Published online Mar 6, 2022. doi: 10.12998/wjcc.v10.i7.2222
Peer-review started: July 3, 2021
First decision: July 14, 2021
Revised: July 15, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 6, 2022
Processing time: 241 Days and 19.7 Hours
Primary hepatic neuroendocrine neoplasm (NEN) is a rare condition, and it is difficult to differentiate between primary and metastatic hepatic NENs. Herein, we report a case of primary hepatic NEN that initially mimicked a hemangioma but showed a gradual increase in size on long-term careful observation.
A 47-year-old woman was incidentally diagnosed with a 12-mm liver mass, suspected to be a hemangioma. Since then, regular follow-up had been carried out. Ten years later, she was referred to our institute due to the tumor (located in segment 4) having increased to 20 mm. Several imaging studies depicted no apparent extrahepatic lesion. Positron emission tomography (PET)/computed tomography exhibited significant accumulation in the mass lesion, which made us consider the possibility of malignancy. Left hepatectomy was performed. The histopathological diagnosis was neuroendocrine tumor grade 2, with somatostatin receptor 2a/5 positivity. Postoperative somatostatin receptor scintigraphy (SRS) showed no other site, leading to the diagnosis of NEN of primary hepatic origin. The gradual growth of the hepatic NEN over 10 years suggested that it was likely to be a primary liver tumor.
In this case, positivity on PET and postoperative SRS may have helped determine whether the tumor was primary or metastatic.
Core Tip: The clinical diagnosis of primary hepatic neuroendocrine neoplasm (NEN) remains challenging due to its rarity and difficulty in differentiating between primary and metastatic NENs. We present a case of primary hepatic NEN that presented with exceedingly gradual growth over 10 years, initially mimicking a hemangioma. Close preoperative observation, positron emission tomography findings, and postoperative somatostatin receptor scintigraphy findings greatly contributed to the final diagnosis. This case highlights the importance of close preoperative observations of NENs. In addition, the clinical usefulness of these modalities for correct diagnosis has been suggested, although regular postoperative follow-up is required.
- Citation: Akabane M, Kobayashi Y, Kinowaki K, Okubo S, Shindoh J, Hashimoto M. Primary hepatic neuroendocrine neoplasm diagnosed by somatostatin receptor scintigraphy: A case report. World J Clin Cases 2022; 10(7): 2222-2228
- URL: https://www.wjgnet.com/2307-8960/full/v10/i7/2222.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i7.2222
Primary hepatic neuroendocrine neoplasm (NEN) is a rare condition with unclear clinical features. Obtaining a preoperative diagnosis of primary hepatic NEN can pose a challenge. In this report, we describe a case of hepatic NEN that initially mimicked hemangioma but gradually increased in size after close observation for 10 years. Immunohistological staining showed that the tumor cells were positive for somatostatin receptor (SSTR) 2a/5. Postoperative somatostatin receptor scintigraphy (SRS) revealed no other significant site, which supported the idea that the tumor was of primary hepatic origin with gradual tumor progression. This case report was written in accordance with the SCARE guidelines[1].
The patient had no complaints.
A 47-year-old Japanese woman underwent abdominal ultrasound for a medical check-up, which revealed a 12-mm hyperechoic hepatic mass in segment 4. Because the mass was suspected to be a hemangioma, the patient regularly underwent follow-up annual ultrasound examinations to assess its size. Ten years later, the mass had increased to 20 mm. The patient was then referred to our institute because the mass was suspected to be malignant.
The patient’s past medical history was not significant, except for uterine myomas.
The patient had no remarkable family history. She denied any specific personal history of other diseases.
At her first presentation to our clinic, physical examination revealed a soft, flat, and non-tender abdomen.
Her laboratory data indicated that her renal and liver functions were normal. The Child-Pugh score was categorized as class A, and the indocyanine green retention at 15 min was 11.3%. The patient was negative for hepatitis B or C infection. The serum tumor markers, carcinoembryonic antigen, carbohydrate antigen 19-9, alpha-fetoprotein, and protein induced by vitamin K antagonist-II, were all within their normal ranges. All electrolytes were within normal limits.
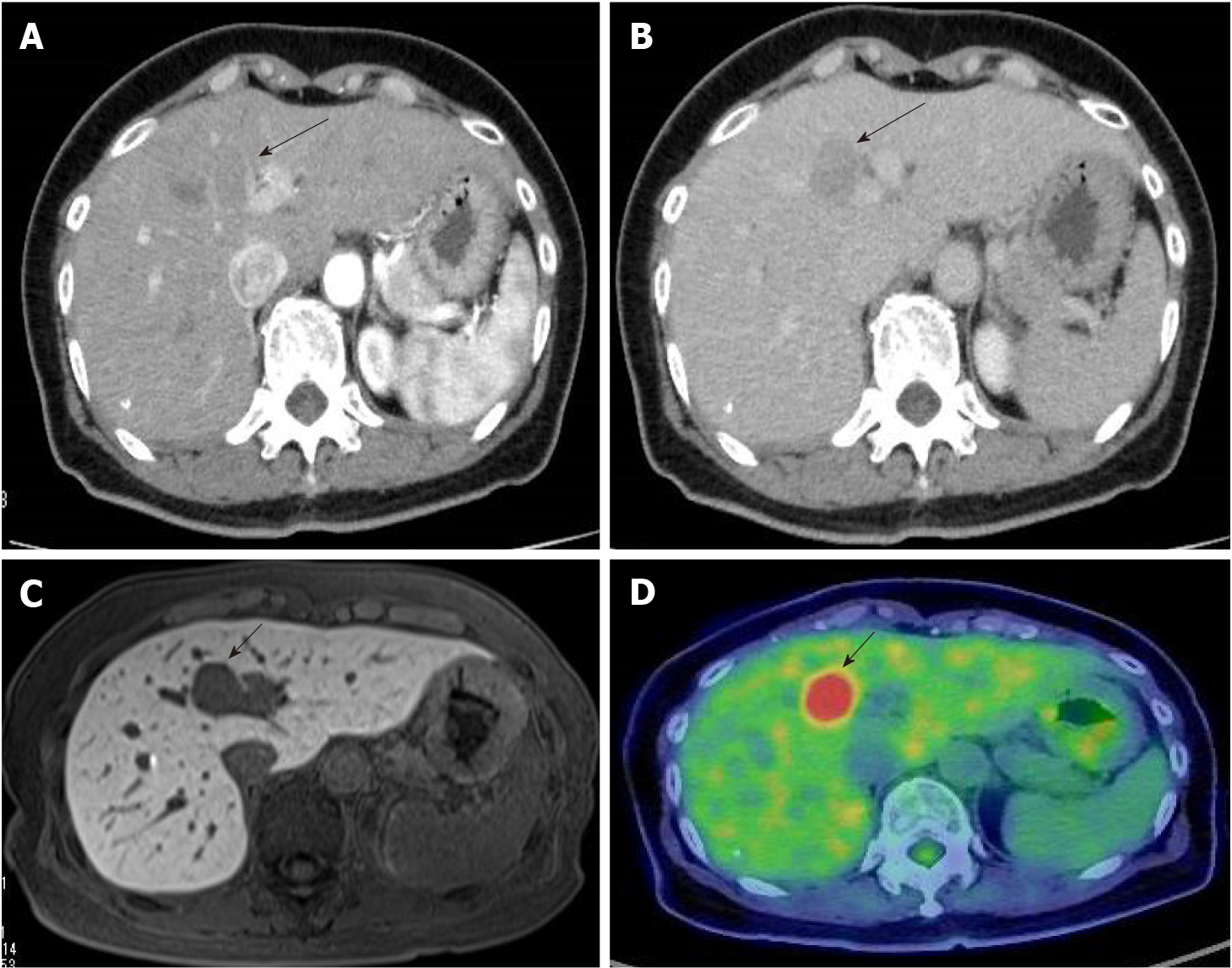
Contrast-enhanced computed tomography (CT) revealed a 23-mm liver mass in segment 4, adjacent to the hepatic hilum. The mass exhibited a slight ring-like enhancement in the arterial phase. In the portal and delayed phases, the signal intensity of the mass was lower than that of the surrounding liver tissue (Figure 1). Contrast-enhanced magnetic resonance imaging (MRI) demonstrated a low signal on T1-weighted images and an intermediate signal on T2-weighted images. In the early phase, peripheral enhancement with a low signal was observed inside the mass. Diffusion-weighted images showed a significantly high signal, and the apparent diffusion coefficient (ADC) map demonstrated a low signal. A decreased uptake of gadoxetate sodium was also observed. Positron emission tomography (PET)/CT showed significant accumulation, with a maximum standardized uptake value of 10.1 at the mass. No other abnormal accumulations were observed. Esophagogastroduodenoscopy and colonoscopy revealed no malignant lesions.
Considering these preoperative imaging studies, malignancy was considered as a differential diagnosis.
Surgical resection was planned as a treatment option. Intraoperatively, we found no ascites, dissemination, or distant metastasis. Although intraoperative ultrasound demonstrated an apparent capsule around the mass in segment 4, the mass was located just on the hilar plate and was stiffly attached to Glisson’s capsule. Therefore, a left hepatectomy was performed to obtain a surgical margin. The postoperative clinical course was unremarkable, and the patient was discharged on postoperative day 14.
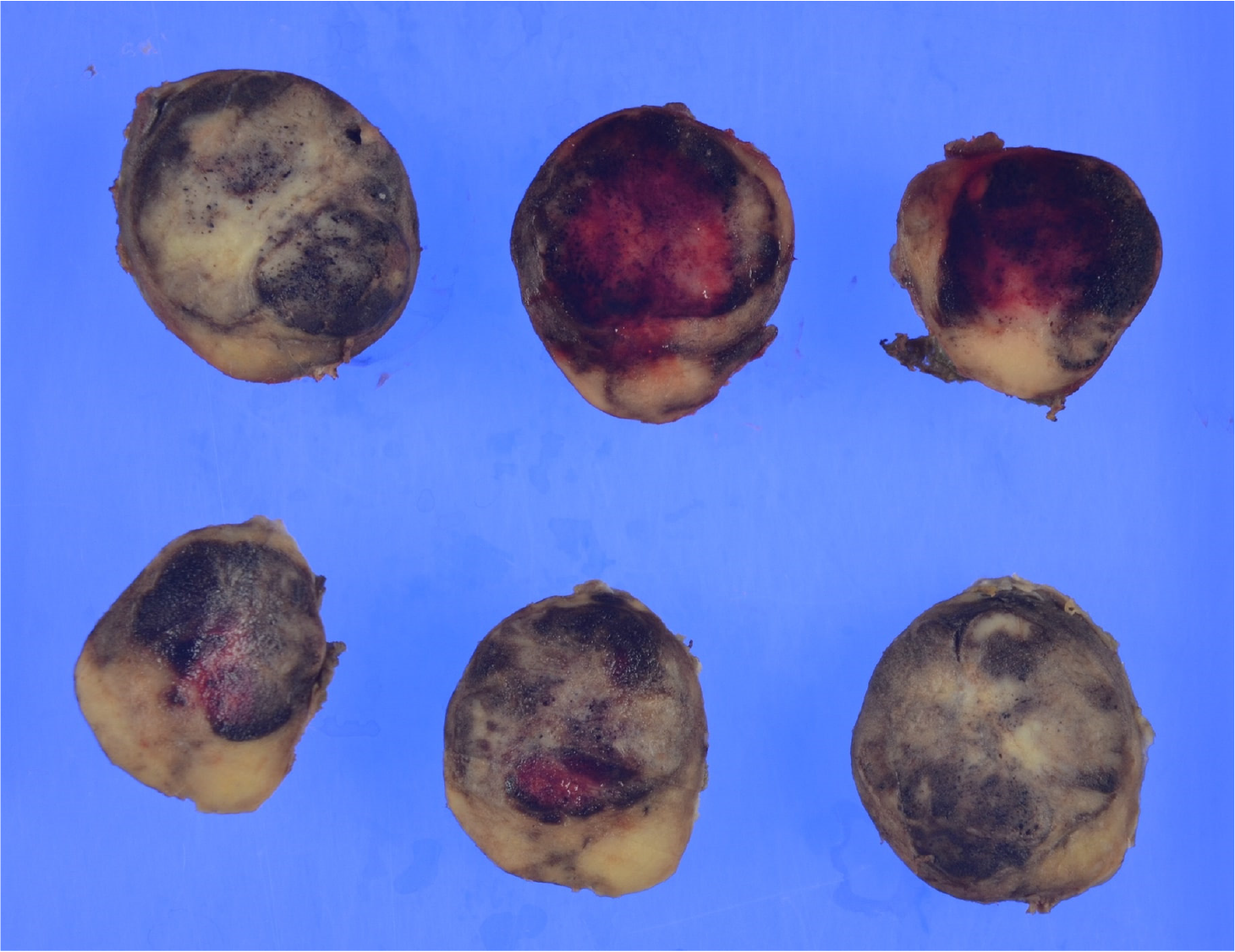
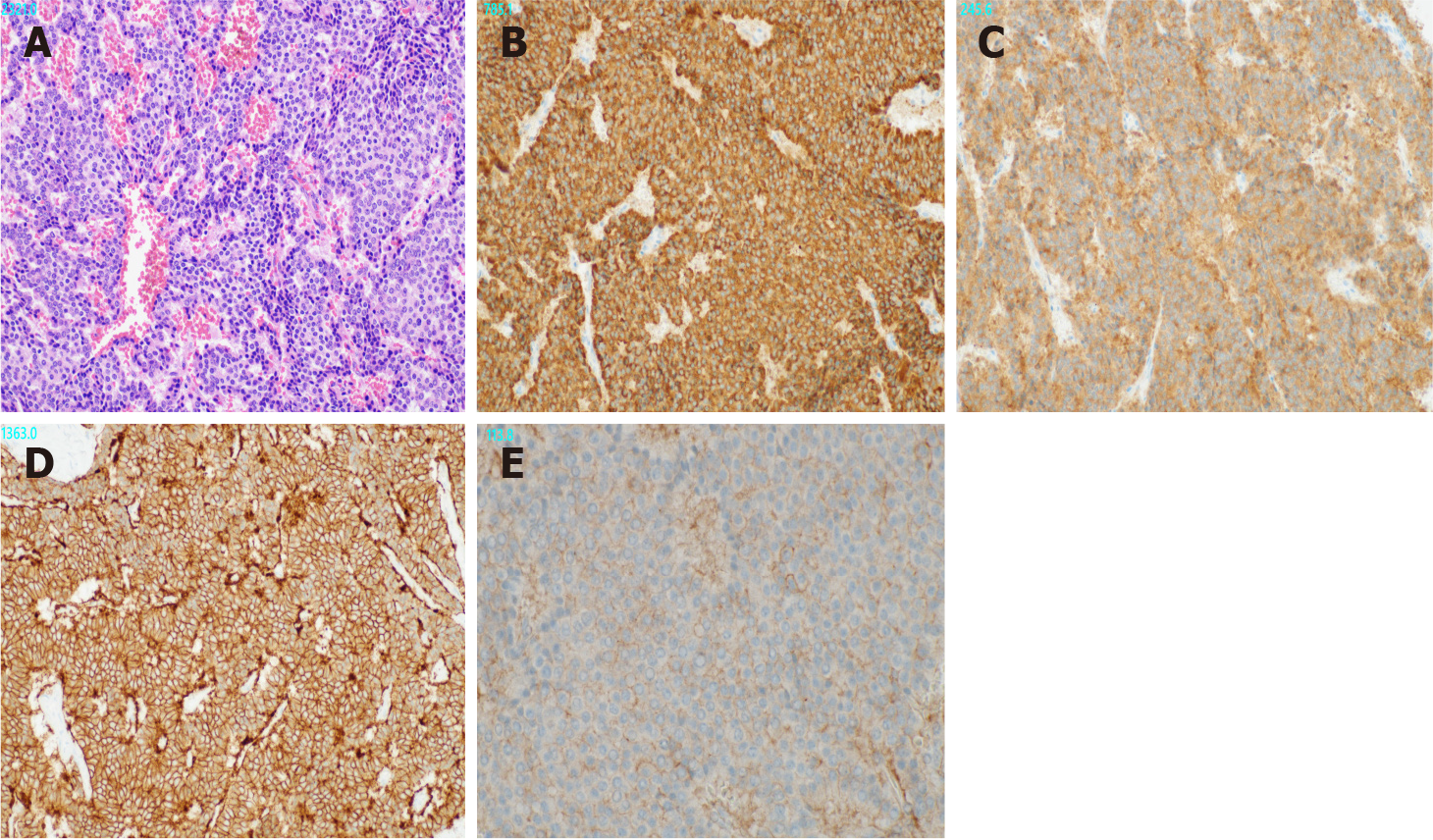
Gross examination revealed a 2.5 cm × 2.0 cm × 3.0 cm yellowish-white mass with hemorrhage (Figure 2). Histologically, atypical cells with small round nuclei and eosinophilic cytoplasms were arranged in an alveolar, reticular, or trabecular pattern. The cells were characterized by nuclear division, with 2 per 10 high-power fields. Immunohistological staining showed that the tumor cells were positive for chromogranin A/synaptophysin and negative for CD56t. The Ki-67 labeling index was found to be 7%. Thus, a diagnosis of hepatic NEN (grade 2) was made. With respect to SSTR, the scores were 3 for SSTR2a and 2 for SSTR5 (Figure 3). SRS performed in the outpatient clinic identified no other significant accumulation, which cast doubt on the possible existence of primary sites other than the liver. Taking these findings into account, we concluded that the tumor originated in the liver; however, continuous follow-up is essential to completely rule out the possible existence of other primary sites or to identify the appearance of new hepatic lesions in the future. Regular follow-up with imaging studies has been performed since the operation, considering possible recurrence. At the 1-year follow-up, the patient was in good health and free from recurrence.
NEN is a primary malignant tumor arising from neuroendocrine cells throughout the body. The most common primary sites of NEN are the gastrointestinal tract (67.5%) and the lung/bronchus (25.3%)[2]. Within the gastrointestinal tract, the small intestine (25.3%), rectum (27.4%), and stomach (8.7%) are the most commonly involved organs[2]. Primary hepatic NEN is extremely rare (0.4%)[2] and has no specific imaging findings or biomarkers. Thus, the diagnosis of hepatic NEN is generally difficult. The fact that the liver is frequently the metastatic site of NEN makes this diagnosis even more challenging. Furthermore, early diagnosis is often difficult because patients may have nonspecific symptoms or be asymptomatic, and the growth of NEN is generally slow. In the present case, the doubling time of the mass was calculated to be 46.4 mo, and such a gradual increase might have caused a delay in diagnosis. To the best of our knowledge, the longest observation period before surgery of a hepatic NEN in previous reports was 26 years[3].
Regarding imaging modalities for the diagnosis of primary hepatic NEN, contrast-enhanced CT is often nonspecific because it shows the contrast effect in the arterial phase and washout in the portal phase, which is similar to the classic contrast pattern of hepatocellular carcinoma[4,5]. MRI can depict a low signal on ADC maps/T1-weighted images and a high signal on T2-weighted images[4]. The detection rate of NENs on PET/CT is 25%-73%, which is not very high and may be explained by the fact that NENs have relatively low tumor growth activity compared to carcinomas[6]. However, PET has been reported to be useful in the search for metastasis and the diagnosis of recurrence in patients with high-grade tumors, such as those with abnormal accumulation in the primary lesion[7]. Thus, PET/CT may be useful for differentiating metastatic NENs in the liver. In the present case, peripheral enhancement on CT and MRI and high accumulation on PET/CT also raised the possibility of intrahepatic cholangiocarcinoma with marginal vascular regeneration around the tumor. However, the extremely slow increase in tumor rate made us doubtful of the possibility of cholangiocarcinoma. The present case is noteworthy in that primary hepatic NEN exhibited significant accumulation on PET/CT. To the best of our knowledge, there are no reports showing accumulation at the site of primary hepatic NEN on PET/CT.
Although no clear clinical guidelines have been established for the treatment of primary hepatic NEN, surgical resection remains the basic treatment when feasible[8]. Knox et al[9] reported a 5-year postoperative survival rate of 74%-78%, 10-year survival rate of 68%, and recurrence rate of 18%. Although it is often difficult to determine whether NEN is a primary or metastatic lesion preoperatively, there are reports of improved prognosis after tumor reduction surgery even in unresectable or recurrent cases, regardless of whether the tumor is primary or metastatic[10]. Therefore, aggressive surgical resection should always be considered.
Tumors of neuroendocrine origin usually have cell surface receptors with an affinity for somatostatin[11]. SRS is an imaging technique in which gamma-ray emitting radionuclides are labeled on octreotide, which shows an affinity for SSTR expressed on the cell membrane of lesions. Thus, SSTR is expressed in lesions with accumulation on SRS. SSTR has five subtypes, and subtypes 2a and 5 are characteristic of NEN. Volante et al[12] defined scores of 2 (membranous reactivity in less than 50% of tumor cells) and 3 (circumferential membranous reactivity in more than 50% of tumor cells) as positive. Hasegawa et al[13], who analyzed 16 cases of NEN, reported that the concordance rate between accumulation in a lesion on SRS and pathological SSTR2a expression was 93.8%.
SRS has been reported to have an 89% sensitivity to NEN[14]. However, there are some reports of high accumulation in meningiomas and small cell lung cancer[15], as well as accumulation in non-tumorous conditions including pneumonia, surgical wounds, and the breast[16]. When SRS is performed to investigate the possibility that the primary tumor is located in another organ, as in this case, the interpretation of whether the site of accumulation is the primary site or a false positive should be carefully made in consideration of the other imaging modalities.
In the present case, SRS was performed to identify the presence of a primary site in another organ after histological diagnosis. Scigliano et al[17] reported high sensitivity, specificity, and accuracy (89%, 94%, and 91%, respectively) for SRS as a detection method for recurrence. Considering the gradual clinical course and postoperative investigation for another primary site, we thought that the hepatic tumor was most likely to be of primary hepatic origin. In this case, SRS revealed no possible primary or metastatic sites in other organs, and recurrence could be detected by comparative appraisal with the present results of SRS in the future. Therefore, continuous follow-up and preoperative observation are essential. Several reports[18,19] on the usefulness of SRS as a preoperative staging assessment for pancreatic and gastrointestinal NENs have been reported. However, to the best of our knowledge, SRS has not reportedly been performed in a patient with suspected primary hepatic NEN to postoperatively evaluate the possible existence of other primary lesions.
We observed a long course of gradual tumor growth of hepatic NEN, considered the primary origin, for 10 years. Close preoperative observations, including PET/CT studies, enabled surgical decision-making and curative resection. Postoperative SRS after histological studies may be beneficial in making a final diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ergun Y, Jensen RT S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Agha RA, Fowler AJ, Saeta A, Barai I, Rajmohan S, Orgill DP; SCARE Group. The SCARE Statement: Consensus-based surgical case report guidelines. Int J Surg. 2016;34:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1517] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 2. | Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1851] [Article Influence: 84.1] [Reference Citation Analysis (1)] |
| 3. | Meng XF, Pan YW, Wang ZB, Duan WD. Primary hepatic neuroendocrine tumor case with a preoperative course of 26 years: A case report and literature review. World J Gastroenterol. 2018;24:2640-2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Wang LX, Liu K, Lin GW, Jiang T. Primary hepatic neuroendocrine tumors: comparing CT and MRI features with pathology. Cancer Imaging. 2015;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Li R, Tang CL, Yang D, Zhang XH, Cai P, Ma KS, Guo DY, Ding SY. Primary hepatic neuroendocrine tumors: clinical characteristics and imaging features on contrast-enhanced ultrasound and computed tomography. Abdom Radiol (NY). 2016;41:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Modlin IM, Latich I, Zikusoka M, Kidd M, Eick G, Chan AK. Gastrointestinal carcinoids: the evolution of diagnostic strategies. J Clin Gastroenterol. 2006;40:572-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Carideo L, Prosperi D, Panzuto F, Magi L, Pratesi MS, Rinzivillo M, Annibale B, Signore A. Role of Combined [68Ga]Ga-DOTA-SST Analogues and [18F]FDG PET/CT in the Management of GEP-NENs: A Systematic Review. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Foster DS, Jensen R, Norton JA. Management of Liver Neuroendocrine Tumors in 2018. JAMA Oncol. 2018;4:1605-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Knox CD, Anderson CD, Lamps LW, Adkins RB, Pinson CW. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10:1171-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Saxena A, Chua TC, Sarkar A, Chu F, Liauw W, Zhao J, Morris DL. Progression and survival results after radical hepatic metastasectomy of indolent advanced neuroendocrine neoplasms (NENs) supports an aggressive surgical approach. Surgery. 2011;149:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Kumbasar B, Kamel IR, Tekes A, Eng J, Fishman EK, Wahl RL. Imaging of neuroendocrine tumors: accuracy of helical CT vs SRS. Abdom Imaging. 2004;29:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C, De Rosa G, Dogliotti L, Colao A, Papotti M. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20:1172-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Hasegawa S, Kobayashi N, Tokuhisa M, Goto A, Takano S, Takada Y, Kaneta T, Mori R, Matsuyama R, Endo I, Yamanaka S, Nakajima A, Inoue T, Ichikawa Y. Clinical Usefulness of Somatostatin Receptor Scintigraphy in Japanese Patients with Gastroenteropancreatic Neuroendocrine Tumors. Digestion. 2017;96:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Binderup T, Knigge U, Loft A, Mortensen J, Pfeifer A, Federspiel B, Hansen CP, Højgaard L, Kjaer A. Functional imaging of neuroendocrine tumors: a head-to-head comparison of somatostatin receptor scintigraphy, 123I-MIBG scintigraphy, and 18F-FDG PET. J Nucl Med. 2010;51:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A, Vitek P, Sundin A, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology. 2009;90:184-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Gibril F, Reynolds JC, Chen CC, Yu F, Goebel SU, Serrano J, Doppman JL, Jensen RT. Specificity of somatostatin receptor scintigraphy: a prospective study and effects of false-positive localizations on management in patients with gastrinomas. J Nucl Med. 1999;40:539-553. [PubMed] |
| 17. | Scigliano S, Lebtahi R, Maire F, Stievenart JL, Kianmanesh R, Sauvanet A, Vullierme MP, Couvelard A, Belghiti J, Ruszniewski P, Le Guludec D. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Kolasińska-Ćwikła AD, Konsek SJ, Buscombe JR, Maciejkiewicz K, Cichocki A, Roszkowska-Purska K, Sawicki Ł, Tenderenda M, Cwikla JB. The Value of Somatostatin Receptor Scintigraphy (SRS) in Patients with NETG1/G2 Pancreatic Neuroendocrine Neoplasms (p-NENs). Nucl Med Rev Cent East Eur. 2019;22:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Savelli G, Lucignani G, Seregni E, Marchianò A, Serafini G, Aliberti G, Villano C, Maccauro M, Bombardieri E. Feasibility of somatostatin receptor scintigraphy in the detection of occult primary gastro-entero-pancreatic (GEP) neuroendocrine tumours. Nucl Med Commun. 2004;25:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |