Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2393
Peer-review started: August 14, 2021
First decision: October 16, 2021
Revised: October 30, 2021
Accepted: February 16, 2022
Article in press: February 16, 2022
Published online: March 16, 2022
Processing time: 208 Days and 16.9 Hours
Pancreatic adenocarcinoma is one of the most common malignant tumors of the digestive system. More than 80% of patients with pancreatic adenocarcinoma are not diagnosed until late stage and have distant or local metastases.
To investigate the value of computed tomography (CT) perfusion imaging in the evaluation of angiogenesis in pancreatic adenocarcinoma patients.
This is a retrospective cohort study. Patients with pancreatic adenocarcinoma and volunteers without pancreatic diseases underwent CT perfusion imaging from December 2014 to August 2017 in Huashan Hospital, Fudan University Shanghai, China.
A total number of 35 pancreatic adenocarcinoma patients and 33 volunteers were enrolled. The relative blood flow (rBF), and relative blood volume (rBV) were significantly lower in patients with pancreatic adenocarcinoma than in the control group (P < 0.05). Conversely, the relative permeability in patients with pancreatic adenocarcinoma was significantly higher than that in controls (P < 0.05). In addition, rBF, rBV, and the vascular maturity index (VMI) were significantly lower in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). Vascular endothelial growth factor (VEGF), CD105-MVD, CD34-MVD, and angiogenesis rate (AR) were significantly higher in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). Significant correlations between rBF and VEGF, CD105-MVD, AR, and VMI (P < 0.01) were observed. Moreover, the levels of rBV were statistically significantly correlated with those of VEGF, CD105-MVD, CD34-MVD, and VMI (P < 0.01).
Perfusion CT imaging may be an appropriate approach for quantitative assessment of tumor angiogenesis in pancreatic adenocarcinoma.
Core Tip: A total of 35 pancreatic adenocarcinoma patients and 33 volunteers were enrolled in the study. The relative blood flow, relative blood volume, and relative peak enhancement were significantly lower in patients with pancreatic adenocarcinoma than in the control group (P < 0.05). Conversely, the relative permeability in patients with pancreatic adenocarcinoma was significantly higher than that in controls (P < 0.05).
- Citation: Liu W, Yin B, Liang ZH, Yu Y, Lu N. Computed tomography perfusion imaging evaluation of angiogenesis in patients with pancreatic adenocarcinoma. World J Clin Cases 2022; 10(8): 2393-2403
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2393.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2393
Pancreatic adenocarcinoma is one of the most common malignant tumors of the digestive system. The prognosis of pancreatic adenocarcinoma is poor, with 5-year survival rates lower than 5%[1]. Importantly, more than 80% of patients with pancreatic adenocarcinoma are not diagnosed until late stage and have distant or local metastases[1,2]. Therefore, early detection of pancreatic adenocarcinoma is critical for improving prognosis outcomes.
Accumulating evidence indicates that vascularity is crucially involved in the tumorigenesis and drug responsiveness of pancreatic adenocarcinoma[3,4]. Thus, the evaluation of angiogenesis in pancreatic adenocarcinoma is of considerable significance for the diagnosis, treatment, and prognosis[5-8]. Computed tomography (CT) perfusion imaging provides information on tissue hemodynamics, which facilitates the more effective characterization and identification of pancreatic adenocarcinoma[9-11]. For instance, perfusion CT imaging has been widely applied in brain tumors, and the perfusion parameters have been proven to be of great significance in brain disease diagnosis[12-15]. However, relative perfusion parameters in pancreatic adenocarcinoma diagnosis have not yet been reported.
Therefore, in the present study, we performed perfusion CT imaging to explore the correlations between CT perfusion parameters and immunohistochemical angiogenesis indices, and their application for evaluating their diagnostic value in pancreatic adenocarcinoma.
This retrospective cohort study was conducted in Fudan University from December 2014 to August 2017. Subjects with pancreatic ductal adenocarcinoma and volunteers without pancreatic diseases were enrolled. Pancreatic adenocarcinoma patients with other pancreatic diseases were excluded. This study protocol was approved by the Institutional Review Board of Fudan University, Shanghai, China (2014-04-02). Written informed consent was obtained from each participant.
Perfusion CT imaging was performed using a 64-slice spiral CT scanner (SOMATOM Sensation 64, Siemens Medical Solutions, Forchheim, Germany). The baseline unenhanced CT acquisition provided wide coverage of the whole organ of interest. The field of view of perfusion CT imaging was positioned to include the maximum visible area of the tumor and a relevant arterial vessel. The abdominal aorta was used as an arterial input. An abdominal bandage was utilized to reduce the artifacts caused by respiratory motion. CT perfusion examinations were then performed in a continuous volume scan pattern using the following parameters: tube voltage 100 kV, tube current 80 mA, and a matrix of 512 × 512 pixels. The reconstructed slice thickness was 7.2 mm; the acquisition collimation was 7.2 mm, with 280 slices in each dataset. An average radiation dose of 9.3 mGy was applied. A volume of 50 mL of Omnipaque® 300 (GE Healthcare, Shanghai, China) was administered at a high flow rate (5 mL/s) using a high-pressure syringe. The contrast medium bolus was followed immediately by 15 mL of normal saline flush to increase the peak arterial enhancement. Stationary CT scans were then acquired every 1 s over a period of 70 s, with a delay of 4 s.
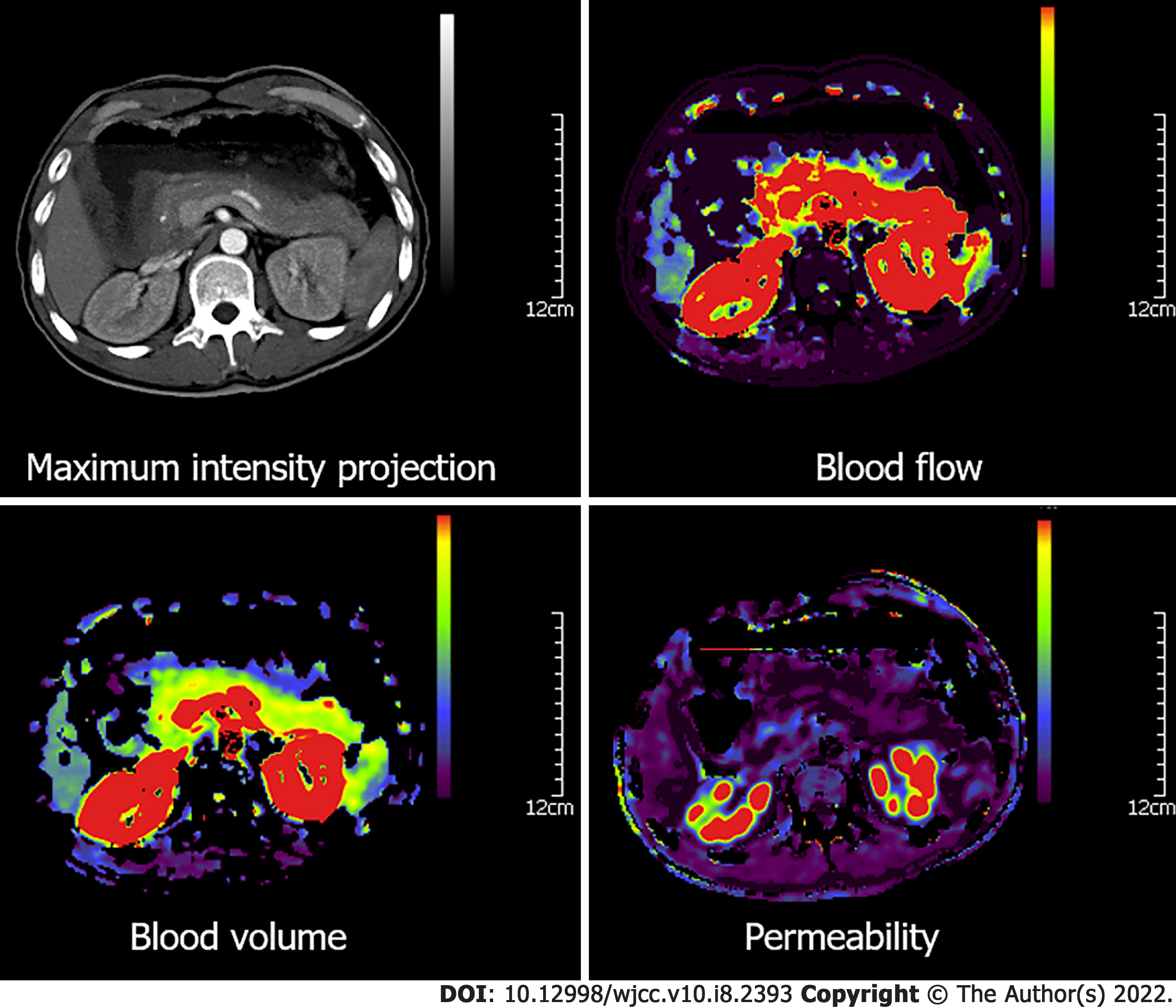
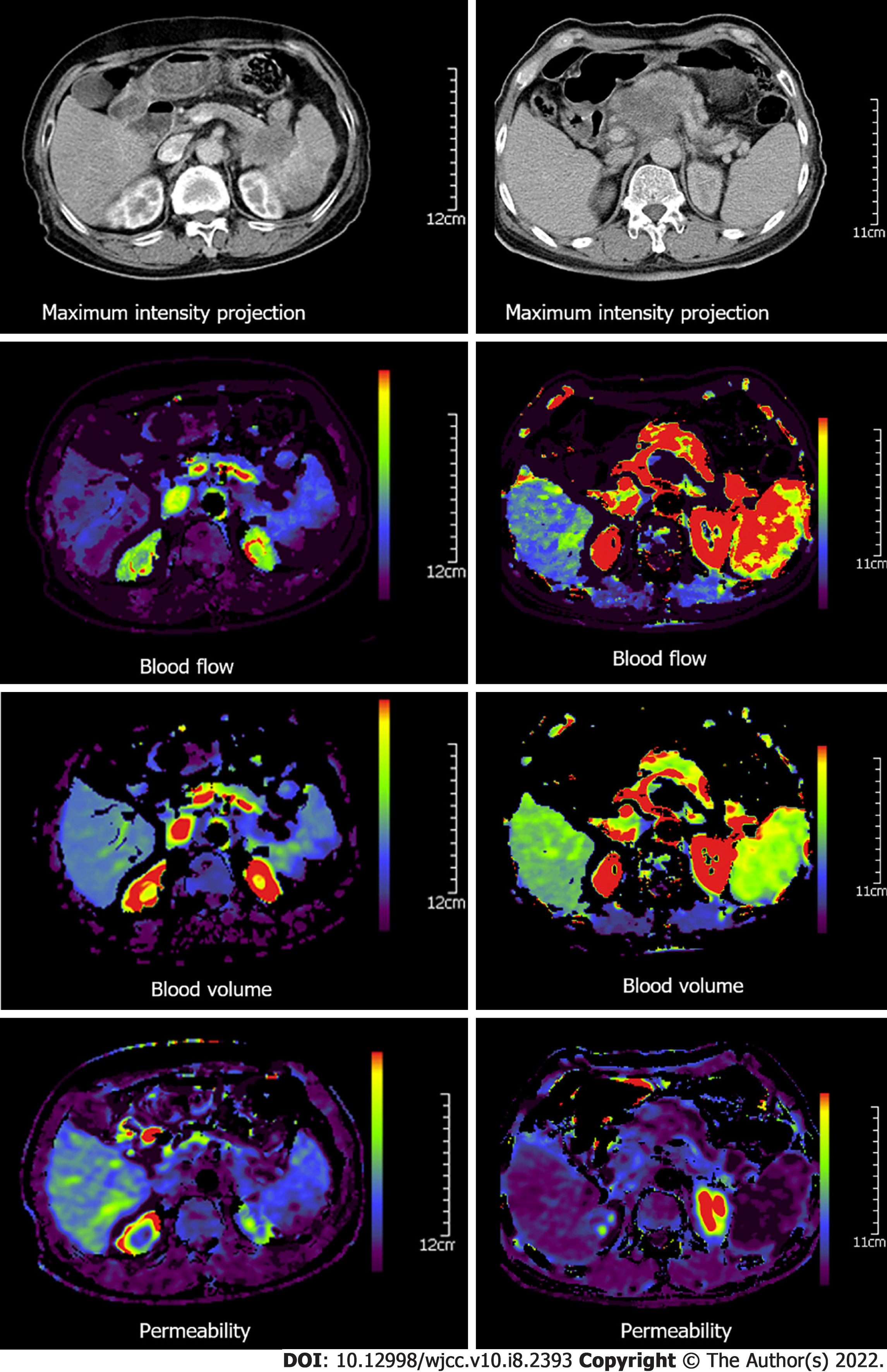
The obtained images were independently evaluated by two radiologists with more than 10 years’ experience. Dynamic CT perfusion data were analyzed by the pancreatic perfusion CT software package (Syngo, Siemens, Erlangen, Germany). Based on the maximum-slope method, color maps of CT perfusion parameters, including blood flow (BF), blood volume (BV), permeability, time to peak and mean transit time, maximum-density-projection and contrast-enhanced CT images were extracted. Furthermore, regions of interest (ROIs) were positioned on the highest intensity projection of tumor parenchyma to avoid selecting the vascular or the necrotic areas, and normal pancreas tissue in patients with pancreatic adenocarcinoma. For large heterogeneous tumors, the average value of three ROIs in the tumor parenchyma was used. In the control group, ROIs were located on the pancreatic head and cauda. Each CT perfusion parameter was measured three times, and the mean values were used. Relative CT perfusion parameters, including relative BF (rBF), relative BV (rBV), relative permeability (rPermeability), relative peak enhancement (rPE), and relative time to peak (rTTP) were calculated as follows: Relative CT perfusion parameters = parameters of pancreatic adenocarcinoma tumor parenchyma/parameters of adjacent relatively normal pancreatic tissue. The relative CT perfusion parameters in the controls were calculated as parameters of the pancreatic head/parameters of the pancreatic cauda.
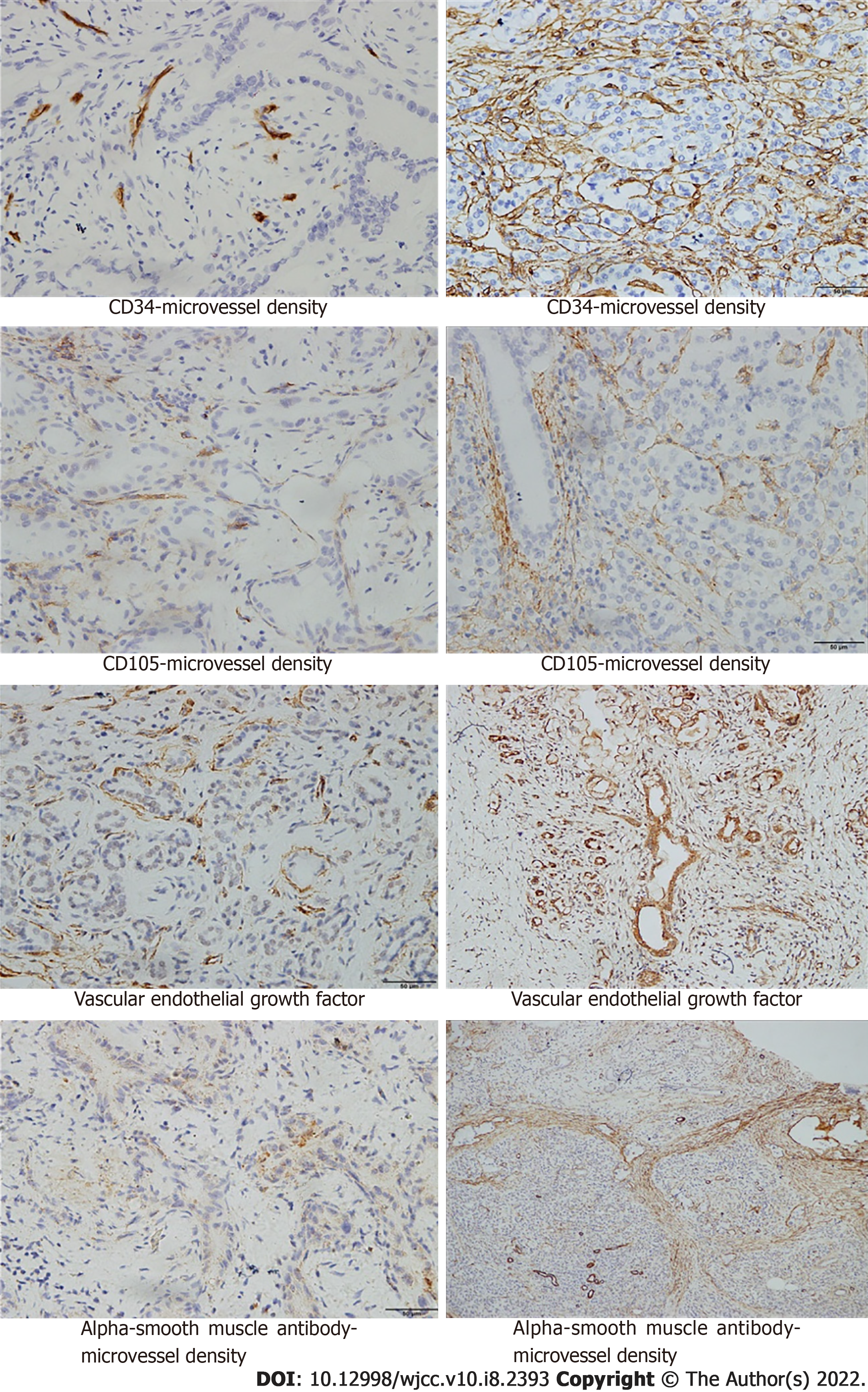
Tumor specimens were resected and the expression of vascular endothelial growth factor (VEGF), CD105, CD34, and alpha-smooth muscle actin (α-SMA) was detected by immunohistochemical staining. Yellow and brown yellow were used to indicate positive cells on the premise of excluding non-specific staining. Five random visual fields were selected at high magnification, and 100 cells in each visual field of each section were observed. Microvascular density (MVD) was determined by counting the total number of positive vessel walls in each tumor section. MVD was then graded using a scale of 0-5: 0 point, the proportion of chromogenic cells was less than 5%; 1 point, chromogenic cells ranged from 5% to 25%; 2 points, chromogenic cells ranged from 25% to 50%; 3 points, chromogenic cells ranged from 50% to 75%; 4 points, the proportion of chromogenic cells was more than 75%; 5 points, all cells were positive. Angiogenesis rate (AR) and vascular maturity index (VMI) are important indicators of tumor angiogenesis. AR was calculated using the following formula: AR = (CD105-MVD/CD34-MVD) × 100%. VMI was calculated according to the formula: VMI = (α-SMA-MVD/CD34-MVD) × 100%.
Demographic characteristics including age, gender, and tumor grade were collected at enrollment.
Continuous data conforming to a normal distribution are expressed as mean ± standard deviation (SD). Continuous data with non-normal distribution are presented as median (interquartile range, IQR); these data were analyzed using the independent t-test or Mann-Whitney U-test where appropriate. Categorical data are presented as count (percentage) and compared using the χ2 test. Pearson correlation coefficients were employed to assess the correlations between relative CT perfusion parameters and immunohistochemical indices. Statistical analysis was performed using the SPSS 17.0 package (SPSS Inc., Chicago, IL, United States), and two-tailed P < 0.05 was considered statistically significant.
A total of 68 subjects were enrolled in our analysis: 35 cases (17 males, age range 46-79 years, 25 cases with grade I-II, 10 cases with grade III-IV) in the pancreatic adenocarcinoma group and 33 cases (20 males, age range 28-68 years) in the control group. The rBV, rBF, and rPE values of the tumor parenchyma in patients with pancreatic adenocarcinoma were significantly lower than those in the control group (P < 0.01), and the rTTP and rPermeability values of the tumor parenchyma were significantly higher than those of the controls (P < 0.01) (Table 1).
| Pancreatic adenocarcinoma (n = 35) | Controls (n = 33) | P value | |
| Age (yr) | 61.5 (46-79) | 48 (28-68) | < 0.001 |
| Gender | |||
| Male | 17 (48.6%) | 20 (60.6%) | 0.342 |
| Female | 18 (51.4%) | 13 (39.4%) | |
| rBF | 0.222 ± 0.089 | 1.000 ± 0.023 | < 0.001 |
| rBV | 0.453 ± 0.193 | 0.993 ± 0.076 | < 0.001 |
| rPE | 0.576 ± 0.278 | 1.003 ± 0.008 | < 0.001 |
| rPermeability | 6.000 ± 1.395 | 0.949 ± 0.165 | < 0.001 |
| rTTP | 1.917 ± 0.208 | 1.014 ± 0.039 | < 0.001 |
In addition, the relative CT perfusion parameters of patients with grade I-II pancreatic adenocarcinoma (n = 25) and those with grade III-IV pancreatic adenocarcinoma (n = 10) (P < 0.01) were significantly different. RBF and rBV values were significantly lower in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.01) (Table 2). Patients with pancreatic adenocarcinoma had a lower density on the maximum-density projection images, as well as lower values of blood flow, blood volume, and permeability than those of the adjacent relatively normal pancreatic tissue and those in the control group (Figures 1 and 2).
| Grade I-II pancreatic adenocarcinoma (n = 25) | Grade III-IV pancreatic adenocarcinoma (n = 10) | P value | |
| rBF | 0.266 ± 0.057 | 0.111 ± 0.042 | < 0.001 |
| rBV | 0.546 ± 0.127 | 0.223 ± 0.123 | < 0.001 |
| rPE | 0.586 ± 0.265 | 0.552 ± 0.321 | 0.750 |
| rPermeability | 5.841 ± 1.413 | 6.393 ± 1.336 | 0.297 |
| rTTP | 1.919 ± 0.208 | 1.911 ± 0.218 | 0.915 |
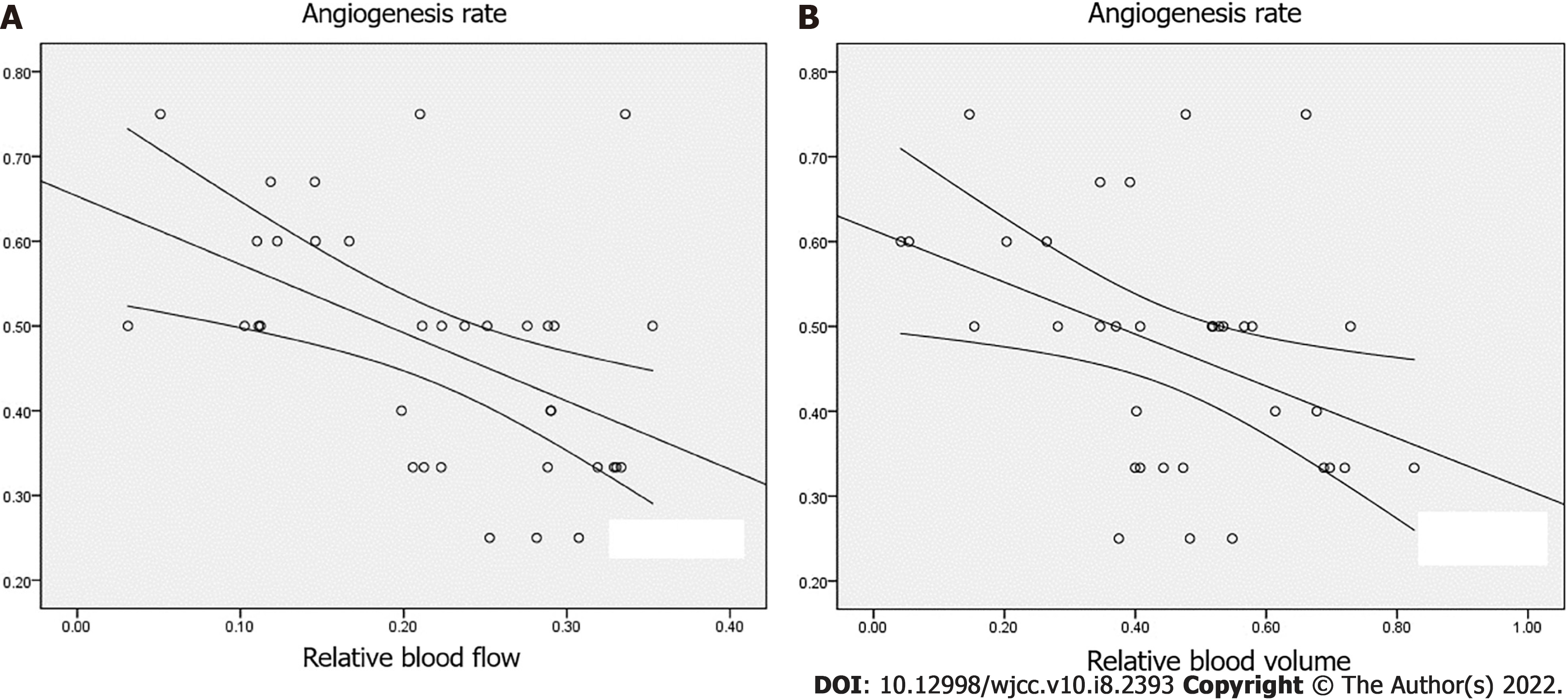
Additionally, VMI values were significantly lower in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). VEGF, CD105-MVD, CD34-MVD, and AR showed significantly higher values in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). However, no significant difference was observed in (α-SMA)-MVD between grade I-II pancreatic adenocarcinoma and grade III-IV pancreatic adenocarcinoma (P > 0.05) (Figure 3). Furthermore, the levels of VEGF, CD105-MVD, and CD34-MVD were significantly higher in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma. No significant difference was found in (α-SMA)-MVD between grade I-II pancreatic adenocarcinoma and grade III-IV pancreatic adenocarcinoma. A significant correlation was detected between rBF and VEGF, CD105-MVD, AR, and VMI (P < 0.01) and between rBV and VEGF, CD105-MVD, CD34-MVD, and VMI (P < 0.01). There was a moderate correlation between rBV and AR (r = -0.412, P < 0.05), and CD34-MVD (r = -0.407, P < 0.05), as depicted in Table 3 and Figure 4A and 4B. No significant correlations were observed between rPer
In the present study, we found a correlation between the relative CT perfusion parameters and the immunohistochemical indicators. These findings indicate that CT perfusion parameters may be a useful noninvasive tool for pancreatic adenocarcinoma diagnosis.
CT perfusion imaging is performed on the basis of the central volume principle by monitoring the first pass of a bolus of iodinated contrast agent through the cerebral vasculature[16-19]. The quantitative parameters from perfusion CT can reflect the pancreatic tissue vascularity directly and can thus be utilized as a tool for detecting disturbance of the pancreatic microcirculation[20]. The relative CT perfusion parameters are beneficial for the reduction of the individual differences in pancreatic perfusion. The results of this investigation indicated that relative CT perfusion quantitative parameters may be valuable for detecting disturbances in the pancreatic microcirculation in pancreatic adenocarcinoma.
Here, we found that the rBF and rBV values in patients with pancreatic adenocarcinoma were lower than those in the controls. The rBF and rBV values in grade III-IV pancreatic adenocarcinoma were significantly lower than those in grade I-II pancreatic adenocarcinoma. Considering that the rBF and rBV values could reveal blood perfusion in pancreatic adenocarcinoma to some extent, we suggest that low rBF and rBV values may be associated with fibrosis and arteriolosclerosis in pancreatic adenocarcinoma. Therefore, rBF and rBV values could provide useful information for the evaluation of angiogenesis in patients with pancreatic adenocarcinoma.
VEGF, CD34, CD105, and AR are frequently used indicators to evaluate tumor angiogenesis. VEGF is critically involved in angiogenesis induction[7,21,22]. CD34 is a total vascular endothelial cell marker, which is present in the vast majority of the blood vessels in the tumor[23]. CD105 is a member of the transforming growth factor-β superfamily that participates in angiogenesis and maintaining vascularity, which is highly expressed in the endothelial cells of nascent tumor blood vessels and the vascular endothelial cells of the tumor margin. CD105 was considered an ideal target in tumor therapy for suppression of tumor angiogenesis[24]. CD105-MVD was found to be an independent prognostic marker for most solid tumors[25]. AR represents the percentage of CD105-MVD/CD34-MVD, reflecting the proportion of neovascularization. In the present study, we found that VEGF, CD105-MVD, CD34-MVD, and AR in grade III-IV pancreatic adenocarcinoma were significantly higher than those in grade I-II pancreatic adenocarcinoma, which is in accordance with the general characteristics of malignant tumors, that is, tumor angiogenesis is more pronounced in pancreatic adenocarcinoma with higher malignancy. Negative correlations were found between VEGF, CD105-MVD, AR, and rBF, as well as between VEGF, CD105-MVD, CD34-MVD, and rBV. These results might have been due to the decreased amount of residual pancreatic tissue.
Previous results demonstrated that VMI played a major role in tumor blood supply[26]. Our results showed that VMI was significantly lower in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). A positive correlation was observed between rBV, rBF, and VMI. These results could be attributed to larger quantities of mature vessels in grade I-II pancreatic adenocarcinoma than in grade III-IV pancreatic adenocarcinoma[27]. It was reported that the percentage of tumor vessels with function was less than 5% and absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlated with hematogenous metastasis and prognosis of the neoplasm[28]. Accumulating evidence has shown that rBF and rBV correlate with angiogenesis markers to some extent; however, further research is required to confirm these findings.
The relative permeability of the tumor tissue in patients with pancreatic adenocarcinoma was higher than that in normal controls, which is similar to previously reported findings[16,29]. Furthermore, this outcome is consistent with the influence of the increased immature neovascularization in pancreatic adenocarcinoma. The incomplete endothelium of immature tumor vessels augmented the permeability of the blood vessel walls. However, certain controversies have been reported. For example, Ho et al[20] found no significant difference between the permeability of pancreatic adenocarcinoma and that of normal tissues. Additionally, Matsusaki et al[30] reported that the permeability of tumor tissue in patients with pancreatic adenocarcinoma was lower than that in normal controls. Perhaps these results were associated with the existence of fibrosis and sclerosis in pancreatic adenocarcinoma.
This study is not without limitations. The sample size was relatively small, and thus a future larger study is warranted to confirm the present results. In addition, there may be selection bias due to the single center design of our investigation despite our attempts to consecutively include potential subjects for analysis.
In conclusion, the rBF and rBV values of pancreatic adenocarcinoma are correlated with the immunohistochemistry indices of angiogenesis to a certain extent. These findings suggest that perfusion CT imaging may be an appropriate technique for quantitative assessments of pancreatic adenocarcinoma microvasculature.
Pancreatic adenocarcinoma is one of the most common malignant tumors of the digestive system. More than 80% of patients with pancreatic adenocarcinoma are not diagnosed until late stage and have distant or local metastases.
To investigate the value of computed tomography (CT) perfusion imaging in the evaluation of angiogenesis in pancreatic adenocarcinoma patients.
To investigate the value of computed tomography (CT) perfusion imaging in the evaluation of angiogenesis in pancreatic adenocarcinoma patients.
This is a retrospective cohort study. Patients with pancreatic adenocarcinoma and volunteers without pancreatic diseases underwent CT perfusion imaging from December 2014 to August 2017 in Huashan Hospital, Fudan University Shanghai, China.
A total of 35 pancreatic adenocarcinoma patients and 33 volunteers were enrolled. The relative blood flow (rBF), and relative blood volume (rBV) were significantly lower in patients with pancreatic adenocarcinoma than in the control group (P < 0.05). Conversely, the relative permeability in patients with pancreatic adenocarcinoma was significantly higher than that in controls (P < 0.05). In addition, rBF, rBV, and the vascular maturity index (VMI) were significantly lower in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). Vascular endothelial growth factor (VEGF), CD105-MVD, CD34-MVD, and angiogenesis rate (AR) were significantly higher in grade III-IV pancreatic adenocarcinoma than in grade I-II pancreatic adenocarcinoma (P < 0.05). Significant correlations between rBF and VEGF, CD105-MVD, AR, and VMI (P < 0.01) were observed. Moreover, the levels of rBV were statistically significantly correlated with those of VEGF, CD105-MVD, CD34-MVD, and VMI (P < 0.01).
Perfusion CT imaging may be an appropriate approach for the quantitative assessment of tumor angiogenesis in pancreatic adenocarcinoma.
Further research on perfusion CT imaging for quantitative assessment of tumor angiogenesis in pancreatic adenocarcinoma is warranted.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Naserian S, Setiawati R, Shamseldeen AA S-Editor: Wang LL L-Editor: Webster JR P-Editor: Wang LL
| 1. | Nishikawa Y, Tsuji Y, Isoda H, Kodama Y, Chiba T. Perfusion in the tissue surrounding pancreatic cancer and the patient's prognosis. Biomed Res Int. 2014;2014:648021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 859] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 3. | Iordache S, Angelescu R, Filip MM, Costache MI, Popescu CF, Gheonea DI, Sãftoiu A. Power Doppler endoscopic ultrasound for the assessment of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2012;1:150-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Kim SI, Shin JY, Park JS, Jeong S, Jeon YS, Choi MH, Choi HJ, Moon JH, Hwang JC, Yang MJ, Yoo BM, Kim JH, Lee HW, Kwon CI, Lee DH. Vascular enhancement pattern of mass in computed tomography may predict chemo-responsiveness in advanced pancreatic cancer. Pancreatology. 2017;17:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Longo V, Brunetti O, Gnoni A, Cascinu S, Gasparini G, Lorusso V, Ribatti D, Silvestris N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget. 2016;7:58649-58658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Craven KE, Gore J, Korc M. Overview of pre-clinical and clinical studies targeting angiogenesis in pancreatic ductal adenocarcinoma. Cancer Lett. 2016;381:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Georgiadou D, Sergentanis TN, Sakellariou S, Filippakis GM, Zagouri F, Vlachodimitropoulos D, Psaltopoulou T, Lazaris AC, Patsouris E, Zografos GC. VEGF and Id-1 in pancreatic adenocarcinoma: prognostic significance and impact on angiogenesis. Eur J Surg Oncol. 2014;40:1331-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388:518-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 631] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 9. | Delrue L, Blanckaert P, Mertens D, Van Meerbeeck S, Ceelen W, Duyck P. Tissue perfusion in pathologies of the pancreas: assessment using 128-slice computed tomography. Abdom Imaging. 2012;37:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Grözinger G, Grözinger A, Horger M. The role of volume perfusion CT in the diagnosis of pathologies of the pancreas. Rofo. 2014;186:1082-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ha J, Choi SH, Byun JH, Kim KW, Kim SY, Kim JH, Kim HJ. Meta-analysis of CT and MRI for differentiation of autoimmune pancreatitis from pancreatic adenocarcinoma. Eur Radiol. 2021;31:3427-3438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Xie Y, Huang H, Guo J, Zhou D. Relative cerebral blood volume is a potential biomarker in late delayed radiation-induced brain injury. J Magn Reson Imaging. 2018;47:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kameda K, Uno J, Otsuji R, Ren N, Nagaoka S, Maeda K, Ikai Y, Gi H. Optimal thresholds for ischemic penumbra predicted by computed tomography perfusion in patients with acute ischemic stroke treated with mechanical thrombectomy. J Neurointerv Surg. 2018;10:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Payabvash S, Oswood MC, Truwit CL, McKinney AM. Acute CT perfusion changes in seizure patients presenting to the emergency department with stroke-like symptoms: correlation with clinical and electroencephalography findings. Clin Radiol. 2015;70:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Smitha KA, Gupta AK, Jayasree RS. Relative percentage signal intensity recovery of perfusion metrics—an efficient tool for differentiating grades of glioma. Br J Radiol. 2015;88:20140784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Chen A, Shyr MH, Chen TY, Lai HY, Lin CC, Yen PS. Dynamic CT perfusion imaging with acetazolamide challenge for evaluation of patients with unilateral cerebrovascular steno-occlusive disease. AJNR Am J Neuroradiol. 2006;27:1876-1881. [PubMed] |
| 17. | Lu N, Feng XY, Hao SJ, Liang ZH, Jin C, Qiang JW, Guo QY. 64-slice CT perfusion imaging of pancreatic adenocarcinoma and mass-forming chronic pancreatitis. Acad Radiol. 2011;18:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Lu N, Di Y, Feng XY, Qiang JW, Zhang JW, Wang YG, Guo QY. Comparison between acetazolamide challenge and 10% carbon dioxide challenge perfusion CT in rat C6 glioma. Acad Radiol. 2012;19:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lu N, Di Y, Feng XY, Qiang JW, Zhang JW, Wang YG, Liu Y. CT perfusion with acetazolamide challenge in C6 gliomas and angiogenesis. PLoS One. 2015;10:e0121631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Li HO, Sun C, Xu ZD, Miao F, Zhang DJ, Chen JH, Li X, Wang XM, Liu C, Zhao B. Low-dose whole organ CT perfusion of the pancreas: preliminary study. Abdom Imaging. 2014;39:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Zhou R, Curry JM, Roy LD, Grover P, Haider J, Moore LJ, Wu ST, Kamesh A, Yazdanifar M, Ahrens WA, Leung T, Mukherjee P. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene. 2016;35:5608-5618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Costache MI, Iordache S, Costache CA, Dragos E, Dragos A, Saftoiu A. Molecular Analysis of Vascular Endothelial Growth Factor (VEGF) Receptors in EUS-guided Samples Obtained from Patients with Pancreatic Adenocarcinoma. J Gastrointestin Liver Dis. 2017;26:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Miyata Y, Mitsunari K, Asai A, Takehara K, Mochizuki Y, Sakai H. Pathological significance and prognostic role of microvessel density, evaluated using CD31, CD34, and CD105 in prostate cancer patients after radical prostatectomy with neoadjuvant therapy. Prostate. 2015;75:84-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Huang YK, Liu H, Wang XZ, Zhu S. Annexin A2 and CD105 expression in pancreatic ductal adenocarcinoma is associated with tumor recurrence and prognosis. Asian Pac J Cancer Prev. 2014;15:9921-9926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Zhou L, Yu L, Ding G, Chen W, Zheng S, Cao L. Overexpressions of DLL4 and CD105 are Associated with Poor Prognosis of Patients with Pancreatic Ductal Adenocarcinoma. Pathol Oncol Res. 2015;21:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | D'Onofrio M, Gallotti A, Mantovani W, Crosara S, Manfrin E, Falconi M, Ventriglia A, Zamboni GA, Manfredi R, Pozzi Mucelli R. Perfusion CT can predict tumoral grading of pancreatic adenocarcinoma. Eur J Radiol. 2013;82:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Karamysheva AF. Mechanisms of angiogenesis. Biochemistry (Mosc). 2008;73:751-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 28. | Gilead A, Meir G, Neeman M. The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Cancer. 2004;108:524-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Yonenaga Y, Mori A, Onodera H, Yasuda S, Oe H, Fujimoto A, Tachibana T, Imamura M. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncology. 2005;69:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Li Y, Li P, Jin M, Jiang C, Gao Z. Docetaxel-encapsulating small-sized polymeric micelles with higher permeability and its efficacy on the orthotopic transplantation model of pancreatic ductal adenocarcinoma. Int J Mol Sci. 2014;15:23571-23588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |