormone receptor-positive and HER2-positive infiltrating duct carcinoma in her left breast.
The patient felt a painless lump in the left breast during a physical examination. After several examinations, she underwent breast-conserving surgery, and sentinel lymph node biopsy was resultingly found to be 1/4 positive.
The patient’s prior medical history was unremarkable. The patient did not demonstrate any history of drug allergies and had no history of ear, nose, and throat (ENT), migraine or other central nervous system diseases.
The patient gave birth at the age of 25 and breastfed her infant. She experienced regular menstrual cycles and had no family history of cancer.
A physical examination of the patient revealed a 1.0 cm × 1.0 cm non-tender mass in the upper outer quadrant of the left breast. Her physical examination confirmed no signs of ENT diseases, central nervous system diseases or cerebral metastasis. And she had a body mass index (BMI) of 30.4.
Laboratory examinations (routine blood analysis, liver biochemical analysis, renal function, tumor markers, etc.) were normal.
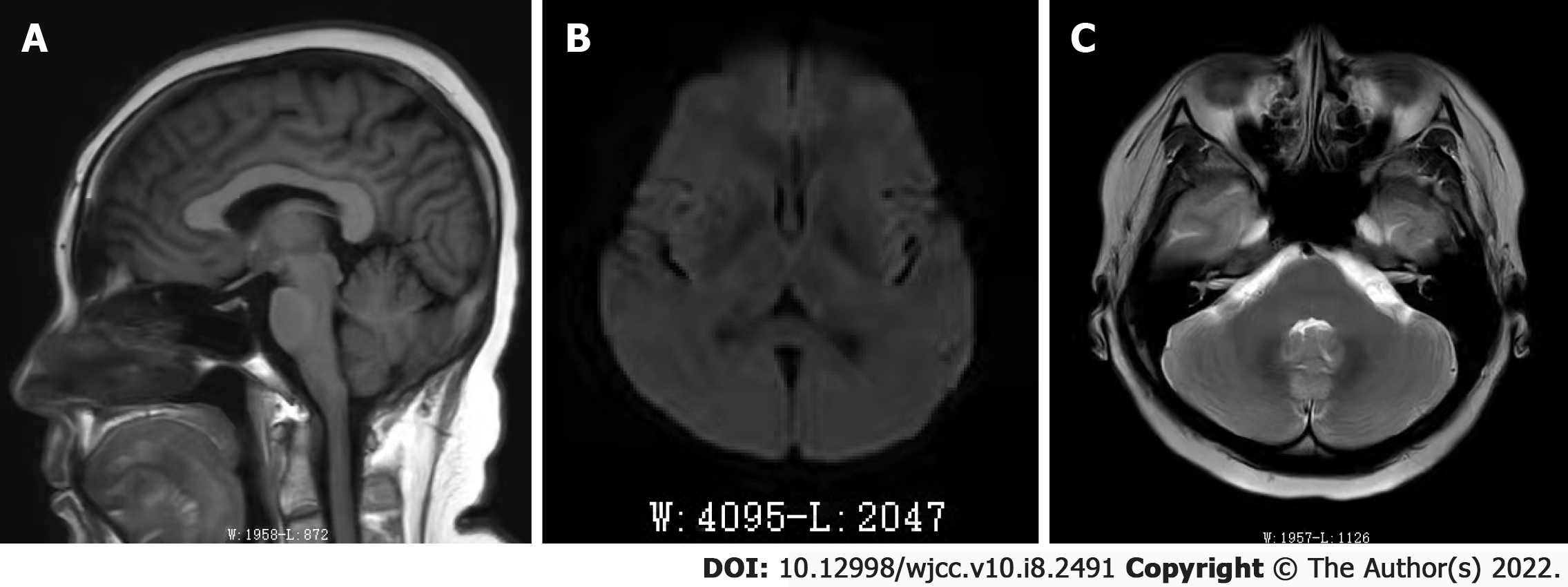
The patient’s lungs, bones and liver were normal. Imaging examination did not demonstrate any evidence of distant metastases. A cerebral magnetic resonance imaging scan revealed no sign of intracranial or skeletal cranial metastases or any vascular disorders (Figure 1).
The final diagnosis of the presented case was primary breast cancer at stage IIB (pT2N1M0). The tumor was estrogen- and progesterone-receptor-positive and HER2-positive. Fluorescent in-situ hybridization revealed HER2 amplification in the patient.
After surgery, a standard dose regimen of adjuvant chemotherapy and targeted therapy was initiated. This therapy consisted of four cycles of epirubicin (90 mg/m2) and cyclophosphamide (600 mg/m2) administration every three weeks followed by four cycles of docetaxel(100 mg/m2) administration in combination with trastuzumab (8 mg/kg and 6 mg/kg) plus pertuzumab (840 mg and 420 mg) every three weeks. We planned to follow the treatment regimen consisting of trastuzumab plus pertuzumab for one year.
The premedication agent (dexamethasone; 8 mg every 12 h for three doses beginning 12 h before administration of docetaxel) was administered before docetaxel to decrease the occurrences of anaphylactic reactions (ARs). Because ARs have been frequently reported with taxanes, drug administration occurred in the order of trastuzumab, pertuzumab and docetaxel.
During the first cycle of targeted therapy, the patient received 468 mg of trastuzumab monotherapy at a rate of 3 mg/min. Approximately 30 min after the administration of 90 mg of trastuzumab, the patient complained of severe tinnitus and left-sided migraine headache. The infusion of trastuzumab monotherapy was discontinued immediately, and the symptoms disappeared after 10 min. After resolution of severe tinnitus and migraine, the patient was administered trastuzumab for the second time. However, 20 min following trastuzumab re-administration, severe tinnitus and migraine headache again developed, and trastuzumab was therefore stopped. Steroid therapy was successfully used to combat these reactions. Both severe tinnitus and migraine headache diminished after 10 min. The patient’s vital signs were carefully monitored during drug administration, and body temperature, pulse rate, respiratory rate, and blood pressure were normal. Subsequent investigation revealed that the white blood cell count, hemoglobin level, and platelet count as well as the liver and renal functions were also normal. There were no signs of a rash. Other causes of tinnitus were excluded by consultation with an otolaryngologist and a neurosurgeon, and the possibility that the headache could be attributed to a disorder of the ears was also ruled out. External auditory canal was eumorphic, and the ear drum membrane was in keeping with physiological obliquity. The patient’s limbs were not swollen, and she maintained free movement of all four limbs with normal muscle force and strength. No pathological reflection of Babinski's sign was induced. There was no evidence of acute stroke, intracranial hemorrhage, or hydrocephalus.
Multidisciplinary team members carefully assessed, communicated and informed the patient of appropriate treatment benefit and risk. Subsequently, pertuzumab (840 mg) and docetaxel (100 mg/kg) therapy was initiated, and no adverse events were observed. The patient’s symptoms were not likely a result of bacterial contamination, as the residual liquid bacteria culture was negative. Therefore, the severe tinnitus and migraine headache were significantly associated with trastuzumab rather than with pertuzumab or docetaxel.
Given the HER2-positive nature of the patient’s cancer and the clinical course, administration of trastuzumab retreatment was attempted 21 d later. She received subsequent infusions of trastuzumab every three weeks afterward, and none of the previous adverse reactions recurred.
Trastuzumab is a monoclonal antibody used as a standard treatment for breast cancer when the cancer cells overexpress HER2. Trastuzumab is typically well-tolerated; chills, flu-like symptoms, fever, nausea, skin rash, and cardiac toxicity are the most commonly reported adverse effects, and, less frequently, severe thrombocytopenia, hepatotoxicity, and systemic capillary leak syndrome have been described by some studies[3-5].
Here, we describe a case of severe tinnitus and migraine headache induced by trastuzumab. The symptoms recurred on two occasions during trastuzumab usage and re-administration when the patient received her first cycle treatment of trastuzumab. Our case shows, for the first time, that severe tinnitus and migraine headache can be simultaneously induced by trastuzumab infusion. The first case of trastuzumab-related strictly unilateral headache was reported in 2003: a 59-year-old patient experienced severe headache, back pain, fatigue, and a decrease in blood pressure after trastuzumab administration[6]. The second case of trastuzumab-induced throbbing headache was reported in 2009[7]. A 31-year-old woman experienced a very strictly unilateral headache with photophobia, nausea, and vomiting following infusion of trastuzumab. The authors stated that the nature of the migraine headache was not entirely understood[7]. Subsequently, other reports discussed the theory that monoclonal antibodies such as trastuzumab could possibly induce aseptic meningitis, which seemed to be part of an infusion-related reaction or immune-mediated hypersensitivity phenomenon[8,9].
Tinnitus is a subjective complaint defined as a sound in the head or ears that occurs in the absence of any external acoustical source. Studies report tinnitus prevalence ranging from 5.1%-42.7% by different age groups and generally showing an increase in prevalence as age increases. Young adults with migraines are more likely to suffer from tinnitus[10]. There is limited knowledge of direct biological links between migraine and tinnitus. One cross-sectional study showed that headache was associated with tinnitus, and the association was stronger for individuals reporting migraine with aura[11]. Ear injuries, central sensitization, and visual snow can cause tinnitus and may be related to the occurrence of migraines[12,13]. Migraine, tinnitus, anxiety and depression are prevalently comorbid disorders and have been frequently reported in patients with visual snow. Visual snow may start during or shortly after migrant aura. One theory suggests that there is a bidirectional relationship among depression, visual snow and migraine[14]. But in this case, the young patient did not have any past histories and clinical symptoms in the later following-up.
Studies suggest that ototoxicity is a possible adverse effect during treatment with taxanes[15]. Although Sarafraz and Ahmadi[16] did not observe tinnitus or hearing loss as the significant side-effects of taxanes, Xuan et al[17] presented two cases of ototoxicity caused by docetaxel-based chemotherapy regimens and speculated that docetaxel may result in degeneration of nerve fibers through disrupted axon transportation. They also suggest that clinicians note the adverse effect on the audiovestibular system caused by neurotoxic chemotherapy[17].
Immunolabeling patterns of HER2 have been described in the utricle and cochlea of rat P3 cultures[18]. Zhang et al[19] showed the pattern of HER2 Labeling in the utricle, saccule, organ of Corti, lateral wall, and spiral ganglion region of adult chinchillas. Whether the severe tinnitus and headache associated with the use of trastuzumab are related to the overexpression of HER2 in the human inner ear remains unclear.
To our knowledge, severe tinnitus and migraine headache with single-agent trastuzumab use are so rare that they had not yet been reported as adverse events in the existing literature. This case therefore has some limitations because only a single case has been reported so far, but more cases may be reported in the future. Because of the widespread use of this drug, we should pay more attention to its adverse reactions, especially because more serious complications may be prevented when adverse events are detected early. Because other patients may experience tinnitus induced by trastuzumab infusion, it is necessary to inform them of the potential adverse events before the use of trastuzumab; these events can also be specified in the drug instructions. The severe tinnitus and migraine headache induced by trastuzumab are noteworthy, and further studies are needed to evaluate whether targeted therapy treatment strategies affect migraine and tinnitus and to determine the mechanisms associated with the symptoms.
In conclusion, although trastuzumab is widely used and well-tolerated in most patients, we still should pay attention to the risk of severe tinnitus and migraine induced by trastuzumab. This case report serves as a reminder to be aware of adverse reactions of the breast cancer drugs.
We thank the patient, her family, and the personnel involved in the treatment of this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasan A, Menendez-Menendez J S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD, Giordano SH, Goetz MP, Goldstein LJ, Hurvitz SA, Isakoff SJ, Jankowitz RC, Javid SH, Krishnamurthy J, Leitch M, Lyons J, Matro J, Mayer IA, Mortimer J, O'Regan RM, Patel SA, Pierce LJ, Rugo HS, Sitapati A, Smith KL, Smith ML, Soliman H, Stringer-Reasor EM, Telli ML, Ward JH, Wisinski KB, Young JS, Burns JL, Kumar R. NCCN Guidelines® Insights: Breast Cancer, Version 4.2021. J Natl Compr Canc Netw. 2021;19:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 2. | Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-3865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 3. | Jones AL, Barlow M, Barrett-Lee PJ, Canney PA, Gilmour IM, Robb SD, Plummer CJ, Wardley AM, Verrill MW. Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br J Cancer. 2009;100:684-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Mantzourani M, Gogas H, Katsandris A, Meletis J. Severe thrombocytopenia related to trastuzumab infusion. Med Sci Monit. 2011;17:CS85-CS87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Zhang F, Yang J, Li Z. Trastuzumab-induced systemic capillary leak syndrome in a breast cancer patient. Pathol Oncol Res. 2014;20:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tada K, Ito Y, Hatake K, Okudaira T, Watanabe J, Arakawa M, Miyazato M, Irie T, Mizunuma N, Takahashi S, Aiba K, Horikoshi N, Kasumi F. Severe infusion reaction induced by trastuzumab: a case report. Breast Cancer. 2003;10:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | van Rooij FG, Dorresteijn LD, Van Bokhoven MM, Verstappen CC. A throbbing pain in the head: trastuzumab-induced migraine. Anticancer Res. 2009;29:4223-4225. [PubMed] |
| 8. | Olesen J. The international classification of headache disorders. 2nd edition (ICHD-II). Rev Neurol (Paris). 2005;161:689-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Cologno D, Buzzi MG, Carlesimo GA, Cicinelli P, Costa A, Fadda L, Formisano R, Marconi B, Pero S, Caltagirone C. Psychiatric disorders and pain location in unilateral migraineurs. J Headache Pain. 2005;6:227-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | McCormack A, Edmondson-Jones M, Somerset S, Hall DA. Corrigendum to "A systematic review of the reporting of tinnitus prevalence and severity" [Hear. Res. 337 (2016) 70-79]. Hear Res. 2016;339:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Guichard E, Montagni I, Tzourio C, Kurth T. Association Between Headaches and Tinnitus in Young Adults: Cross-Sectional Study. Headache. 2016;56:987-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Langguth B, Hund V, Busch V, Jürgens TP, Lainez JM, Landgrebe M, Schecklmann M. Tinnitus and Headache. Biomed Res Int. 2015;2015:797416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | García A, Madrigal J, Castillo M. Vestibular Migraine and Tinnitus: A Challenging Narrative. Cureus. 2021;13:e15998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Lugo A, Edvall NK, Lazar A, Mehraei G, Lopez-Escamez JA, Bulla J, Uhlen I, Canlon B, Gallus S, Cederroth CR. Relationship between headaches and tinnitus in a Swedish study. Sci Rep. 2020;10:8494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Dong Y, Ding D, Jiang H, Shi JR, Salvi R, Roth JA. Ototoxicity of paclitaxel in rat cochlear organotypic cultures. Toxicol Appl Pharmacol. 2014;280:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Sarafraz M, Ahmadi K. Paraclinical evaluation of side-effects of Taxanes on auditory system. Acta Otorhinolaryngol Ital. 2008;28:239-242. [PubMed] |
| 17. | Xuan L, Sun B, Meng X, Liu C, Cong Y, Wu S. Ototoxicity in patients with invasive ductal breast cancer who were treated with docetaxel: report of two cases. Cancer Biol Ther. 2020;21:990-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999;28:901-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Zhang M, Ding D, Salvi R. Expression of heregulin and ErbB/Her receptors in adult chinchilla cochlear and vestibular sensory epithelium. Hear Res. 2002;169:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |