Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2268
Peer-review started: July 21, 2021
First decision: October 16, 2021
Revised: October 27, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: March 6, 2022
Processing time: 224 Days and 11.4 Hours
Mixed neuroendocrine-nonneuroendocrine neoplasm (MiNEN) is a rare tumor that occurs in the gastrointestinal tract and pancreas, usually composed of adenocarcinoma and neuroendocrine carcinoma. MiNEN occurring in ampulla is even rarer. We report 4 cases of MiNEN in ampulla, combined with literature review to summarize the clinical features and treatment of the disease, in order to improve the understanding of the disease.
A retrospective analysis was performed in 4 cases of MiNEN of the ampulla diagnosed by pathology from 2014 to 2021. The 4 patients were all male, aged 67-81 years (average 72.25 years). Among them, 2 patients had jaundice, 1 patient had abdominal pain, and 1 patient had jaundice with abdominal pain as the first symptom. All 4 patients underwent enhanced CT or MRI, which all indicated that the tumors were located in the ampulla. Two patients underwent duodenoscopy, and a biopsy revealed ampullary adenocarcinoma. All 4 patients underwent radical pancreaticoduodenectomy. Four cases were followed up: One patient developed severe complications after the operation, his condition deteriorated, and he survived for 1 mo. In the other 3 patients, tumor recurrence was observed during follow-up, and 2 of them survived for 29 mo and 22 mo respectively. One case survived and is still being followed up.
MiNEN of the ampulla are extremely rare, lacking typical clinical symptoms and imaging features, and are usually diagnosed after postoperative histopathological and immunohistochemical examinations. The main treatment is radical surgical resection, which can be combined with chemotherapy. The best method of diagnosis and treatment needs further research.
Core Tip: Mixed neuroendocrine-nonneuroendocrine neoplasm (MiNEN) is a rare tumor that occurs in the gastrointestinal tract and pancreas. MiNEN occurring in ampulla is extremely rare. We report 4 cases of MiNEN of the ampulla, summarizes these cases and reviews related literature.
- Citation: Wang Y, Zhang Z, Wang C, Xi SH, Wang XM. Mixed neuroendocrine-nonneuroendocrine neoplasm of the ampulla: Four case reports. World J Clin Cases 2022; 10(7): 2268-2274
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2268.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2268
According to the latest World Health Organization (WHO) classification of gastrointestinal tumors in 2019, tumors with nonneuroendocrine and neuroendocrine components are named mixed neuroendocrine-nonneuroendocrine neoplasms (MiNENs), and each component must account for at least 30% of the neoplasm composition[1]. A MiNEN of the digestive tract is a rare tumor that can be found in the pancreas, bile duct, stomach, colon and rectum, but a MiNEN that occurs in the ampulla is very rare[2]. This article reports the clinical data of 4 cases of MiNEN in the ampulla, reviews the relevant literature, and summarizes the clinical characteristics and treatment of the disease to improve the understanding of MiNEN in the ampulla.
The 4 cases were all male, and the age of onset was 67-81 years old. Patients 1 and 2 developed jaundice, patient 3 had abdominal pain, and patient 4 had both jaundice and abdominal pain and was admitted to the hospital.
Three of the patients were treated at the local hospital, but they did not respond to therapy. Therefore, they were admitted to our hospital for further treatment. Patient 4 did not receive treatment before surgery.
Patients 1 and 2 had no significant medical history, and patient 3 had a history of an open fracture of the right lower limb. Patient 4 had a history of hypertension, diabetes, and hepatitis C.
One patient had hypertension and diabetes. None of the patients had any family members that had similar diseases.
Patients 1, 2, and 4 developed jaundiced and varying degrees of anemia. Patients 1 and 4 developed epigastric tenderness. The rest of the examinations showed no obvious abnormalities.
None of the 4 patients had an increase in white blood cells, except for patient 3, who had varying degrees of anemia and an increase in total bilirubin. All patients had increased transaminase and decreased albumin. There were no abnormalities in the tumor indicators, alpha fetal protein and carcinogenic embryonic antigen, in any of the patients, and the tumor markers, carbohydrate antigen 199, of patients 1 and 3 were increased.
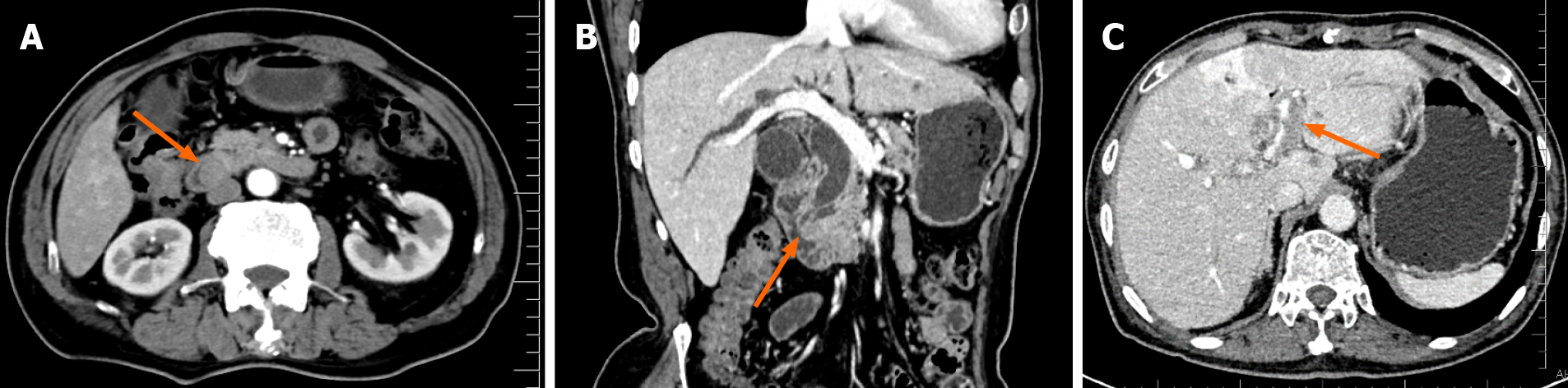
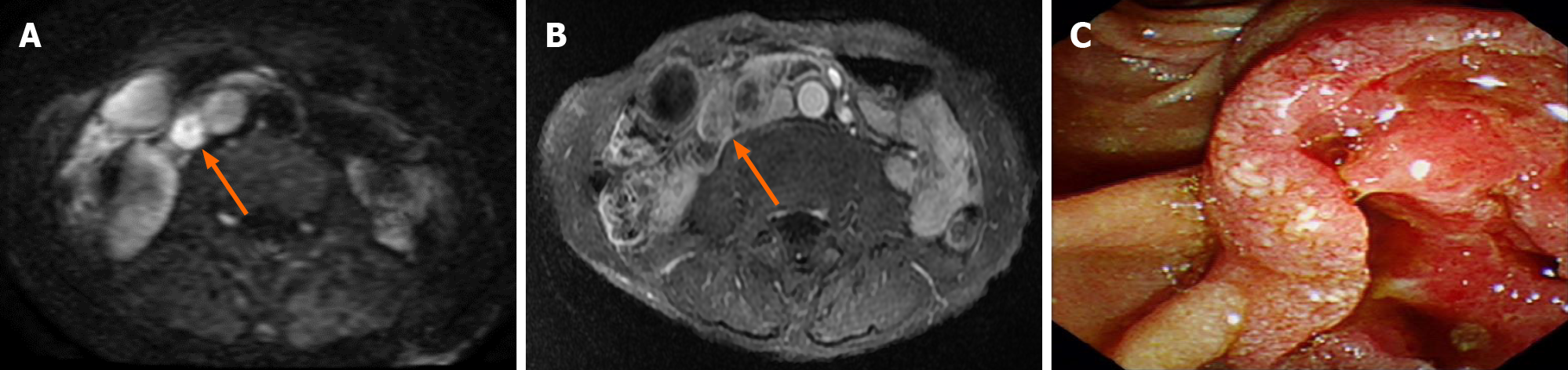
Enhanced computed tomography (CT) and magnetic resonance imaging (MRI) of the abdomen showed that all patients had lesions in the ampulla, which caused a dilation of the bile ducts and pancreatic ducts in and out of the liver. No distant metastases were found in the preoperative imaging examinations of any of the patients. Patients 2 and 3 underwent further duodenoscopy. The lesions were found in the ampulla, and both pathological biopsies revealed adenocarcinoma. Table 1 and Figures 1 and 2 show the characteristics of these ampullary lesions.
| Serial number | Image | Duodenal endoscopy and biopsy | Tumor markers | Ratio (%); ADC:NEN | Immunohisto-chemistry | Ki-67 |
| Patient 1 | Enhanced CT | No | CA19-9 rise; CEA, AFP normal | 30:70 | Component (NEN): CgA(-), Syn(+), CD56(+) | 30% |
| Patient 2 | Enhanced MRI | Yes, ADC | CA19-9, CEA, AFP normal | 60:40 | Component (NEN): CgA(+), Syn(+), CD56(+) | 50% |
| Patient 3 | Enhanced MRI | Yes, ADC | CA19-9 rise; CEA, AFP normal | 60:40 | Component (NEN): CgA(+/-), Syn(+), CD56(+) | 70% |
| Patient 4 | Enhanced CT | No | CA19-9, CEA, AFP normal | 40:60 | Component (NEN): CgA(-), Syn(+), CD56(+) | 70% |
All patients were diagnosed with MiNENs by postoperative pathology and immunohistochemistry. All sections were reread and diagnosed by pathologists, which further confirmed the histopathological diagnoses.
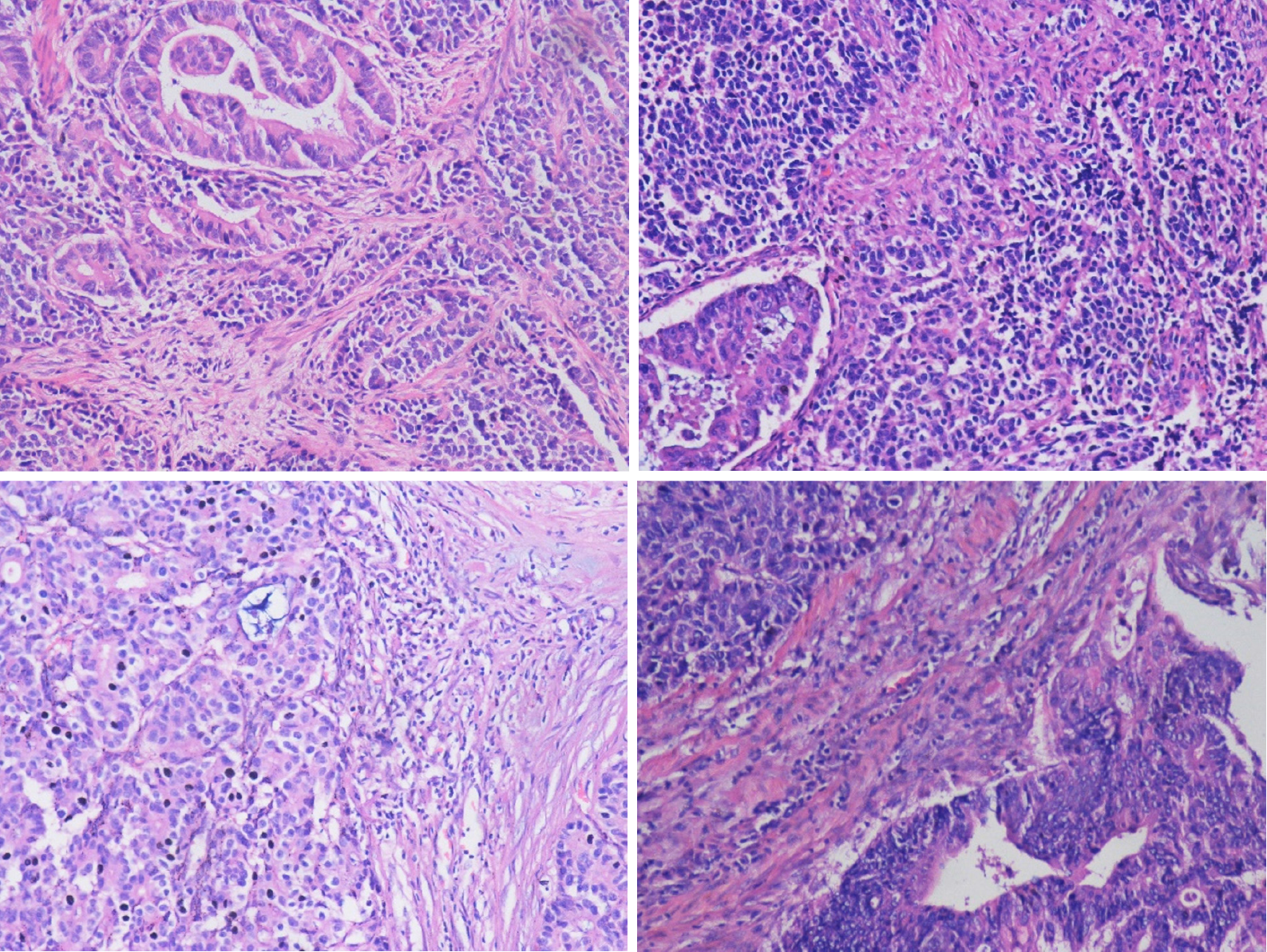
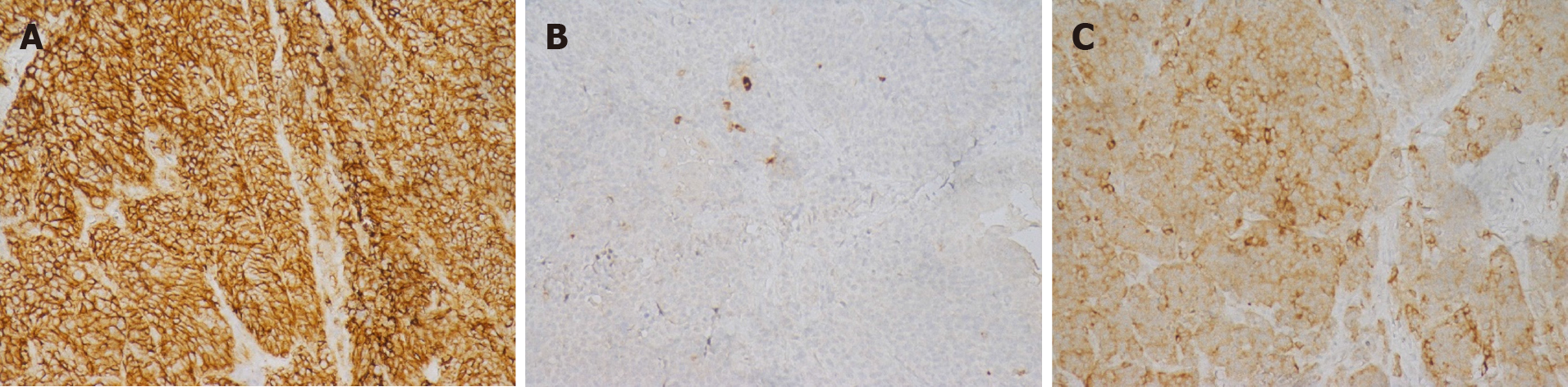
Four patients were hospitalized for surgical treatment, and all underwent radical pancreaticoduodenectomy. Patient 1 developed complications of pancreatic leakage, intestinal anastomotic leakage, abdominal cavity and lung infections and was discharged after refusing further treatment. Patient 2 developed infection after the operation and was discharged after symptomatic treatment improved the patient’s condition. Patients 3 and 4 had no perioperative complications. Pathologically confirmed MiNEN of the ampulla (Figure 3), and immunohistochemistry is shown in Figure 4. Patient 1 did not receive chemotherapeutic treatment postoperatively; Patient 2 received a total of 14 cycles of chemotherapy after surgery: 1 cycle of oxaliplatin + 5-fluorouracil + leucovorin, 6 cycles of etoposide + nedaplatin, and 7 cycles of irinotecan + nedaplatin. Patient 3 received a total of 7 cycles of chemotherapy: Capecitabine + oxaliplatin regimen. Patient 4 received hepatic arterial chemoembolization with cisplatin + etoposide when he developed a liver metastasis postoperatively.
All patients were followed up postoperatively. Patient 1 died of postoperative complications in the 1st postoperative month; Patient 2 developed tumor liver metastasis in the 22nd postoperative month and eventually died of systemic metastases in the 29th month; Patient 3 developed tumor liver metastasis in the 4th postoperative month and eventually died of systemic metastasis in the 22nd month; and patient 4 developed tumor liver metastasis in the 4th postoperative month and is currently still alive Table 2.
| Serial number | Sex | Age | First symptoms | Treatment | Chemo-therapy | Follow-up | Postoperative overall survival (mo) |
| Patient 1 | Male | 81 | Jaundice | Pancreatico-duodenectomy | No | Death | 1 |
| Patient 2 | Male | 69 | Jaundice | Pancreatico-duodenectomy | Yes | Death | 29 |
| Patient 3 | Male | 67 | Abdominal pain | Pancreatico-duodenectomy | Yes | Death | 22 |
| Patient 4 | Male | 72 | Jaundice;Abdominal pain | Pancreatico-duodenectomy | Yes | Survival | - |
In 2010, the WHO classification of gastrointestinal tumors defined tumors with exocrine and neuroendocrine components as mixed adenoneuroendocrine carcinoma (MANEC), and each component had to account for at least 30% of the tumor composition and being malignant. In 2017, the WHO classification of gastrointestinal tumors renamed MANEC in the pancreas to MiNEN. In the latest 2019 WHO classification of gastrointestinal tumors, the use of MiNEN has been expanded to all gastrointestinal and pancreatic tumors that meet the diagnostic criteria. Among them, the nonneuroendocrine component is mainly adenocarcinoma, and squamous cell carcinoma is rare. The neuroendocrine component is a neuroendocrine tumor or cancer, and each component must still account for at least 30% of the tumor[1,3]. At present, the pathogenesis in MiNEN's biphasic morphology is not clear. There are three main theories: the first theory suggests that two components are produced independently from different precursor cells in a synchronous or different time; the second theory suggests that the two components are from common pluripotent stem cells or naive cells; and the third theory suggests that the neuroendocrine differentiation develops from the original nonneuroendocrine cells[4].
MiNEN is a rare tumor commonly found in the appendix and colorectum in the digestive tract, while MiNENs that occur in the ampulla are very rare. The literature search found only 16 cases,and most of them were case reports[1,2,5]. Patients with a MiNEN of the ampulla usually present with nonspecific clinical symptoms, such as abdominal pain, jaundice, nausea, vomiting, and upper abdominal discomfort[6]. Jaundice and pain are the most common. Although some tumors secrete hormones, endocrine symptoms are rare or mild[7]. Auxiliary examinations such as CT, MRI, endoscopy and ultrasound can provide a certain value in the diagnosis, but the diagnosis mainly depends on postoperative histopathology and immunohistochemical examinations. Immunohistochemical examinations have showed that the neuroendocrine components CgA, Syn and CD56 were usually positive. To determine the neuroendocrine nature of the tumor, there must be two of the three commonly used neuroendocrine markers above[6,8]. It is also difficult to diagnose by duodenoscope tissue biopsy before surgery. Currently, only 4 cases of MiNEN in the ampulla have been diagnosed by biopsy. One of the histopathological features of MiNEN is that the mucosa usually contains adenocarcinoma, so it is most likely to be diagnosed as adenocarcinoma before surgery[2]. However, the use of fine needle aspiration for deep biopsy under ultrasound endoscopy combined with immunohistochemical examination can improve the accuracy of diagnosis[9].
At present, the best treatment for MiNEN is not clear[5]. It has been reported that for MiNEN without distant metastases, radical surgery can be the first choice for treatment, and palliative chemotherapy can be selected when the patient has distant metastases[10]. Regarding chemotherapy, the treatment target of MiNEN should be mainly toward the more aggressive tumor components. If the neuroendocrine components of MiNEN are well differentiated and the malignant behavior is benign or low-grade, then chemotherapy should focus on the more aggressive exocrine components. In contrast, in patients with neuroendocrine cancer, the neuroendocrine cancer will become the main target for treatment[11]. Among the reported cases of MiNEN in the ampulla, most cases undergo surgical radical resection (pancreaticoduodenectomy). Postoperative chemotherapy regimens are different, such as oxaliplatin-based combination chemotherapy and S-1, and chemotherapy for NEC components is usually recommended[2,5].Among the 3 patients undergoing chemotherapy in this study, the regimen was different from the previously reported regimen. After the tumor recurrence was observed in these patients, they were treated with chemotherapeutic drugs, and the reduction of metastases was observed, suggesting that it may be effective. Regarding the regimen and efficacy of chemotherapy, further research is needed in the future. We report 4 cases of MiNEN in the ampulla, summarize the clinical features and treatment of these cases, help to better understand the disease, and provide references for further research on the disease.
Studies have shown that the prognosis of MiNEN in the digestive tract is generally poor, with a median survival time of only 13.2 mo. and its prognosis depends on the low-differentiated neuroendocrine components. The Ki67 index is the main prognostic factor of MiNEN, and a percentage that higher than 55% indicates a poor prognosis[12]. Studies have also shown that the prognoses between MiNEN and pure NEC in the gastrointestinal tract are different and are related to the tumor site. Compared with pure NEC, MiNENs in the small intestine and appendix have a worse prognosis; however, there is no significant difference in survival rates between NEC and MiNEN in other parts of the gastrointestinal tract[13]. In our case, the Ki-67 index of 2 patients was greater than 55%, and distant metastases appeared earlier after surgery, suggesting a poor prognosis. However, there are only a few cases of MiNEN of the ampulla in this review, and the treatment measures taken after the operation are not the same, which can create certain limitations in this study. The prognosis of these tumors needs further study.
MiNEN of the ampulla are extremely rare, lacking typical clinical symptoms and imaging features, and are usually diagnosed after postoperative histopathological and immunohistochemical examinations. The main treatment is radical surgical resection, which can be combined with chemotherapy. In order to obtain an accurate preoperative diagnosis and the best treatment, further research on the disease is needed.
Manuscript source: Unsolicited Manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Yamanaka K S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Frizziero M, Chakrabarty B, Nagy B, Lamarca A, Hubner RA, Valle JW, McNamara MG. Mixed Neuroendocrine Non-Neuroendocrine Neoplasms: A Systematic Review of a Controversial and Underestimated Diagnosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (2)] |
| 2. | Yoshimachi S, Ohtsuka H, Aoki T, Miura T, Ariake K, Masuda K, Ishida M, Mizuma M, Hayashi H, Nakagawa K, Morikawa T, Motoi F, Kanno A, Masamune A, Fujishima F, Sasano H, Kamei T, Naitoh T, Unno M. Mixed adenoneuroendocrine carcinoma of the ampulla of Vater: a case report and literature review. Clin J Gastroenterol. 2020;13:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Huang YC, Yang NN, Chen HC, Huang YL, Yan WT, Yang RX, Li N, Zhang S, Yang PP, Feng ZZ. Clinicopathological features and prognostic factors associated with gastroenteropancreatic mixed neuroendocrine non-neuroendocrine neoplasms in Chinese patients. World J Gastroenterol. 2021;27:624-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Bazerbachi F, Kermanshahi TR, Monteiro C. Early precursor of mixed endocrine-exocrine tumors of the gastrointestinal tract: histologic and molecular correlations. Ochsner J. 2015;15:97-101. [PubMed] |
| 5. | Li X, Li D, Sun X, Lv G. Mixed adenoneuroendocrine carcinoma (MANEC) of the ampulla of Vater in a Chinese patient: A case report. J Int Med Res. 2020;48:300060520947918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Huang Z, Xiao WD, Li Y, Huang S, Cai J, Ao J. Mixed adenoneuroendocrine carcinoma of the ampulla: two case reports. World J Gastroenterol. 2015;21:2254-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 7. | Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol. 2014;42:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Brathwaite S, Rock J, Yearsley MM, Bekaii-Saab T, Wei L, Frankel WL, Hays J, Wu C, Abdel-Misih S. Mixed Adeno-neuroendocrine Carcinoma: An Aggressive Clinical Entity. Ann Surg Oncol. 2016;23:2281-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Yoshioka S, Ebisu Y, Ishida M, Uemura Y, Yanagimoto H, Satoi S, Tsuta K. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla of Vater: A case report with immunocytochemical analyses. Diagn Cytopathol. 2018;46:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Frizziero M, Wang X, Chakrabarty B, Childs A, Luong TV, Walter T, Khan MS, Morgan M, Christian A, Elshafie M, Shah T, Minicozzi A, Mansoor W, Meyer T, Lamarca A, Hubner RA, Valle JW, McNamara MG. Retrospective study on mixed neuroendocrine non-neuroendocrine neoplasms from five European centres. World J Gastroenterol. 2019;25:5991-6005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Yamamoto M, Ozawa S, Koyanagi K, Oguma J, Kazuno A, Ninomiya Y, Yatabe K, Hatanaka K. Mixed adenoneuroendocrine carcinoma of the esophagogastric junction: a case report. Surg Case Rep. 2018;4:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, Vanoli A, Tagliabue G, Pisa E, Messerini L, Centonze G, Inzani F, Scarpa A, Papotti M, Volante M, Sessa F, Fazio N, Pruneri G, Rindi G, Solcia E, La Rosa S, Capella C. Ki67 proliferative index of the neuroendocrine component drives MANEC prognosis. Endocr Relat Cancer. 2018;25:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 13. | Shi H, Qi C, Meng L, Yao H, Jiang C, Fan M, Pang S, Zhang Q, Lin R. Do neuroendocrine carcinomas and mixed neuroendocrine-non-neuroendocrine neoplasm of the gastrointestinal tract have the same prognosis? A SEER database analysis of 12,878 cases. Ther Adv Endocrinol Metab. 2020;11:2042018820938304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |