Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1981
Peer-review started: August 21, 2021
First decision: November 1, 2021
Revised: November 12, 2021
Accepted: January 20, 2022
Article in press: January 20, 2022
Published online: February 26, 2022
Processing time: 186 Days and 5 Hours
Systemic lupus erythematosus (SLE) patients are extremely susceptible to opportunistic infections due to glucocorticoid and immunosuppressive treat
A 46-year-old woman was treated with glucocorticoid and immunosuppressant for SLE involving the hematologic system and kidneys (class IV-G lupus nephritis) for more than one year. She was admitted to hospital due to headache and fever, and was diagnosed with multiple cerebral abscesses. Brain enhanced magnetic resonance imaging showed multiple nodular abnormal signals in both frontal lobes, left parietal and temporal lobes, left masseteric space (left temporalis and masseter region). The initial surgical plan was only to remove the large abscesses in the left parietal lobe and right frontal lobe. After surgery, based on the drug susceptibility test results (a rare pathogen Nocardia asteroides was found) and taking into consideration the patient’s renal dysfunction, a multi-antibiotic regimen was selected for the treatment. The immunosuppressant mycophenolate mofetil was discontinued on admission and the dose of prednisone was reduced from 20 mg/d to 10 mg/d. Re-examination at 3 mo post-surgery showed that the intracranial lesions were reduced, the edema around the lesions was absorbed and dissipated, and her neurological symptoms had disappeared. The patient had no headaches or other neurological symptoms and lupus nephritis was stable during the 2-year follow-up period.
In this report, we provide reasonable indications for immunosuppression, anti-infective therapy and individualized surgery for an SLE patient complicated with multiple cerebral abscesses caused by a rare pathogen, which may help improve the diagnosis and treatment of similar cases.
Core Tip: Infection is a common complication of systemic lupus erythematosus (SLE) treated with glucocorticoids and immunosuppressants, but multiple cerebral abscesses caused by a rare pathogen are uncommon. Conventional treatment includes surgery and antibiotics. However, due to concerns about SLE exacerbation after the reduction of glucocorticoid and immunosuppressant, and poor tolerability to surgery for multiple cerebral abscesses in patients with SLE, treatment is often difficult, and selection of antibiotics sensitive to rare pathogens is challenging. We report a patient who benefited from individualized surgical treatment of multiple cerebral abscesses, and a multi-antibiotic regimen, with good outcomes.
- Citation: Hu QD, Liao LS, Zhang Y, Zhang Q, Liu J. Surgery and antibiotics for the treatment of lupus nephritis with cerebral abscesses: A case report. World J Clin Cases 2022; 10(6): 1981-1990
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1981.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1981
Systemic lupus erythematosus (SLE) is an autoimmune disease that is currently managed by long-term glucocorticoid and immunosuppressive treatments. However, such treatments often render patients extremely susceptible to opportunistic infections, which can negatively impact SLE prognosis [1,2]. The respiratory system, urinary system and skin are most commonly affected by opportunistic pathogens, and multiple cerebral infections are rarely reported[3,4]. We here report an SLE patient with renal involvement and multiple cerebral abscesses who was admitted to our hospital in June 2018. In addition to adjusting glucocorticoid and immunosuppressant therapy, we treated the patient with a combination of surgery and multi-antibiotic therapy to eliminate the abscesses and control the infection. After 2 years of follow-up, the patient's SLE and lupus nephritis (LN) were stable, and the cerebral abscesses resolved. In this case report, we discuss the primary treatment and surgical indications, as well as the postoperative treatment regimen, which may help improve the diagnosis and treatment of similar cases.
A 46-year-old woman was admitted to our hospital due to repeated fever with headache that lasted 10 d and was exacerbated for 1 d.
The patient developed fever without any obvious cause 10 days before admission, with a body temperature of 39 °C, along with headache and vomiting. The patient initially believed that she had upper respiratory infection, and therefore self-administered anti-cold medication, which did not alleviate the symptoms.
SLE involving the hematologic system and kidneys was diagnosed 1 year previously, and renal pathology showed class IV LN. The patient underwent hemodialysis several times due to acute kidney injury. Glucocorticoids and immunosuppressants were maintained. The patient’s past medical record is summarized in Tables 1 and 2.
| Diagnosis items | Result |
| Symptoms | Eyelid and facial edema with skin tightening sensation, pain in multiple joints, hair loss, reduced urine volume, and facial photoallergy |
| Laboratory tests | Urine protein: Positive (+++), WBC: 3.5 × 109/L; Total platelet count: 40x109/L; Albumin: 21.7 g/L; Creatinine: 149 μmmol/L; Thyroid function: Serum free T3: 1.9 pmol, serum free T4: 8.1 pmol/L; Anti-nuclear antibody profile: ANA positive (1:320 fine granular type), RNP/sm: Positive (+), SSA: Positive (+++), RO-52: Positive (+++), SSB: Positive (+++), anti-nucleosome antibody: Positive (++), anti-ribosomal P protein antibody: Positive (++); Complement C3: 0.3 g/L |
| Bone marrow cell test | Bone marrow cell test showed: Accelerated granulocyte maturation and active plasma cells |
| Pathological biopsy | Class IV-G lupus nephritis |
| Diagnosis | Class IV-G lupus nephritis; SLE (involving the hematologic system and organs) SLEDAI score 11; Subclinical hypothyroidism; Acute renal insufficiency |
| Date | Treatment | Drug-specific | |
| Treatment (April-December 2017) | Phase 1 | Primary treatment | Methylprednisolone shock therapy (500 mg/d/3D)/hemodialysis 5 times, once every other day (due to progressive increase in serum creatinine to 455 μmol/L)/intravenous immunoglobulin |
| Adjuvant treatment | Symptomatic treatments to control infection/supplement albumin and supplement thyroxine | ||
| Phase 2 | Primary treatment | Prednisone 60 mg/d QD maintenance/cyclophosphamide shock therapy (European protocol) | |
| Adjuvant treatment | Infection control/platelet infusion | ||
| After discharge (December 2017 – April 2018) | Primary treatment | Oral prednisone 40 mg/d, outpatient follow-up, monthly gradually reduced to 20 mg/d/oral MMF 1.5 g bid | |
| Return for treatment due to lung infection (April 2018) | Primary treatment | The prednisone and MMF schemes remained unchanged /Immunomodulatory and anti-infective therapy for lung infection | |
| After discharge(April-May 2018) | Primary treatment | Oral prednisone 20 mg/d/ oral MMF 1.0 g bid | |
| June 2018 | Admitted to hospital due to multiple brain abscesses | ||
Her personal and family history was unremarkable.
Physical examination upon admission indicated a body temperature 38.7 °C, a conscious but dispirited state, painful expression, Cushing syndrome, anemic appearance, facial edema, butterfly-shaped erythema visible on both maxillofacial regions, and diffused moist rales audible in both lungs. No abnormalities were identified in the heart and abdomen. Nervous system examination indicated neck stiffness (+), Kernig sign (+), Brudzinski’s sign (+), high muscle tension in the lower limbs, and Babinski sign (+).
Routine blood tests showed a white blood cell count of 12.34x109/L, neutrophil % of 83.6%, hemoglobin of 92 g/L, hematocrit of 28.5%, platelets of 123 × 109/L, high sensitivity C reactive protein of 18.85 mg/L, procalcitonin of 0.296 ng/mL, erythrocyte sedimentation rate of 60 mm/h, creatinine of 233 μmol/L, albumin of 22.5 g/L, and a CD4/CD8 ratio of 0.31. A urine test for 24 h urine protein was 5769.4 mg to 9443.5 mg/24 h.
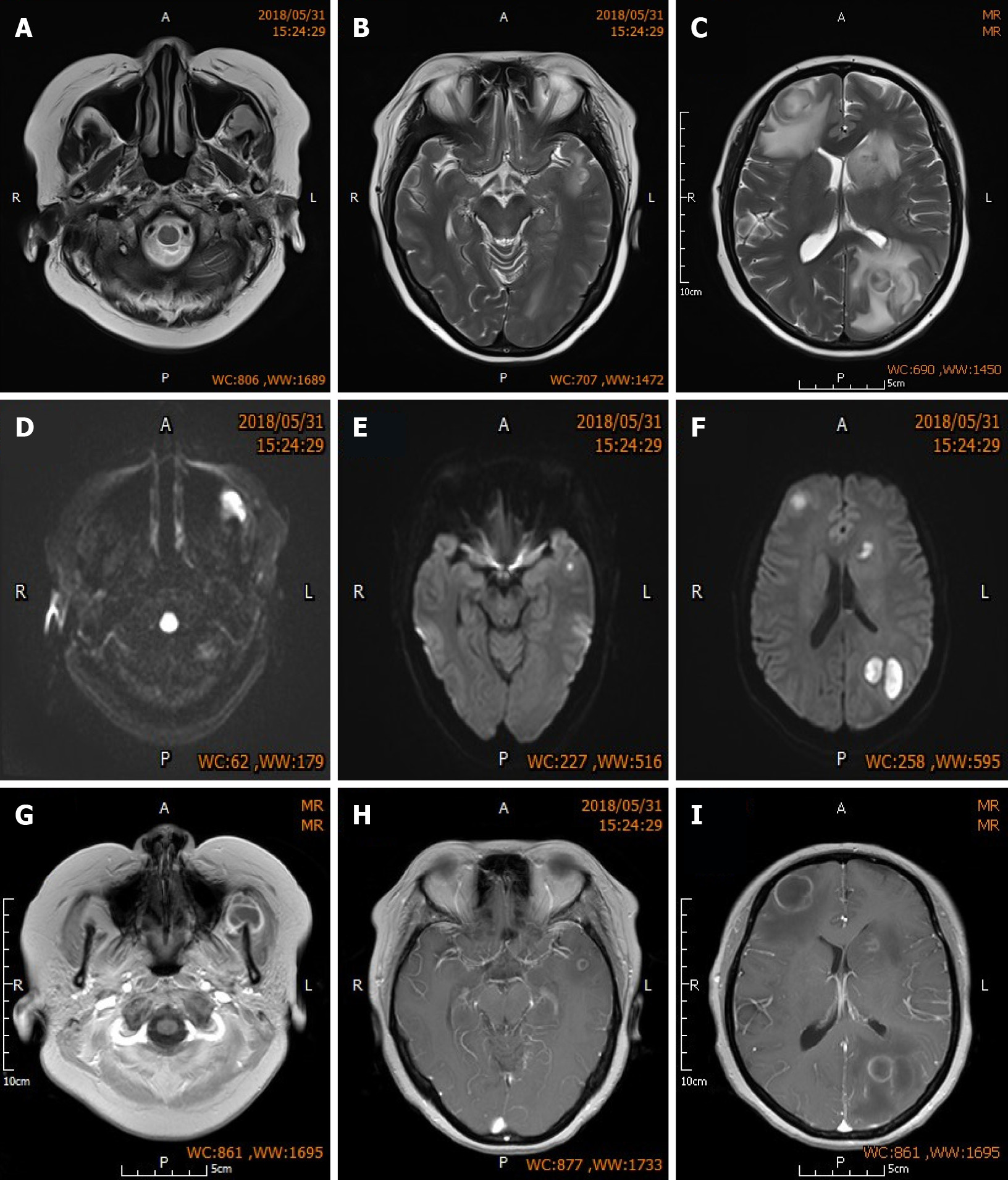
Chest computed tomography indicated dispersed inflammation and fibrotic lesions in both lungs. Brain enhanced magnetic resonance imaging (MRI) (Figure 1) showed multiple nodular abnormal signals in both frontal lobes, left parietal and temporal lobes, and the left masseteric space (left temporalis and masseter region).
Based on the patient’s medical history and laboratory examinations, she was diagnosed with SLE involving the hematologic system and kidneys (class IV-G LN) complicated with multiple infectious cerebral abscesses.
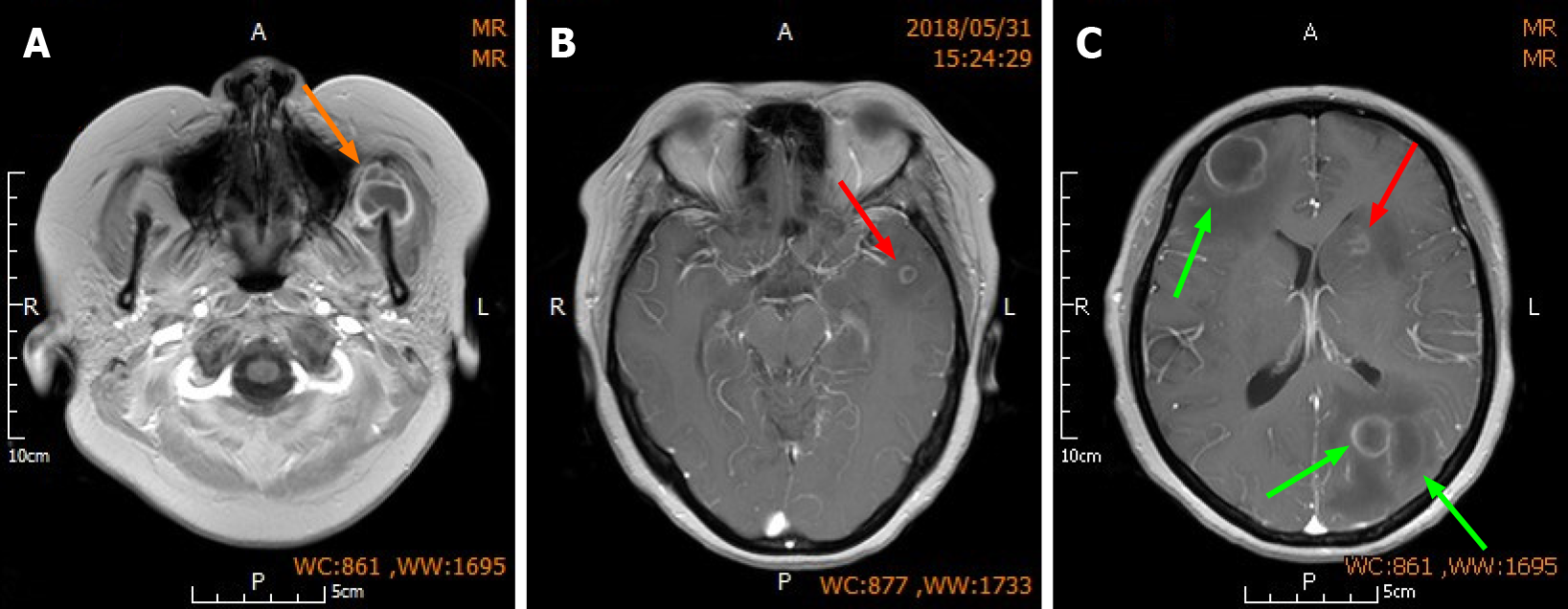
Upon admission, the patient was given dehydration therapy to lower intracranial pressure. Given her history of renal impairment caused by LN, glycerin fructose alternating with furosemide were used as dehydrating agents instead of mannitol and albumin to avoid exacerbation of renal impairment. Mycophenolate mofetil (MMF) was discontinued immediately due to severe infection, but the prednisone dose (20 mg/d) was maintained. The surgical contraindications of anemia and electrolyte imbalance were corrected by red blood cell transfusion and fluid infusion, respectively. The surgery was performed immediately after correcting the preoperative contraindications 3 days post-admission. Large abscesses in the left parietal lobe and right frontal lobe were resected via the left parietal approach and right frontal approach (Figure 2), respectively. Other small abscesses in the deeper part of the brain were not removed. Local linear incision was made. Through the scalp, bone valve and endocranium, after accurate positioning, brain tissue edema was observed. During brain histostomy, the abscess wall was found intact and tough, and no important cerebrovascular trunk was found around the abscess, and light yellow pus was aspirated from the abscess. The abscess cavity was completely removed, the blood vessels on the abscess wall were cut off, and electrocoagulation hemostasis was performed (Supplementary Figure 1). During the surgery, abscess wall should be removed completely to avoid pus overflow and reduce the damage to the normal vascular and cerebral tissue around the abscess. Finally, the aspirated light yellow pus was sent for bacterial culture and drug susceptibility testing.
The patient reported significant relief from headache after surgery, and neurological symptoms such as hemiplegia, paresthesia and aphasia were not observed. The patient was given routine postoperative treatments including dehydration therapy (as described previously) and fluid infusion. The bacterial culture obtained on postoperative day 4 indicated Nocardia asteroides. Based on the drug susceptibility test results and taking into consideration the patient’s renal dysfunction, a multi-antibiotic regimen was selected consisting of sulfamethoxazole-trimethoprim (30 mg/kg, q12h, oral), ceftriaxone (2 g, q12h, i.v.) and amikacin (6 mg/kg, q24h, i.v.) for the first 3 wk post-surgery, and switched to sulfamethoxazole-trimethoprim (30 mg/kg, q12h, oral) and minocycline (0.1 g, bid, oral) based on the patient’s symptoms and vital signs, imaging and laboratory test results, and nephrotoxicity of the antibiotics. The estimated duration of the entire treatment regimen was one year.
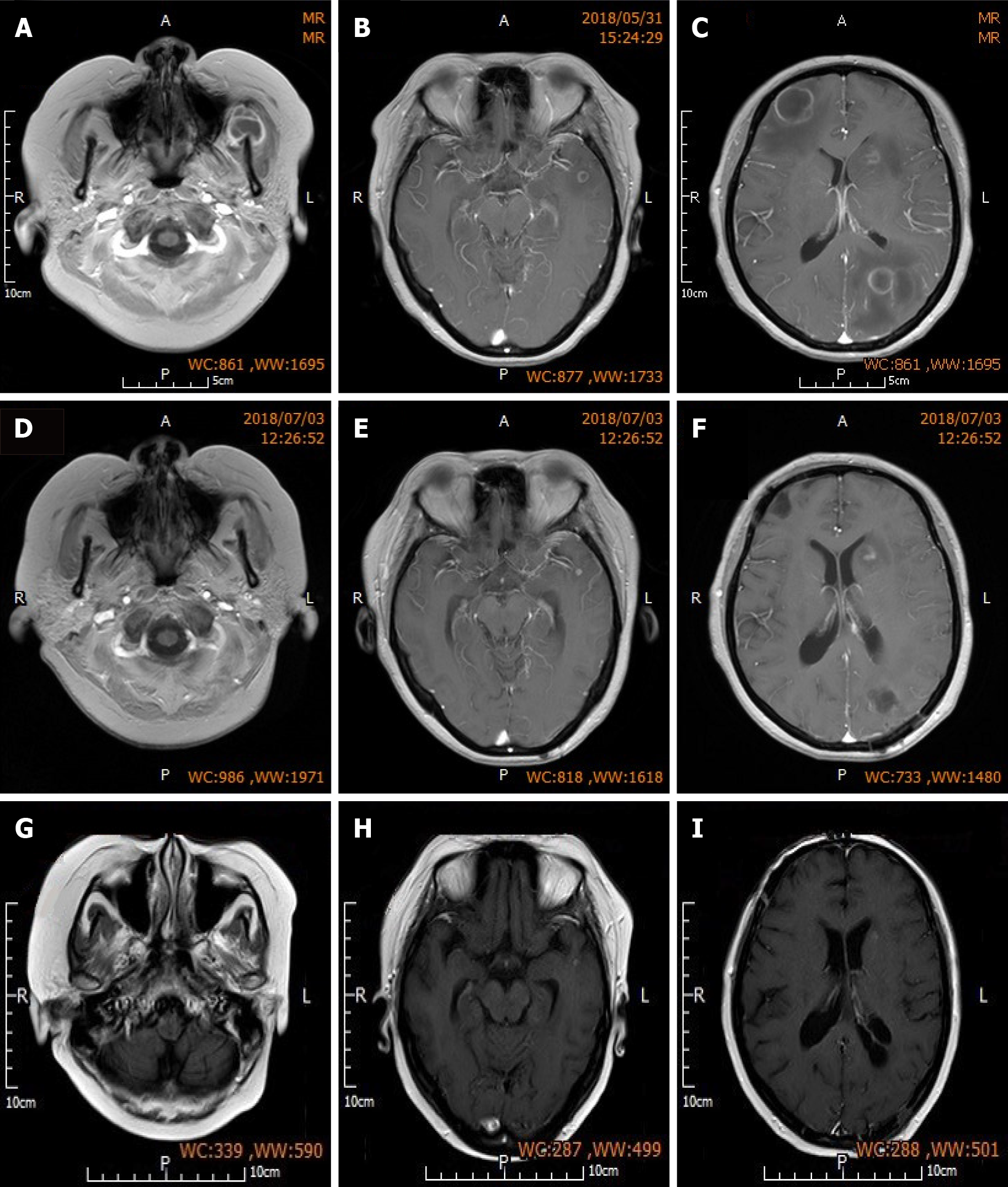
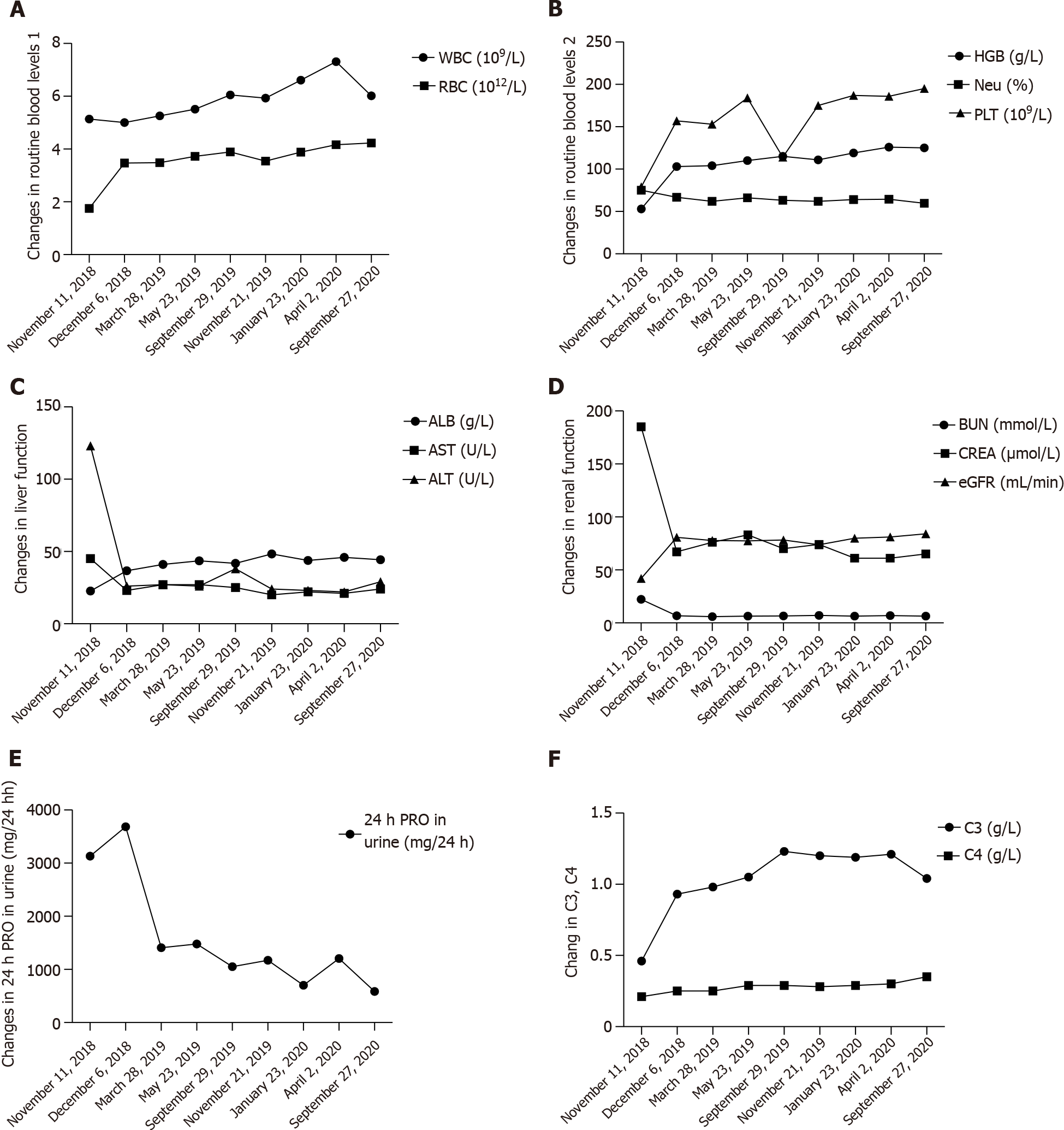
Head MRI one month post-surgery indicated complete resolution of abscesses in the left parietal lobe and right frontal lobe, slight perilesional edema, reduced size of small non-resected abscesses in the left frontal lobe and left caudate nucleus, and significant alleviation of edema around these small lesions. The patient was discharged one month after surgery but continued to receive the prescribed antibiotics (see above), along with prednisone (10 mg/d) maintenance treatment and regular follow-up assessments. Re-examination at 3 mo post-surgery showed further reduction in the size of non-resected lesions in the left frontal and temporal lobes compared to that at 1 mo post-surgery, absorption and dissipation of perilesional edema (Figure 3), and complete disappearance of neurological symptoms. Fortunately, renal function was not affected by the administration of multiple antibiotics (Table 3). Conservative treatment was continued after the patient was updated on her condition. No headaches or other neurological symptoms occurred and LN was stable during the 2-year follow-up period (Table 4 and Figure 4).
| At admission | Before surgery | 4 d post-surgery | 3 d post-antibiotic adjustment | 3 wk post-multi-antibiotic treatment | |
| White blood cell (109/L) | 12.34 | 6.26 | 5.23 | 6.54 | 3.73 |
| Percentage of neutrophils (Neu%) | 83.60 | 75.40 | 73.60 | 71.90 | 70 |
| High-sensitivity C-reactive protein (mg/L) | 18.85 | 20.3 | 6.19 | 25.99 | < 0.499 |
| Creatinine (CREA, umol/L) | 233 | 193 | 198 | 166 | 158 |
| Urea nitrogen (BUN, mmol/L) | 14.9 | 10.7 | 11.2 | 10.8 | 9.4 |
| Date | WBC (109/L) | Neu (%) | RBC (1012/L) | HGB (g/L) | PLT (109/L) | ALB (g/L) | AST (U/L) | ALT (U/L) | BUN (mmol/L) | CREA(μmol/L) | eGFR (mL/min) | 24 h PRO in urine (mg/24h) | C3 (g/L) | C4 (g/L) | ESR (mm/h) | CD4/CD8 |
| November 11, 2018 | 5.14 | 75.1 | 1.75 | 53 | 79 | 22.7 | 45 | 123 | 22.29 | 185 | 41.95 | 3131 | 0.46 | 0.21 | 7 | 0.31 |
| December 16, 2018 | 5.0 | 66.8 | 3.47 | 103 | 157 | 36.7 | 23 | 26 | 6.76 | 67 | 80.74 | 3684 | 0.93 | 0.25 | 28 | / |
| March28, 2019 | 5.26 | 62 | 3.48 | 104 | 153 | 41.1 | 27 | 27 | 5.85 | 76 | 77.73 | 1409 | 0.98 | 0.25 | 36 | / |
| May 23, 2019 | 5.51 | 66.2 | 3.72 | 110 | 184 | 43.6 | 27 | 26 | 6.49 | 83 | 77.46 | 1479 | 1.05 | 0.29 | 25 | / |
| September 29, 2019 | 6.05 | 63.2 | 3.89 | 115 | 114 | 41.8 | 25 | 38 | 6.50 | 70 | 78.27 | 1051 | 1.23 | 0.29 | 16 | / |
| November 21,2019 | 5.93 | 61.9 | 3.54 | 111 | 175 | 48.3 | 20 | 24 | 7.00 | 74 | 73.81 | 1173 | 1.20 | 0.28 | 19 | / |
| January 23, 2020 | 6.61 | 64.1 | 3.88 | 119 | 187 | 43.8 | 22 | 23 | 6.36 | 61 | 79.87 | 700 | 1.19 | 0.29 | 19 | / |
| April 2,2020 | 7.31 | 64.4 | 4.16 | 126 | 186 | 45.9 | 21 | 22 | 6.92 | 61 | 81.08 | 1204 | 1.21 | 0.30 | 23 | / |
| September 27, 2020 | 6.02 | 59.6 | 4.23 | 125 | 195 | 44.3 | 24 | 29 | 6.43 | 65 | 84.03 | 586 | 1.04 | 0.35 | 19 | 0.88 |
SLE is an autoimmune disease that affects multiple organs and systems, and is primarily characterized by dysfunctional humoral immunity, reduced CD8+ T cell cytotoxicity, defective CD4+ T cell proliferation, and impaired antigen presentation by mononuclear cells[5,6]. The current primary treatment for SLE includes high doses of corticosteroids and immunosuppressants. Although these treatments can significantly increase the 5-year and 10-year survival of SLE patients, their prolonged use can exacerbate immunodeficiency and increase the risk of infections, especially intracranial infections, which are one of the major causes of SLE-associated mortality[7]. Fever, headache and meningeal irritation are the major symptoms in SLE patients with intracranial infection. However, they can often become atypical after prolonged use of corticosteroids and immunosuppressants, resulting in misdiagnosis and missed diagnosis. Sometimes, these patients are even diagnosed with lupus encephalopathy.
Therefore, in addition to clinical symptoms, imaging and laboratory tests are essential for accurate diagnosis of cerebral involvement in SLE. Cerebrospinal fluid culture and smear are important diagnostic tests, but their positive detection rates are low. Once the abscess is identified by imaging, biochemical, microbiological and pathological testing of pus are useful to determine the subsequent treatment of patients[8].
Nocardia is a genus of obligate aerobic actinomycetes that are widely found in soil, and most species are non-pathogenic parasites that are found in rotting organic matter. The latter are not part of the normal human microflora and generally do not cause endogenous infections. However, they can cause exogenous conditional infections in patients with late stage progressive disease or immune disorders, especially those with Cushing syndrome, diabetes or under long-term usage of corticosteroids, immunosuppressants and broad-spectrum antibiotics[9]. A similar case was reported previously. After treatment with methylprednisone and cyclophosphamide, a 24-year-old woman with LN developed severe pleural pneumonia and occipital abscess, both caused by Nocardia asteroides. Although she was treated with multiple antibiotics, she ultimately died[10]. Our patient was diagnosed with SLE and LN one year before presenting at our hospital, and had been receiving corticosteroids and immunosuppressants, and had undergone hemodialysis due to acute kidney injury. Therefore, she was highly likely to be immuno-deficient and was considered Nocardia-susceptible. In addition, the patient had facial pigmentation along both sides of the jaw and previous skin damage, together with an infectious lesion in her left masseteric space. Puncture aspiration indicated Nocardia infection. We believe that the left jaw was the source of infection, from where the bacterium entered the circulation and eventually reached the brain, forming multiple cerebral abscesses.
A previous study demonstrated that sufficient dosage and duration of antibiotic treatment combined with surgery can effectively treat a Nocardia-induced cerebral abscess[11]. However, the treatment has not yet been standardized based on patients’ physical conditions, surgical approach, and selection and timing of postoperative antibiotics. We believe that the satisfactory outcome of our patient was the result of two factors: the development of a personalized surgical procedure, and immediate identification of the pathogen and its antibiotic susceptibility following surgery. Resection of the two largest cerebral abscesses rapidly resolved the primary symptom (severe headache) of the patient at diagnosis, and prevented further complications that might have been caused by an increase in intracranial pressure. Our patient developed multiple non-uniform cerebral abscesses, most of which were located in important functional regions of the brain. However, preoperative head MRI revealed that the patient’s intracranial pressure was mainly caused by the two large abscesses and perilesional edema in the left parietal lobe and right frontal lobe. Given that the patient was in poor physical condition before surgery (multi-organ damage due to SLE), we selectively resected the two lesions in the left parietal lobe and right frontal lobe, and applied conservative treatment for the smaller lesions in the deeper parts of the brain. A phase II resection strategy was also planned, to be performed when necessary. In addition, we developed a rational anti-microbial regimen taking into account the patient’s renal function. Testing of pus samples aspirated from the cerebral abscesses and maxillofacial region confirmed Nocardia asteroides infection. As a review of the literature indicated that sulfonamides are currently the first-line treatment for Nocardia[12,13], we selected a combination of sulfamethoxazole-trimethoprim, ceftriaxone and amikacin to ensure treatment efficacy. After 3 wk of intravenous administration, the patient no longer experienced fever, and routine blood testing during follow-up indicated resolution of the infection. To reduce nephrotoxicity of the drugs and prevent further renal impairment, we switched to continuous oral sulfamethoxazole-trimethoprim plus minocycline for one year[14,15]. The antibiotic regimen described above showed good results and avoided secondary surgery. In addition, stopping immunosuppressants was also the right decision.
In summary, we can draw three conclusions from this case. First, SLE patients often have secondary infections due to corticosteroid and immunosuppressive treatments, and due to more emphasis on the lungs, the brain and other organs are often not screened. Second, timely discontinuation of MMF during infection was conducive to improving the patient's own immunity to fight the infection. This also indirectly relieved SLE due to reduction of the systemic inflammatory response, rather than suddenly aggravated by withdrawal of MMF. This also reflects the need for a holistic immune balance in the body. Third, timely identification of the pathogen and source of infection, treatment adjustment, by means of surgery, and development of a personalized antibiotic regimen can result in a satisfactory treatment outcome for rare diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Swai J S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Tektonidou MG, Wang Z, Dasgupta A, Ward MM. Burden of Serious Infections in Adults With Systemic Lupus Erythematosus: A National Population-Based Study, 1996-2011. Arthritis Care Res (Hoboken). 2015;67:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Keeling SO, Alabdurubalnabi Z, Avina-Zubieta A, Barr S, Bergeron L, Bernatsky S, Bourre-Tessier J, Clarke A, Baril-Dionne A, Dutz J, Ensworth S, Fifi-Mah A, Fortin PR, Gladman DD, Haaland D, Hanly JG, Hiraki LT, Hussein S, Legault K, Levy D, Lim L, Matsos M, McDonald EG, Medina-Rosas J, Pardo Pardi J, Peschken C, Pineau C, Pope J, Rader T, Reynolds J, Silverman E, Tselios K, Suitner M, Urowitz M, Touma Z, Vinet E, Santesso N. Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus. J Rheumatol. 2018;45:1426-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Vargas PJ, King G, Navarra SV. Central nervous system infections in Filipino patients with systemic lupus erythematosus. Int J Rheum Dis. 2009;12:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Horta-Baas G, Guerrero-Soto O, Barile-Fabris L. Central nervous system infection by Listeria monocytogenes in patients with systemic lupus erythematosus: analysis of 26 cases, including the report of a new case. Reumatol Clin. 2013;9:340-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Rudasill SE, Sanaiha Y, Xing H, Mardock AL, Khoury H, Jaman R, Ebrahimi R, Benharash P. Association of Autoimmune Connective Tissue Disease and Outcomes in Patients Undergoing Transcatheter Aortic Valve Implantation. Am J Cardiol. 2019;123:1675-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Napodano C, Pocino K, Rigante D, Stefanile A, Gulli F, Marino M, Basile V, Rapaccini GL, Basile U. Free light chains and autoimmunity. Autoimmun Rev. 2019;18:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Anagnostou T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis E. Nocardiosis of the central nervous system: experience from a general hospital and review of 84 cases from the literature. Medicine (Baltimore). 2014;93:19-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Penkert H, Delbridge C, Wantia N, Wiestler B, Korn T. Fulminant Central Nervous System Nocardiosis in a Patient Treated With Alemtuzumab for Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2016;73:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Restrepo A, Clark NM; Infectious Diseases Community of Practice of the American Society of Transplantation. Nocardia infections in solid organ transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clin Transplant. 2019;33:e13509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Mc-Nab P, Fuentealba C, Ballesteros F, Pacheco D, Alvarez M, Dabanch J, Cona E. [Nocardia asteroides infection in a patient with systemic lupus erythematosus]. Rev Med Chil. 2000;128:526-528. [PubMed] |
| 11. | Sanna G, Bertolaccini ML, Khamashta MA. Neuropsychiatric involvement in systemic lupus erythematosus: current therapeutic approach. Curr Pharm Des. 2008;14:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Valdezate S, Garrido N, Carrasco G, Medina-Pascual MJ, Villalón P, Navarro AM, Saéz-Nieto JA. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother. 2017;72:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Schlaberg R, Fisher MA, Hanson KE. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother. 2014;58:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Nakamura I, Nagakura T, Fujita H, Fukusima S, Gonoi T. Nocardia elegans infection: a case report and literature review. Int J Infect Dis. 2017;54:15-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 482] [Article Influence: 37.1] [Reference Citation Analysis (0)] |