Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2315
Peer-review started: October 9, 2021
First decision: October 27, 2021
Revised: October 30, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: March 6, 2022
Processing time: 144 Days and 2.7 Hours
Granulocytic sarcoma (GS) is a rare malignant tumor, and relapse is even rarer in the breast and dorsal spine following allogeneic hematopoietic stem cell transplantation. Currently, a standard treatment regimen is not available.
A rare case of GS of the right breast and dorsal spine after complete remission of acute myelogenous leukemia is reported here. A 55-year-old female patient presented with a palpable, growing, painless lump as well as worsening dorsal compressive myelopathy. She had a history of acute myelomonocytic leukemia (AML M4) and achieved complete remission after chemotherapy following allogeneic hematopoietic stem cell transplantation. Imaging examinations showed the breast lump and C7-T1 epidural masses suspected of malignancy. Histologic results were compatible with GS in both the right breast and dorsal spine, which were considered extramedullary relapse of the AML treated 4 years earlier.
A rare case of GS relapse following allogeneic hematopoietic stem cell tran
Core Tip: Granulocytic sarcoma is unusual, and relapse in the breast and dorsal spine after complete remission of acute myeloid leukemia is even rarer. Imaging examinations and histopathologic examinations are essential for the final diagnosis. We reviewed the associated literature and found that the survival rate of the relapse is poor. At present, more prospective studies and clinical trials are needed to standardize its treatment.
- Citation: Li Y, Xie YD, He SJ, Hu JM, Li ZS, Qu SH. Breast and dorsal spine relapse of granulocytic sarcoma after allogeneic stem cell transplantation for acute myelomonocytic leukemia: A case report. World J Clin Cases 2022; 10(7): 2315-2321
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2315.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2315
Granulocytic sarcoma (GS), also known as myeloid sarcoma, is an uncommon extramedullary malignant tumor of immature granulocytic cells[1]. GS is often associated with hematological malignancies, such as acute myeloid leukemia (AML), myelodysplastic syndrome, biphenotypic leukemia and chronic myelomonocytic leukemia, especially following allogeneic hematopoietic stem cell transplant (allo-HSCT)[2]. GS may occur at any site in the body but especially in skin and soft tissues, bones, lymph nodes and the gastrointestinal tract[3]. However, its simultaneous involvement of two organs, breast tissue and dorsal spine, after transplantation is uncommon[4,5].
Here, we report a rare case of a female patient with AML who presented with a painless lump in the right breast and complained of neck pain. The imaging findings of the lesions in the breast and dorsal spine have been described, and this is the first report showing the positron-emission tomography (PET)/magnetic resonance imaging (MRI) findings of GS in the breast and dorsal spine. The present case is extremely rare because the relapse after complete remission upon chemotherapy and allo-HSCT is rare. In addition, the involvement of two organs is unusual.
A 55-year-old female presented to The First Affiliated Hospital of Jinan University (Guangzhou, Guangdong, China) on March 4, 2021 with a palpable, painless mass in the right breast that had been present for 1 year.
The patient presented with a palpable mass on her right breast, which grew rapidly over a month. In addition, she had a 10-mo history of progressive neck pain and upper limb radiating pain with numbness and weakness in both arms.
The patient was diagnosed with AML M4 in our hospital on September 12, 2017 and achieved complete remission after induction chemotherapy followed by consolidation chemotherapy with idarubicin and cytarabine. In January 2018, allo-HSCT was performed, and it stabilized her condition.
No special notes.
Upon physical examination, a movable mass measuring 3.0 cm × 4.0 cm in diameter was palpated on the upper inner quadrant of the right breast, with no palpable axillary lymph nodes. The patient had severe neck pain, limited neck movement and forced postures on admission. Neck tenderness was obvious. Neck compression test, brachial plexus pulling test and bilateral Hoffman test were positive.
No special notes.
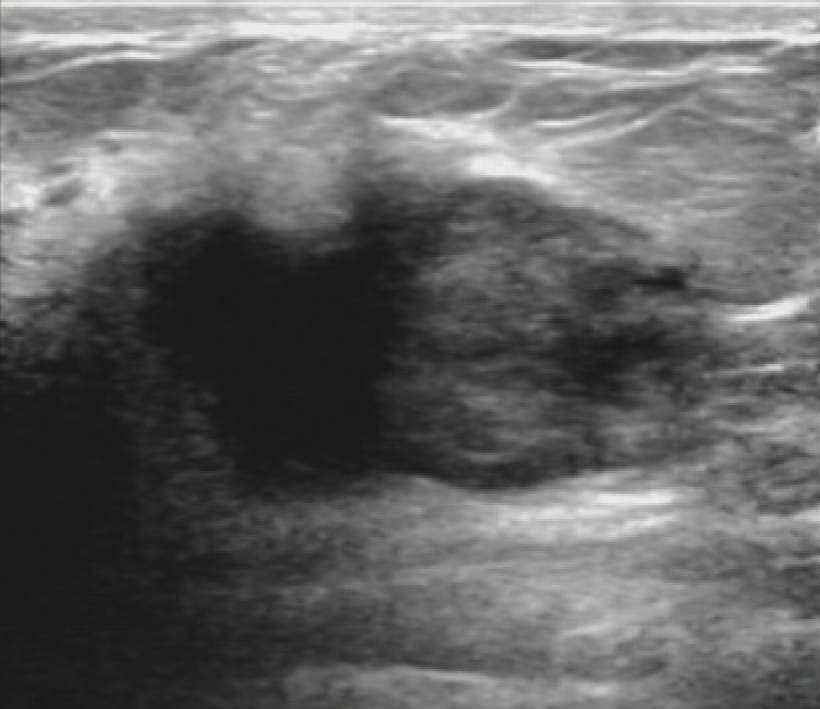
Sonographic images of the breasts showed a single, irregular, heterogeneous hypoechoic mass with ill-defined margins at the upper inner quadrant of the right breast measuring approximately 3.5 cm × 3.8 cm (Figure 1).
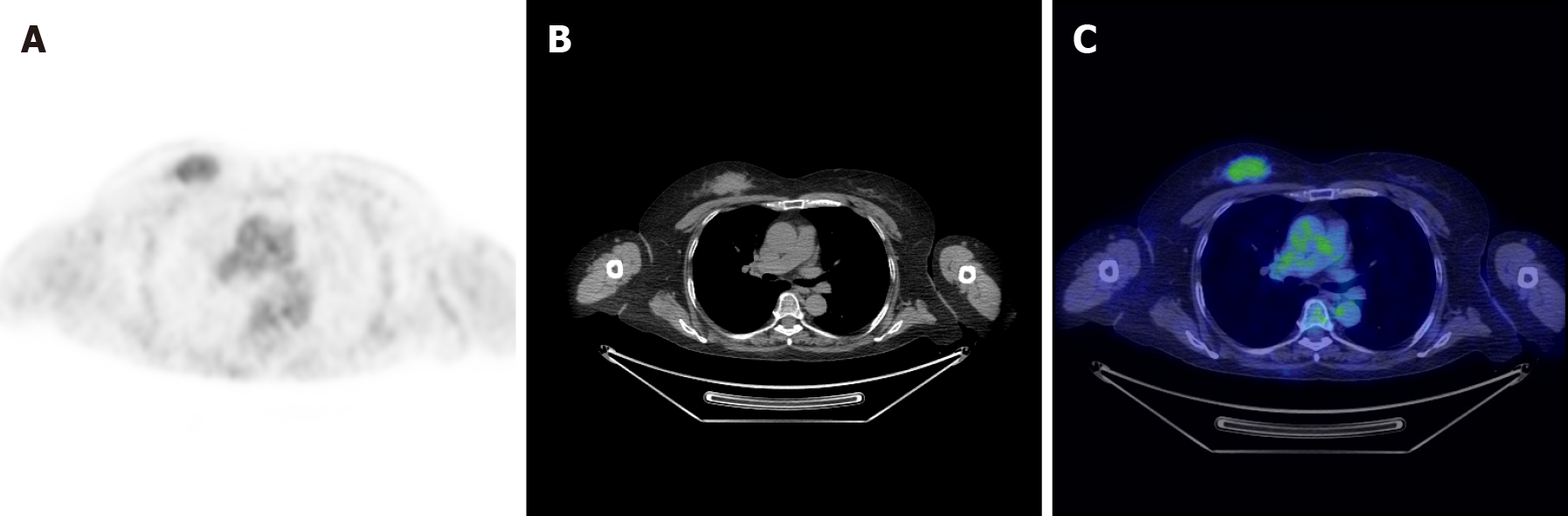
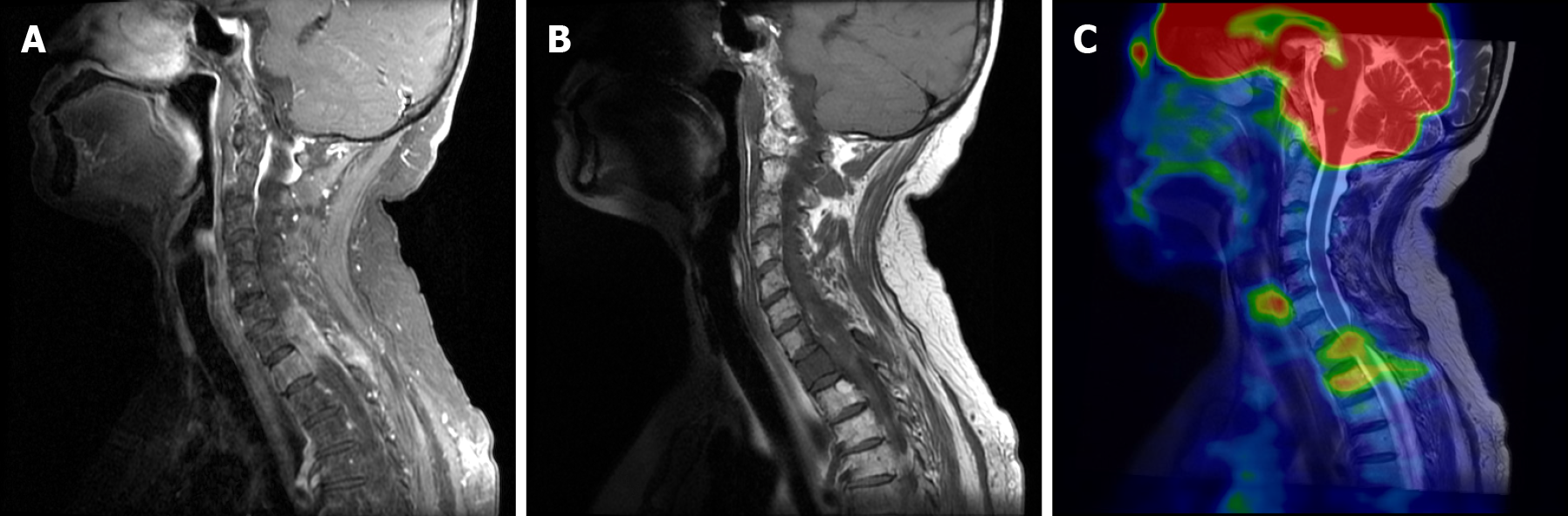
PET/MRI was performed and showed contrast enhancement of a mass on the upper inner quadrant of the right breast. The mass was hyperactive on T1-weighted and T2-weighted images, suggesting malignancy (Figure 2). The right side of the C7 vertebral body, T1 vertebral body and its accessories showed low signal on T1-weighted and T2-weighted images. Soft tissue shadow could be seen around the spinous process and protruded into the spinal canal, resulting in spinal stenosis and partial spinal cord compression (Figure 3).
Core-needle biopsy of the breast lesion was performed, and the patient underwent posterior C7-T2 Laminectomy, nerve root exploration and release, gross total removal of epidural site of lesion, C4-T3 posterolateral bone graft and pedicle screw rod internal fixation.
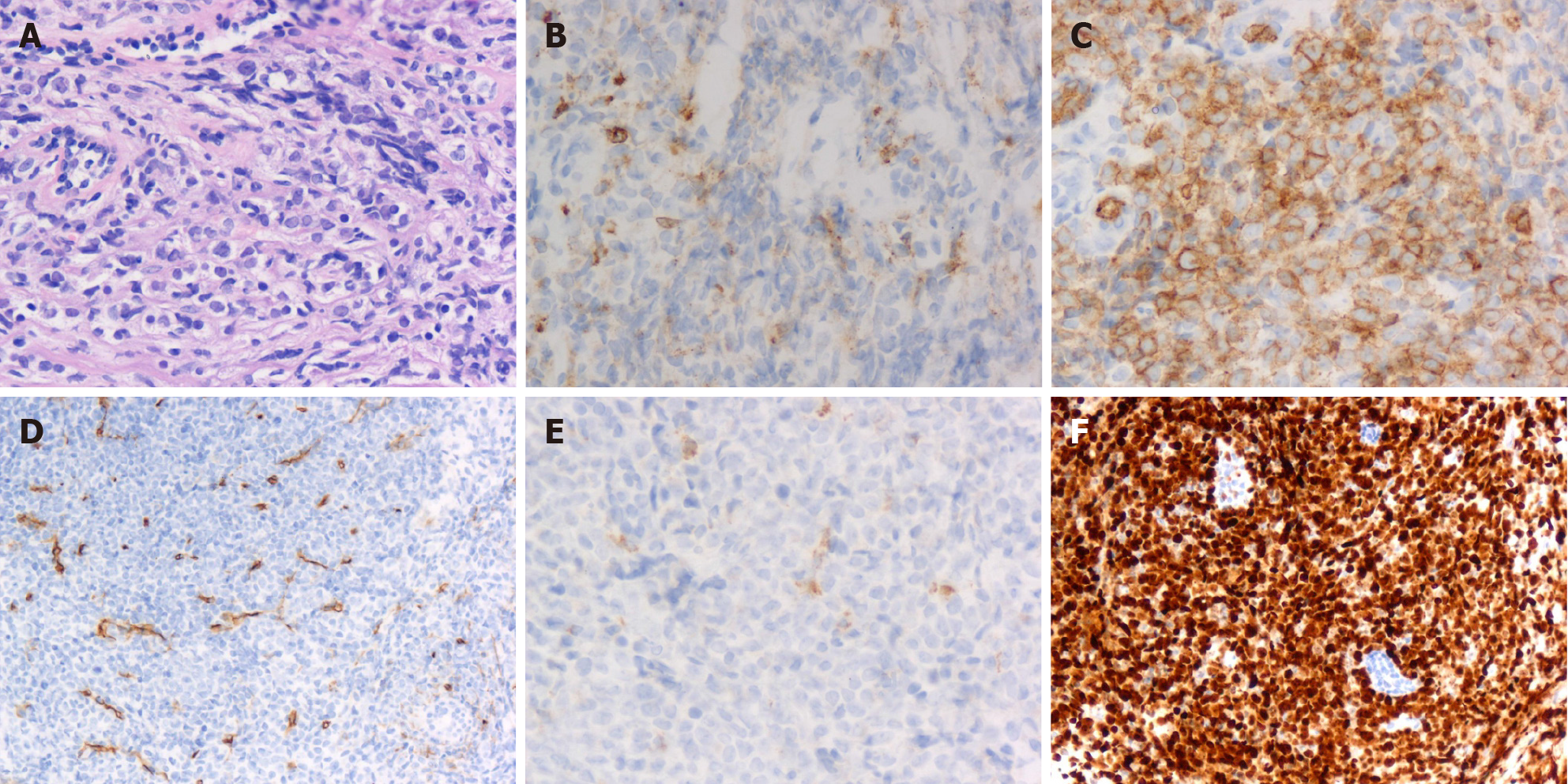
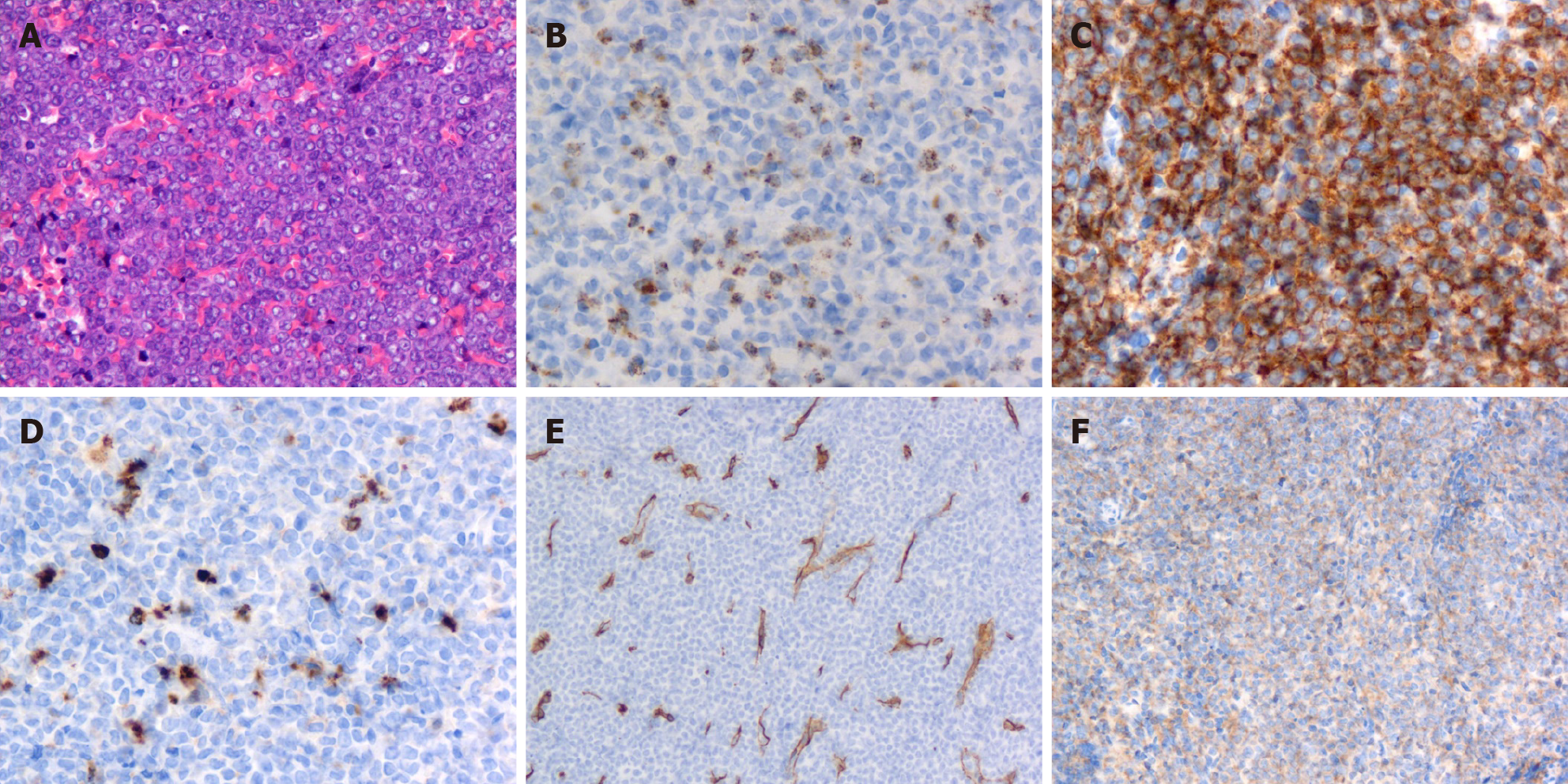
Postoperative histopathology was reported as GS of breast and dorsal spine after microscopic examination and immunohistochemistry. Histological analysis performed at our institution showed the breast tumor was infiltrated with medium-sized cells, finely dispersed chromatin and small distinct nucleoli with scant cytoplasm (Figure 4A). Immunohistochemical examination demonstrated that the tissue was positive for myeloperoxidase, CD117, CD34, CD68 (Figure 4B-E) and P120 and negative for estrogen receptor, progesterone receptor, c-ERBB2 and E-cadherin. The Ki67 proliferation index was approximately 80% (Figure 4F).
The pathological study of the dorsal spine revealed that the tumor cells were round-shaped, small to intermediate in size, with scant cytoplasm and small nucleoli (Figure 5A). According to the immunohistochemical study, the tumor cells showed strong immunoreactivity for myeloperoxidase (Figure 5B), CD117 (Figure 5C) and Ki67. Lysozyme (Figure 5D), CD34 (Figure 5E) and CD38 (Figure 5F) showed weak staining. Terminal deoxynucleotidyl transferase, Kappa, Lambda and CD3 were negative.
Combined with her medical history, the final diagnosis of the case was breast and dorsal spine relapse of GS after allo-HSCT for AML.
After the patient underwent the spinal surgery, we recommended mastectomy and postoperative chemotherapy, but she refused because of her financial circumstances.
The patient died on the 9th day after she was released from the hospital.
Allo-HSCT is an effective post-remission therapy for patients with AML. However, extramedullary relapse occurred after allo-HSCT, resulting in treatment failure and a poor outcome. The incidence of GS relapse after allo-HSCT for AML patients ranges from 0.65% to 9.00%[6], and it is the main cause of death in AML patients. The prognosis after GS relapse is poor, and effective strategies are needed to improve outcomes after transplantation.
Granulocytic sarcoma relapse following allo-HSCT of AML is extremely rare[7]. A European retrospective analysis reported that GS occurred 4-56 mo after HSCT in less than 1% of transplanted patients[8]. GS relapse may occur in any part of the body. The Mayo Clinic reported that only 3% of cases were localized to the breast[8]. The combined involvement of breast and dorsal spine following allo-HSCT treatment is even rarer[9]. To our knowledge, the present case is the first report that described the simultaneous involvement of the breast and dorsal spine following HSCT.
Breast GS is easy to be misdiagnosed as breast carcinoma or lymphoma due to its rare incidence and nonspecific manifestations. The patients often notice a painless, palpable mass in the breast through self-examination. In the present case, the patient exhibited a rapidly growing mass but no other associated symptoms, such as nipple discharge or skin involvement.
Similarly, imaging findings are nonspecific. Previous reports revealed that ultrasonic manifestations of GS lesions were mostly low echoic induced by rich blood flow with well-defined or ill-defined margins[10]. Most mammogram cases may report noncalcified masses with variable size and ill-defined borders[11]. MRI normally shows GS as multiple, ill-defined, heterogeneous and hyperintense masses on T2-weighted images but shows iso- to hypointense on T1-weighted images[12,13]. Unfortunately, MR images do not have characteristic findings of GS either.
MRI findings of the dorsal spine show slightly enhanced extradural masses on T1-weighted images but show intermediate signal intensity on T2-weighted images. Spinal involvement by GS to the extent of producing symptoms of compressive myelopathy are uncommon and limited to case reports.
Single tissue sites of GS have been reported, but the multiple tissue sites of GS have rarely been reported in the medical literature. PET-computed tomography (CT) is more effective in the detection of multiple sites of GS than other diagnostic tools, such as CT or MRI. PET/MRI is a relatively novel technology that combines the anatomic and quantitative strengths of MRI with physiologic information obtained from PET. Compared to PET-CT scans, PET/MRI exposes patients to less radiation. Furthermore, PET/MRI is more effective in studying soft tissues of the human body. Given the higher cost and complexity of operating and interpreting the studies obtained on a PET/MRI system, it has not been applied as widely as PET-CT. To our knowledge, the present case is the first report that describes the PET/MRI of multiple sites of GS.
To confirm the final diagnosis of GS, the pathological study, including histological and immunohistochemical findings are helpful. CD34, a monoclonal antibody directed against the surface glycoprotein on normal myeloid and mixed colony-forming cells, can be used to distinguish GS from malignant lymphoma and help recognize early myeloid cells. However, Mourad et al[14] considered myeloperoxidase to be more sensitive than CD34 because most GS cells are positive for myeloperoxidase. Other CD markers, such as CD13, CD117, CD68 and CD43, were also positive in most cases. Some epithelial markers, including CK7, MNF116, estrogen receptor, progesterone receptor and E-cadherin are often negative in GS cells but positive in normal tissue.
Optimal therapeutic approaches for GS after allo-HSCT have not been well defined, but options include chemotherapy, radiotherapy or a combination of the two. Recurrence after bone marrow transplantation is related to poor prognosis since recurrence reflects resistance or failure to respond to therapy[15]. As for the treatment of GS relapse, there is currently no consensus. Depending on the clinical situation, the treatment for GS may vary from chemotherapy to radiotherapy. However, the survival rate of GS relapse after allo-HSCT is very poor. In the present case, the patient died on the 9th day after hospital discharge.
GS relapse following allo-HSCT is uncommon. Experience in treatment of the disease is currently limited, and further study of combined-modality therapy is required. New prospective studies and clinical trials are needed to generate guidelines for the treatment of GS relapse following allo-HSCT.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Adam CA, Bairwa DBL S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, Bisceglia M, Ponzoni M, Gentile A, Rinaldi P, Franco V, Vincelli D, Pileri A Jr, Gasbarra R, Falini B, Zinzani PL, Baccarani M. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 447] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma. 2006;47:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Kara IO, Sahin B, Paydas S, Kara B. Granulocytic sarcoma of the heart: extramedullary relapse of acute myeloblastic leukemia after allogeneic stem cell transplantation successfully treated by chemotherapy alone. Leuk Lymphoma. 2005;46:1081-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Krishnan P, Bhattacharya C, Roy S; Devleena. Granulocytic sarcoma of the dorsal spine in an aleukemic patient. J Cancer Res Ther. 2015;11:1009-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Campidelli C, Agostinelli C, Stitson R, Pileri SA. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009;132:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Sun X, Rong X, Nie H, Yan X. Isolated retro-orbital granulocytic sarcoma relapse of Acute Myeloid Leukemia after allogeneic hematopoietic stem cell transplantation: a case report. Eur J Ophthalmol. 2020;1120672120976551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kim SJ, Hong WS, Jun SH, Jeong SH, Kang SY, Kim TH, Kang DK, Yim HE, Jung YS, Kim KS. Granulocytic sarcoma in breast after bone marrow transplantation. J Breast Cancer. 2013;16:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Bubulac L, Bardaş A, Popa DC, Vasilache ED, Ionescu BO, Coriu D, Varady Z, Dobrea CM. Breast myeloid sarcoma after allogeneic stem cell transplantation for acute myelomonocytic leukemia - case report. Rom J Morphol Embryol. 2019;60:707-711. [PubMed] |
| 9. | Balleari E, Panarello S, Capello E, Grosso M, Passalia C, Pitto P, Raggi F, Roccatagliata L, Cabiddu F, Ghio R. Granulocytic sarcoma: an unusual cause of spinal cord compression. Int J Clin Oncol. 2007;12:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Thachil J, Richards RM, Copeland G. Granulocytic sarcoma - a rare presentation of a breast lump. Ann R Coll Surg Engl. 2007;89:W7-W9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Kinoshita T, Yokokawa M, Yashiro N. Multicentric granulocytic sarcoma of the breast: mammographic, sonographic, and MR findings. Clin Imaging. 2006;30:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Fernandes Vieira V, Vo QD, Bouquet de la Jolinière J, Khomsi F, Feki A, Hoogewoud HM. Granulocytic Sarcoma Presenting as a Palpable Breast Lump. Front Surg. 2016;3:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Bakst RL, Tallman MS, Douer D, Yahalom J. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 14. | Mourad W, Kfoury H, Al Husseini H. The value of CD34, myeloperoxidase and chloroacetate esterase (Leder) stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med. 2001;21:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Seok JH, Park J, Kim SK, Choi JE, Kim CC. Granulocytic sarcoma of the spine: MRI and clinical review. AJR Am J Roentgenol. 2010;194:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |