Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1952
Peer-review started: August 28, 2021
First decision: November 17, 2021
Revised: December 1, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 26, 2022
Processing time: 179 Days and 2.3 Hours
Eosinophilia is an increase of more than 0.5 × 109/L in the number of eosinophils; it is a systemic condition with an unknown etiology and is often accompanied by multiple impaired organ functions. The clinical manifestations of the disease are highly variable and diverse, rendering identification of the diagnosis challenging; hence, diagnosis and treatment are often delayed. Very few reports of this disease exist globally, especially with rare manifestations of cerebral venous sinus thrombosis and hemorrhage.
A 32-year-old woman with eosinophilia presented to the hospital with bilateral lower-limb edema as the first clinical manifestation, followed by an extensive maculopapular rash throughout the body. She subsequently developed cerebral venous sinus thrombosis along with bilateral lower-limb deep vein thrombosis. Two weeks earlier, she had received a single course of antibiotics from a local hospital for a low-grade fever and sore throat. After various treatments were administered for anticoagulation, maintaining blood circulation, and relieving blood stasis, the lower extremity edema improved significantly; however, the patient’s eosinophil count gradually increased. She experienced cerebral venous sinus thrombosis, cerebral hemorrhage, and deep vein thrombosis of the lower limbs before being declared brain dead. In this case report, we have elaborated the diagnosis and management of deep vein thrombosis manifested as eosinophilia, thrombocytopenia, and elevated D-dimer levels.
Because proper diagnosis is challenging, clinical vigilance is required for patients with eosinophilia, as it can lead to thrombus formation.
Core Tip: A 32-year-old woman with eosinophilia developed venous thromboembolism as the disease progressed, complicated by cerebral venous sinus thrombosis and bleeding, until death. As eosinophilia can cause the blood to be in a hypercoagulable state, this may have been the main cause of the patient’s venous thromboembolism. Therefore, eosinophilia is one of the risk factors of venous thromboembolism, which should arouse clinical attention.
- Citation: Su WQ, Fu YZ, Liu SY, Cao MJ, Xue YB, Suo FF, Liu WC. Eosinophilia complicated with venous thromboembolism: A case report. World J Clin Cases 2022; 10(6): 1952-1960
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1952.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1952
Eosinophilia is defined as an eosinophil count of more than 0.5 × 109/L in healthy individuals. It is a systemic condition with an unknown etiology and is often accompanied by multiple impaired organ functions that include multi-system involvement, such as gastrointestinal, dermatological, central nervous, cardiovascular, and portal venous systems. The most commonly involved organs are the skin, heart, and brain; however, venous thromboembolism (VTE), membranous nephropathy, ocular manifestations, and torticollis have also been reported[1-3]. The clinical manifestations of the disease are highly variable and diverse, rendering the diagnosis challenging; hence, diagnosis and treatment are often delayed. Very few reports of this disease exist globally, especially with rare manifestations such as cerebral venous sinus thrombosis and hemorrhage.
This is a case report of a patient with eosinophilia who initially presented with bilateral lower-limb edema, followed by an extensive maculopapular rash throughout the body. She subsequently developed cerebral venous sinus thrombosis along with bilateral lower-limb deep vein thrombosis. Further, we describe the diagnosis and management of deep vein thrombosis manifested as eosinophilia, thrombocytopenia, and elevated D-dimer levels.
A 32-year-old woman presented to the hospital with the chief complaint of bilateral lower limb swelling for two days.
The patient had developed bilateral lower limb edema two days earlier, with increased severity on the right thigh; however, the superficial veins of the lower extremities were not tortuous. Color Doppler ultrasound of the lower extremities revealed insufficiency of the right common femoral venous valve, and the patient was admitted to our hospital for further treatment.
Two weeks prior, the patient had suffered from a low-grade fever that peaked at 37.3 ℃, which was accompanied by a sore throat. She had received a single course of antibiotics from a local hospital, the specifications of which could not be ascertained. Following symptomatic improvement, no further investigations were performed or treatment administered. The patient did not experience discomfort, such as chills or chest tightness.
There was no history of drug allergy, family history of inherited or systemic diseases, and no history of special exposure.
On admission to the hospital, the patient’s vital signs were within the normal limits, with the presence of bilateral palpable enlarged superficial inguinal lymph nodes. Bilateral lower-limb pitting edema to the mid-thigh was observed, with increased severity on the right side. Furthermore, a mass of approximately 5 cm × 3 cm was palpated on the lateral aspect of the right thigh, which was firm in consistency, non-pulsating, and non-tender. The pulsations of the bilateral dorsal foot arteries were maintained. On cardiopulmonary examination, no abnormalities were detected.
The total leucocyte count in the blood was 10.31 × 109/L, comprising 60.3% neutrophils and 15.5% eosinophils; the eosinophil count was 1.60 × 109/L; hemoglobin level was 124 g/L; and platelet count was 224 × 109/L. The level of high-sensitivity C-reactive protein (hs-CRP) in the blood was 7.31 mg/L; serum electrolyte potassium level was 3.06 mmol/L; and degradation product, D-dimer level was 0.64 mg/L fibrinogen equivalent units (FEU). Liver and kidney function, and routine urine and stool test results were within normal limits; stool test results for liver fluke eggs, fungi, and infectious diseases tests were all negative.
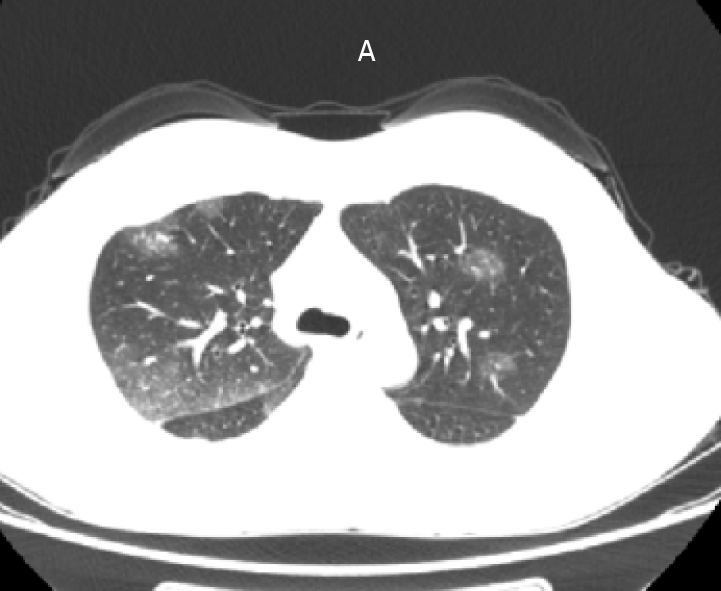
Chest radiography revealed no abnormalities in the heart, lungs, and diaphragm; cardiac color ultrasound revealed minimal tricuspid regurgitation and reduced left ventricular diastolic function (35%), while the left ventricular ejection fraction was normal (63%). Superficial inguinal color ultrasound revealed five hypoechoic nodules in the right groin area, the largest measuring approximately 22 mm × 7 mm, and approximately six enlarged left superficial inguinal lymph nodes, the largest measuring approximately 24 mm × 7 mm.
After admission, the patient received 4000 U of low-molecular-weight heparin once daily to promote blood circulation and relieve stasis, reduce platelet aggregation, improve valvular function, and prevent infection. After treatment, the lower-limb edema reduced; however, the mass on the lateral right thigh was still palpable. On Day 7 of admission, the patient developed pain in the lateral thigh mass of the right lower limb following intermittent pneumatic compression therapy. Emergency bedside color Doppler ultrasound revealed lower limb muscle edema and no obvious abnormalities in the lower limb vessels. The blood tests were repeated and revealed a leucocyte count of 13.77 × 109/L, comprising 59.5% neutrophils and 26.4% eosinophils; hs-CRP, 8.50 mg/L; D-dimer, 1.28 mg/L FEU; and fibrin (proto) degradation product, 5.40 mg/L.
Following the administration of 10 mg morphine hydrochloride injection subcutaneously, the patient’s pain was relieved. Computed tomography (CT) scan of the lower abdomen revealed right thigh subcutaneous edema of approximately 18 cm × 15 cm adjacent to five further enlarged right deep inguinal lymph nodes, the largest measuring approximately 22 mm × 8 mm. Magnetic resonance imaging (MRI) revealed soft tissue swelling and exudative-like changes in the superior portion of the right mid-thigh, with five enlarged deep inguinal lymph nodes adjacent to the iliac vessels, the largest measuring approximately 24 mm × 8 mm.
On Day 10 of admission, the patient developed extensive erythematous macules throughout the body. Anti-allergic treatment was administered, including dexa
The rheumatology and immunology departments were consulted, and color Doppler ultrasound was performed for further examination of the superficial lymph nodes. No obvious enlarged lymph node echo in the bilateral supraclavicular fossae were noticed; however, multiple bilateral axillary lymph nodal echoes were observed, the largest of those measuring approximately 11 mm × 7 mm and 14 mm × 6 mm on the right and left sides, res
Blood tests were repeated again on Day 10 and revealed a leucocyte count of 15.58 × 109/L, comprising 46.5% neutrophils and 36.7% eosinophils; eosinophil count, 5.72 × 109/L; platelet concentration, 108 × 109/L; hs-CRP, 32.52 mg/L; erythrocyte sedimentation rate, 28 mm/h; D-dimer, 4.18 mg/L FEU; fibrin (proto) degradation product, 5.86 mg/L; and aspartate aminotransferase (for myocardial enzyme spectrum) 88 U/L; the remaining parameters were within normal limits. Liver function tests revealed alanine aminotransferase, 69 U/L; aspartate aminotransferase, 88 U/L; and serum potassium, 2.96 mmol/L.
A hematology consultation was recommended to further improve the related parameters. The antineutrophil cytoplasmic antibodies, antinuclear antibody spectrum, anticardiolipin antibodies, and direct Coombs test results were all negative, while thyroid function test results were within normal limits. The immune function tests revealed, immunoglobulin E, 348.81 IU/mL; complement C3, 0.83 g/L; and cytomegalovirus IgG antibody, 279.273 AU/L. The leucocyte count was 12.13 × 109/L, comprising 23.1% neutrophils and 58.1% eosinophils; eosinophil count, 7.06 × 109/L; and platelet count, 120 × 109/L.
On Day 14 of admission, the patient was transferred to the rheumatology department for further treatment. Screening tests for gynecological tumors and respiratory viral infections were negative. Bone marrow aspiration and biopsy results were consistent with high eosinophilia and did not support the morphological changes associated with myelodysplastic syndrome, plasma cell myeloma, or lymphoma; the anti-allergic treatment was continued. On Day 15 of admission, rashes over the entire body subsided significantly. Blood tests revealed a leucocyte count of 15.57 × 109/L, comprising 33.4% neutrophils and 53.3% eosinophils; eosinophil count, 8.3 × 109/L; and platelet count, 80 × 109/L.
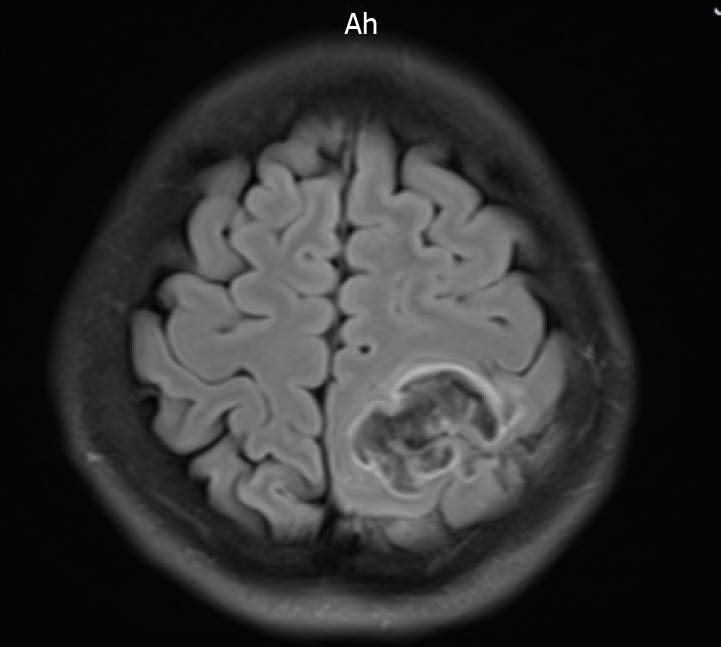
At 3:00 AM on Day 18 of admission, the patient complained of headache, involuntary movements of the right upper limb, and decreased sensation in both the right limbs; she had no other symptoms, such as diplopia and cough. The results of an urgent cranial MRI + magnetic resonance angiography (MRA) + magnetic resonance venography (MRV) revealed the following (Figure 2): (1) Abnormal signals on the left side at the fronto-parietal junction, indicating the formation of a hematoma; (2) Abnormal signals in the right frontal lobe, indicating the possibility of a small amount of hemorrhage; (3) Hypertrophy of the left inferior turbinate; (4) No obvious abnormalities observed on the MRA scan; and (5) Brain MRV revealing (I) Superior sagittal abnormal changes in the blood vessels above the sinus and in the adjacent areas, indicating the possibility of venous sinus thrombosis and (II) Narrowing in the left transverse sinus compared to the opposite side. The patient was transferred to the neurology department for further diagnosis and treatment.
Physical examination following the transfer revealed the following findings: bilateral pupils reactive and equidistant, central position of the tongue, reduced muscle tone of the right limbs, right upper limb muscle strength level 2, and right lower limb muscle strength level 3. The pain on the right side decreased, no pathological reflex was elicited, and meningeal irritation sign was negative. The patient underwent a lumbar puncture; the pressure was 230 mm H2O during the procedure; 20% mannitol injection 125 mL was administered every 8 h and citicoline injection 1.0 g was administered once daily.
On Day 18 of admission, an episode of projectile vomiting occurred, along with gradual loss of consciousness and limb stiffness. An urgent head CT revealed increased left frontal and parietal hemorrhage, subarachnoid hemorrhage of 15 mm × 20 mm, midline-shift, and worsening of the brain swelling; the patient underwent neurosurgery on the same day. During the surgery, brain tissue swelling, multiple subarachnoid hemorrhages on the surface, venous congestion and stasis, disappearance of brain tissue pulsation, and a fistula at the superior frontal region with a dark red hematoma at a depth of approximately 1 cm were observed. Anterior and posterior exploration of the hematoma was performed, and it was drained. Venous blood was oozing from multiple points. The bleeding was controlled while removing the dead brain tissue.
After surgery, the left and right pupils measured 3.5 mm and 2.5 mm, respectively, and bilateral loss of response to light was observed. On Day 19 of admission (postoperative day 1), a venous color Doppler ultrasound revealed no thrombosis in the lower limb, and no obvious abnormalities in the heart were observed on the color Doppler ultrasound. Blood tests revealed a leucocyte count of 13.33 × 109/L, comprising 65.0% neutrophils and 9.3% eosinophils; eosinophil count, 1.24 × 109/L; and platelet count, 40 × 109/L. Liver function tests revealed albumin, 25.4 g/L and alanine aminotransferase, 50 U/L.
On Day 20 of admission (postoperative day 2), the blood test results revealed a leucocyte count of 11.03 × 109/L, comprising 78.7% neutrophils and 8.6% eosinophils; eosinophil count, 0.95 × 109/L; and platelet count, 47 × 109/L.
(1) Secondary (reactive) eosinophilia; (2) VTE; (3) Cerebral venous sinus thrombosis; and (4) Cerebral hemorrhage.
On Day 12 of admission, low-molecular-weight heparin and amoxicillin/potassium clavulanate were discontinued; potassium chloride sustained-release tablets 1 g twice daily and loratadine oral tablets 10 mg once daily were initiated.
On Day 30 of admission (postoperative day 12), lower limb venous Color Doppler ultrasound revealed left lower limb femoral vein, popliteal vein, and posterior tibial vein thrombosis, indicating ineffective anticoagulation treatment due to the occurrence of cerebral hemorrhage; the patient was declared brain dead.
First treatment: Low-molecular-weight heparin 4000 U once daily for 9 d, amoxicillin/potassium clavulanate 1.2 g every 12 h for 12 d.
Second treatment: 20% mannitol injection 125 mL every 8 h, citicoline injection 1.0 g once daily for 2 d.
Third treatment: 20% mannitol injection 125 mL every 8 h, cefoperazone/sulbactam injection 3 g every 8 h, methylprednisolone sodium succinate 3 g every 12 h for 10 d.
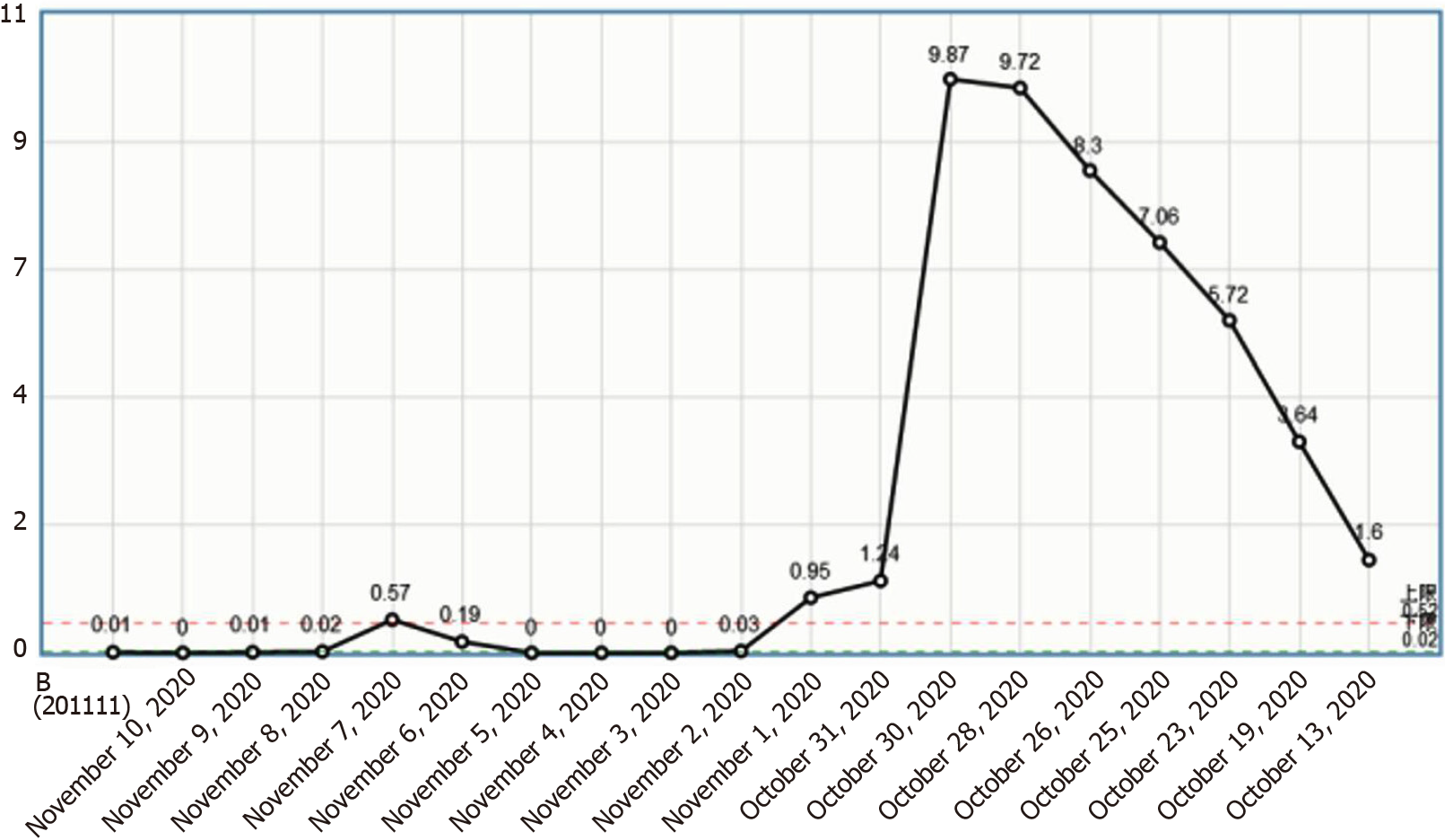
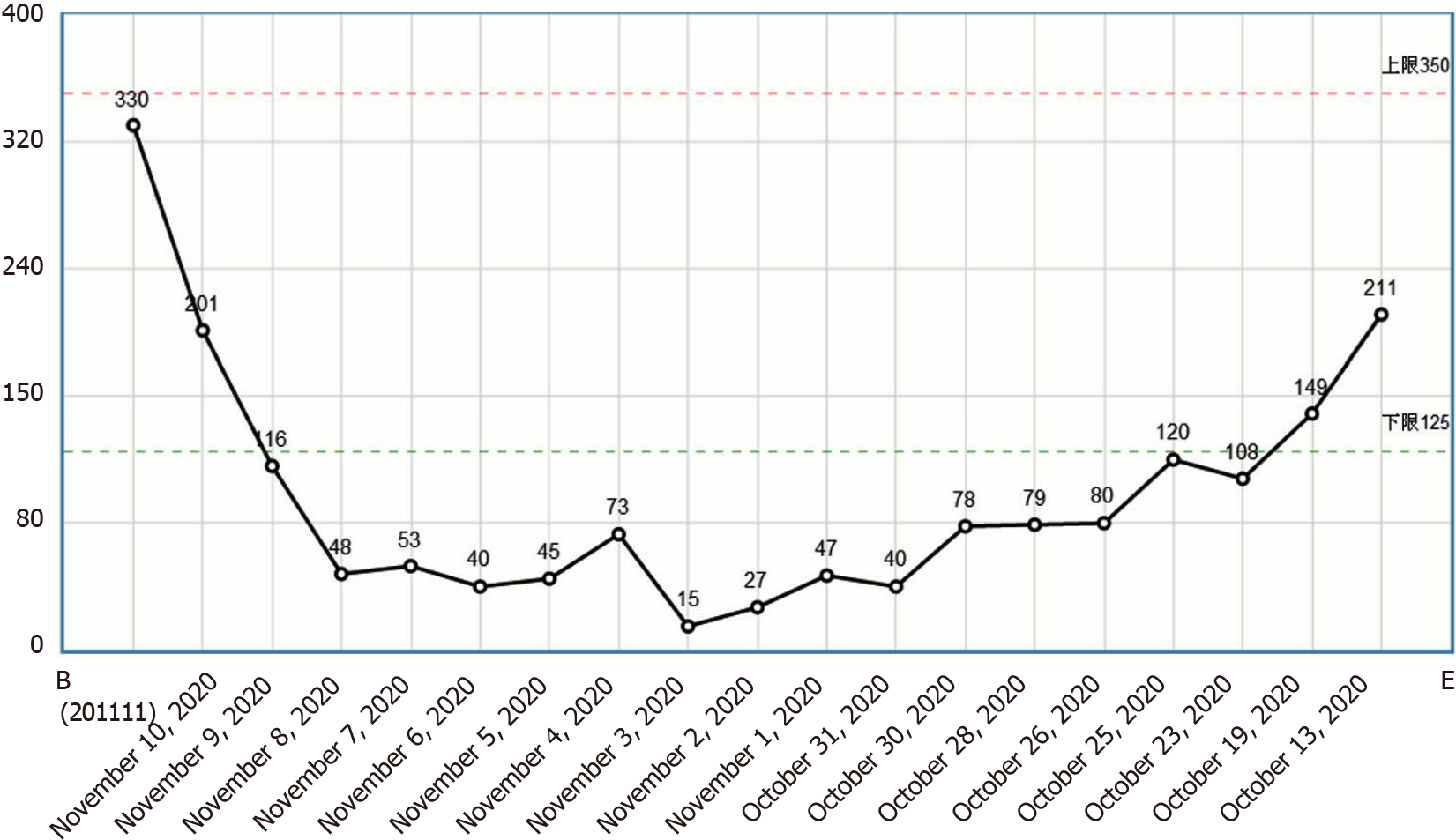
The patient was a young woman with no history of tropical travel; she had a history of pre-infection and was admitted to the hospital with bilateral lower-limb edema as the chief complaint along with the presence of risk factors for thrombosis. After various treatments were administered for anticoagulation, maintaining blood circulation, and relieving blood stasis, the lower extremity edema improved significantly; however, the patient’s eosinophil count gradually increased (Figure 3). She experienced cerebral venous sinus thrombosis, cerebral hemorrhage, and deep vein thrombosis of the lower limbs before being declared brain dead.
There are three elements of VTE: (1) Stagnation of blood flow; (2) Hypercoagulable state of blood; and (3) Injury of blood vessel walls. The initiating factor of VTE in this patient was considered to be reactive eosinophilia. While participating in the normal immune defense response, eosinophils can also cause tissue cell damage. Eosinophils can act on the body's blood coagulation and anticoagulation system. On the one hand, it promotes blood coagulation and on the other hand inhibits anticoagulation, which eventually leads to thrombosis. There are three main mechanisms of eosinophilia that can act on the body through multiple pathways to cause VTE[4]: (1) Major basic protein (MBP), eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), and ethylene glycol dinitrate are released through degranulation. These cytotoxic cations can damage the vascular endothelial cells. At the same time, MBP, ECP, and EPO can also increase the activity of tissue factor, factor VII, factor X, and other coagulation factors; activate the endogenous coagulation pathway; inhibit the production of activated protein C; and cause blood hypercoagulability; (2) Eosinophils can directly activate tissue factors and platelet-activating factors; leukotrienes activate exogenous coagulation pathways, activate and aggregate platelets, and promote thrombosis; and (3) Direct infiltration causes vascular endothelial cell damage. Activated eosinophils can also express CD40 Ligand (CD40L), and the CD40/CD40L system plays a role in inflammation, endothelial cell dysfunction, platelet activation and coagulation activation. Through the effects of all the above aspects, eventually thrombin and fibrin are continuously generated, which eventually leads to thrombus formation.
Eosinophilia is often accompanied by independent diseases; at present, there is no uniform standard for the classification of eosinophil count; values < 1.0 × 109/L, (1.0-5.0) × 109/L, and > 5.0 × 109/L generally indicate mild, moderate, and severe rise, respectively. Hypereosinophilic syndrome (HES) can be divided into four categories: hereditary (familial) HES, primary (tumor) HES, secondary (reactive) HES, and idiopathic HES[5]. We believe that the cause of HES in our patient was drug cross-reactivity due to previous antibiotic administration; thus, the patient developed secondary HES.
The most common secondary causes of eosinophilia are parasitic infections, drug allergies, allergic conditions, skin diseases, and respiratory and gastrointestinal tract diseases; however, the cause of the findings observed in our patient was not clear. The patient underwent several blood tests in our hospital, including tests for parasitic infection, asthma, skin diseases, and tests for other related causes. Prior to hospital admission, the patient’s blood test results revealed a normal eosinophil count, and a single course of antibiotics was administered (the specific medication is unknown). Therefore, based on the relevant adverse drug reactions identified, clinicians should consider the possibility of cross-allergic reactions to drugs in patients with eosinophilia; thus, adverse reactions to drugs during clinical administration should be predicted and evaluated. This emphasizes the importance of understanding and observing drug allergic reactions to minimize the occurrence of drug-induced diseases. In this case, the causes of death were cerebral venous sinus thrombosis and cerebral hemorrhage. Considering that eosinophilia is caused by the hypercoagulation of blood, it should attract clinical attention; however, the mechanism underlying eosinophilia leading to thrombosis has not been fully elucidated. Nevertheless, patients with eosinophilia are considered to be in a prethrombotic state and tend to develop thrombosis[6-8].
The patient’s right thigh was swollen and painful. MRI revealed that the soft tissue in the upper and middle part of the right thigh was swollen with exudative-like changes. Considering the patient’s increased eosinophil count, the edema could have been attributed to eosinophilic fasciitis, a connective tissue disease that is usually caused by overwork and a response to immunosuppression[9]. Biopsy is an important means of diagnosis of eosinophilic fasciitis. Fascial skin biopsy showed evidential fascia thickening and infiltration of lymphocytes and plasma cells, giving rise to inflammatory conditions[10]. Fascia fibrosis was also detected. However, the patient did not undergo a muscle biopsy as she had a decreased eosinophil count during follow-up treatment. Moreover, the symptoms of edema of the right thigh muscle disappeared, which also indirectly supports the possibility of eosinophilia.
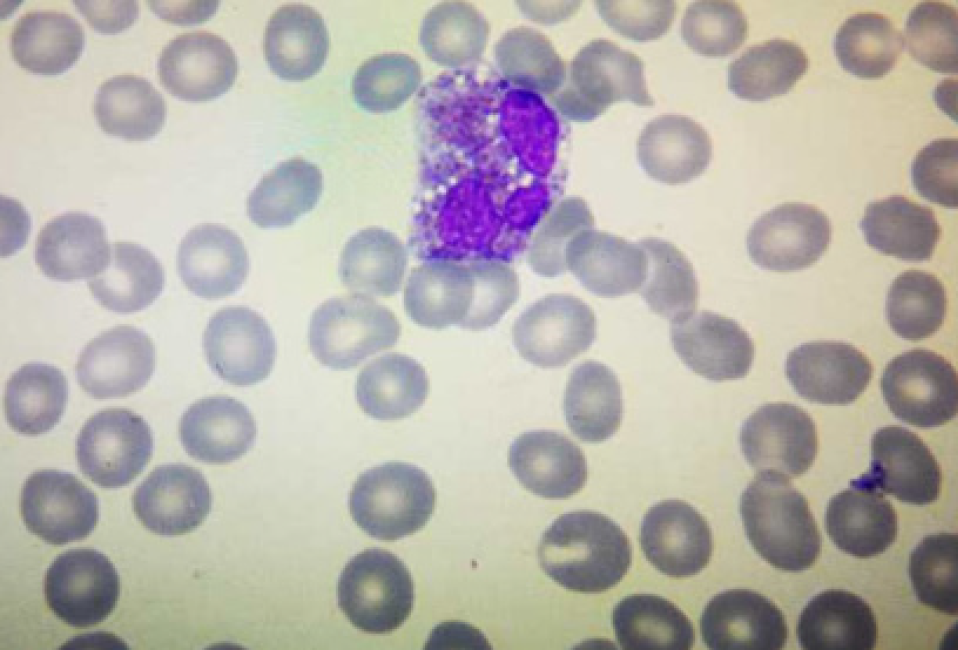
This patient had increased eosinophils and a progressive decline in the platelet count (Figure 4). The mechanism underlying this phenomenon could be that eosinophils inhibit bone marrow megakaryocytes, causing the megakaryocytes to mature and become plaque-producing. The reduction of megakaryocytes reduces the number of platelets circulating in the peripheral blood, and further platelets are consumed by the thrombus, thereby exacerbating thrombocytopenia. On one hand, glucocorticoid therapy can effectively control the eosinophil count and avoid target organ damage; on the other hand, it can also restore the number of platelets[2]. However, the patient’s condition progressed rapidly, and she was not treated with glucocorticoids before hemodynamic diseases were ruled out, which caused platelet activation and aggregation followed by thrombosis. Clinical vigilance is required for eosinophilia because a high eosinophil count can cause vascular endothelial damage and easily lead to thrombus formation[11]; however, the most important cause of VTE events is the release of basic proteins and other substances following degranulation. The eosinophil count indicates the degree of organ damage; therefore, the authors recommend routine peripheral blood smears for patients with eosinophilia to observe whether there are changes in degranulation. If there are changes in degranulation (Figure 5), active treatment to avoid organ damage and VTE events is recommended. For patients with unexplained eosinophilia, active administration of glucocorticoids is recommended in the absence of contraindications for glucocorticoids; similarly, anticoagulation therapy is recommended as soon as possible in the absence of contraindications for anticoagulation therapy. The ultimate goal is to reduce the eosinophil count and to prevent VTE events, especially fatal adverse events such as cerebral venous sinus thrombosis and pulmonary embolism.
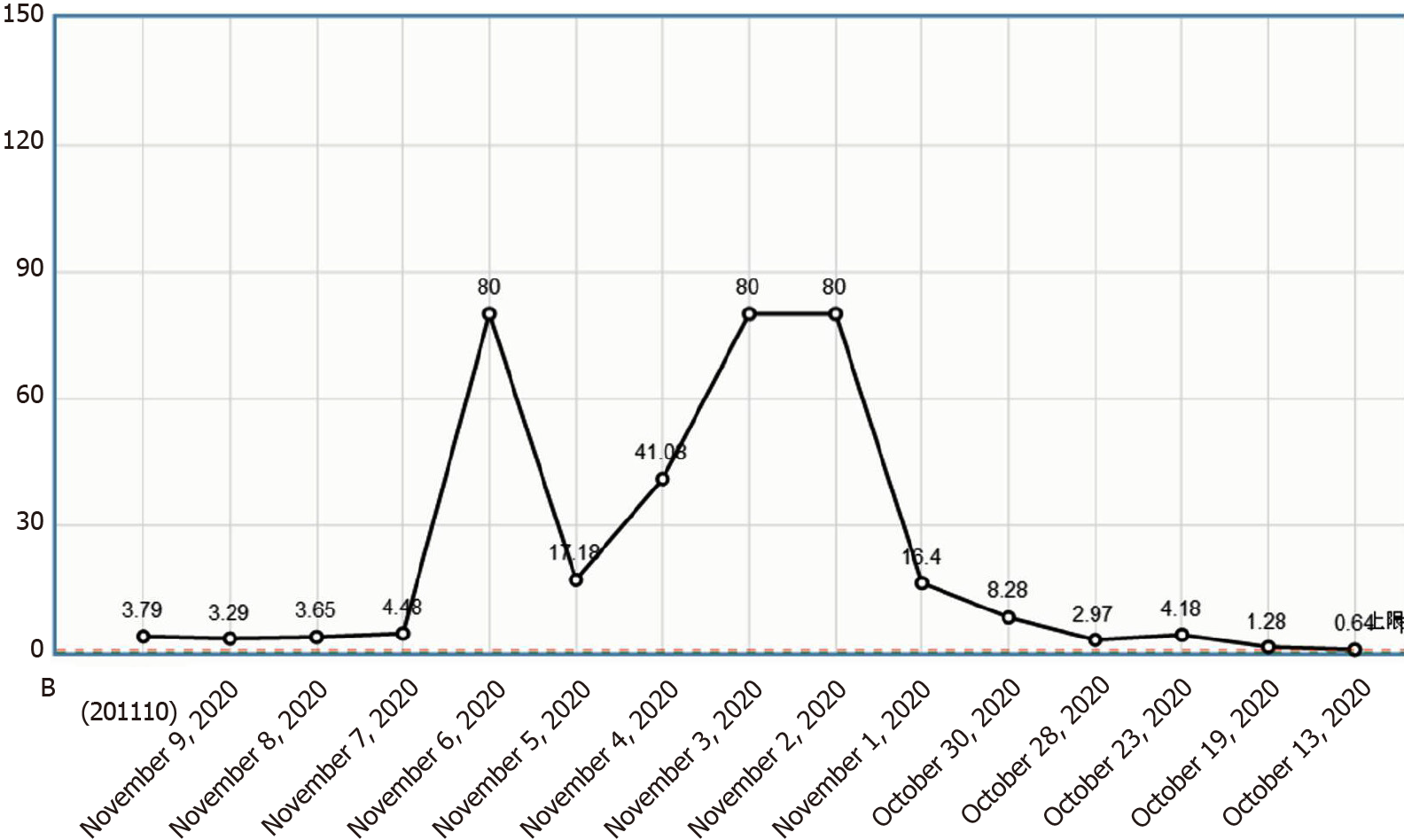
This patient had increased eosinophil and D-dimer levels (Figure 6). She was admitted to the hospital with edema of bilateral lower extremities and administered preventive doses of anticoagulant therapy. Anticoagulation therapy was discontinued for allergen screening during follow-up treatment. Cerebral venous sinus thrombosis and deep vein thrombosis of the lower extremities occurred. Due to the combination of cerebral hemorrhage and thrombocytopenia, anticoagulation therapy was not administered. Anticoagulation therapy may benefit patients in the context of assessing the risk of bleeding; however, further studies are warranted.
In patients with eosinophilia, the skin is one of the most frequently affected organs; pathological features of eosinophilia include eosinophil infiltration and/or eosinophil degranulation[12]. Skin biopsy shows inflammatory changes in blood vessels, and the surrounding blood vessels are mainly infiltrated by mild to moderate eosinophils and monocytes[2]. In 2006, Caputo et al[13] proposed seven classifications for eosinophilic cellulitis: fixed drug eruption, plaque, bullous, granuloma annulare, papule, papule nodular, and urticaria-like. The patient’s skin over the entire body was covered in rashes, which was accompanied by itching, especially at night. Considering that this may be related to diurnal fluctuations in the release of glucocorticoids from the adrenal cortex, the patient was administered symptomatic treatments such as loratadine, levocetirizine, and calcium gluconate; the rash over her body subsided, and the skin and mucous membranes returned to normal.
In conclusion, eosinophilia can cause hypercoagulation of the blood, which can cause thrombosis in the veins of the lower extremities and heart, or even embolisms in the blood vessels of the lungs and brain. Early diagnosis is difficult; hence, vigilance is recommended to prevent a high mortality rate.
Provenance and peer review: Unsolicited article; externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta T, Joalsen I S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Li D, Xu L, Lin D, Jiang S, Feng S, Zhu L. Acute pulmonary embolism and deep vein thrombosis secondary to idiopathic hypereosinophilic syndrome. Respir Med Case Rep. 2018;25:213-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Kim JA, Kim N, Choi HS, Choung HK, Khwarg SI. Eyelid Swelling and Subconjunctival Infiltration as Ophthalmic Manifestations in a Child with Idiopathic Hypereosinophilic Syndrome. Korean J Ophthalmol. 2018;32:517-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Das JK, Gupta K, Deshmukh S, Shrivastava R. A rare case of hypereosinophilic syndrome presenting with unilateral proptosis and torticollis. Indian J Ophthalmol. 2018;66:1508-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Noh HR, Magpantay GG. Hypereosinophilic syndrome. Allergy Asthma Proc. 2017;38:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Leukemia and Lymphoma Group; Chinese Society of Hematology; Chinese Medical Association. . [Chinese expert consensus on the diagnosis and treatment of eosinophilia (2017)]. Zhonghua Xueyexue Zazhi. 2017;38:561-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Maino A, Rossio R, Cugno M, Marzano AV, Tedeschi A. Hypereosinophilic syndrome, Churg-Strauss syndrome and parasitic diseases: possible links between eosinophilia and thrombosis. Curr Vasc Pharmacol. 2012;10:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Sneeboer MMS, Majoor CJ, de Kievit A, Meijers JCM, van der Poll T, Kamphuisen PW, Bel EH. Prothrombotic state in patients with severe and prednisolone-dependent asthma. J Allergy Clin Immunol. 2016;137:1727-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Ames PR. Recurrent abdominal thrombosis despite heparin thromboprophylaxis in a patient with transient eosinophilia. Clin Appl Thromb Hemost. 2011;17:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Dong N, Dong XY. [Pediatric idiopathic hypereosinophilic syndrome with pulmonary embolism: a case report and review of literature]. Zhonghua Er Ke Za Zhi. 2017;55:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ihn H. Eosinophilic fasciitis: From pathophysiology to treatment. Allergol Int. 2019;68:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Sturdy A, Stratton R, Perez-Machado M, Lamb L. Case of eosinophilic fasciitis during military training in a Nepalese British infantry soldier. BMJ Mil Health. 2020;166:277-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Peckruhn M, Elsner P, Tittelbach J. Eosinophilic dermatoses. J Dtsch Dermatol Ges. 2019;17:1039-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Caputo R, Marzano AV, Vezzoli P, Lunardon L. Wells syndrome in adults and children: a report of 19 cases. Arch Dermatol. 2006;142:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |