Published online Feb 26, 2022. doi: 10.12998/wjcc.v10.i6.1775
Peer-review started: September 8, 2021
First decision: October 27, 2021
Revised: November 14, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 26, 2022
Processing time: 168 Days and 8.9 Hours
Although bilirubin is known to be an antioxidant, any relationship with coronary heart disease remains controversial. To the best of our knowledge, no previous study has investigated the association between bilirubin and perioperative myocardial infarction (PMI), including its long-term prognosis.
To investigate the impact of bilirubin levels on PMI in patients undergoing percutaneous coronary intervention (PCI), and long-term prognosis in post-PMI patients.
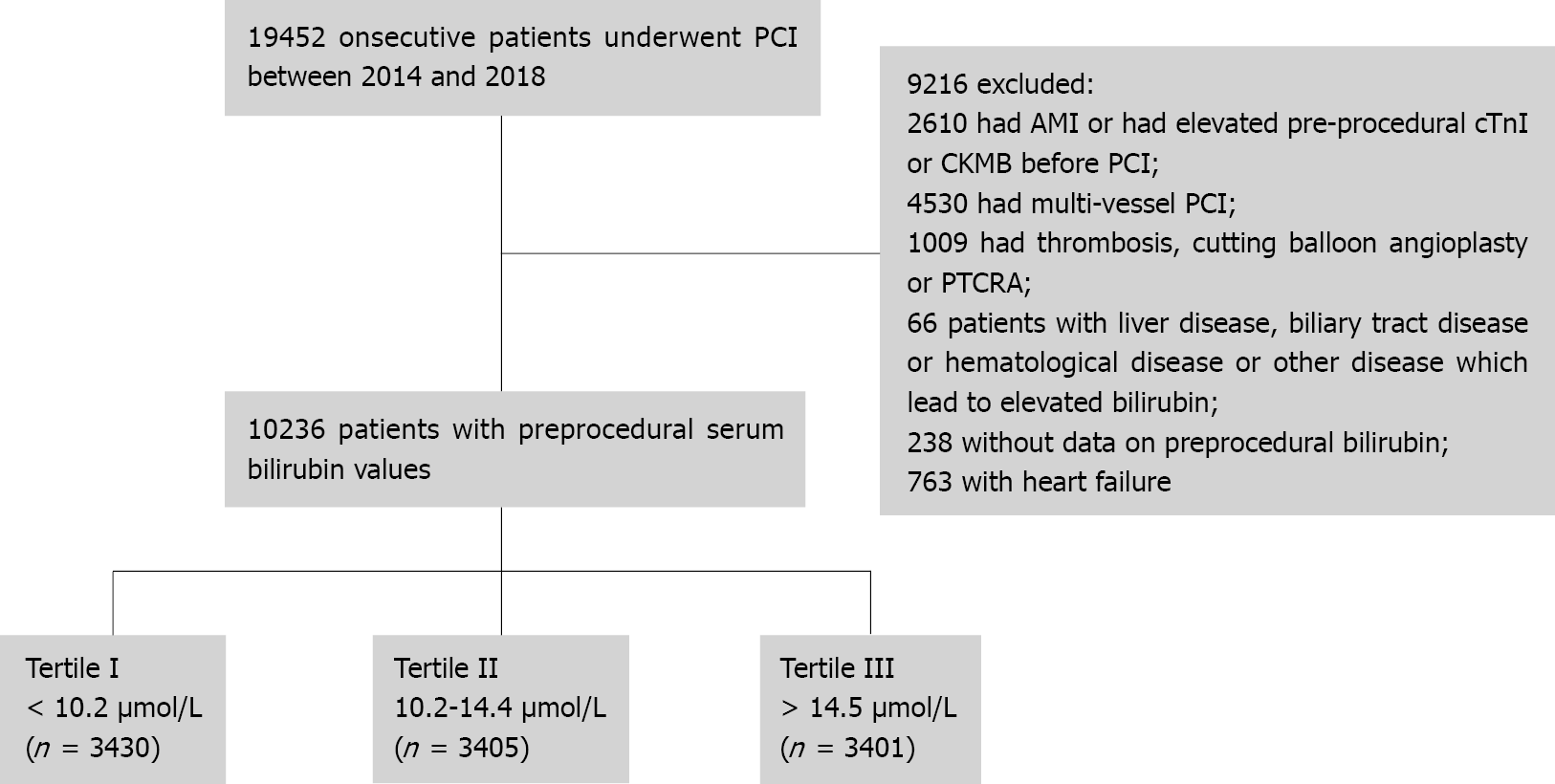
Between January 2014 and September 2018, 10236 patients undergoing elective PCI were enrolled in the present study. Total bilirubin (TB) and cardiac troponin I (cTnI) levels were measured prior to PCI and cTnI at further time-points, 8, 16 and 24 h after PCI. Participants were stratified by pre-PCI TB levels and divided into three groups: < 10.2; 10.2-14.4 and > 14.4 μmol/L. PMI was defined as producing a post-procedural cTnI level of > 5 × upper limit of normal (ULN) with normal baseline cTnI. Major adverse cardiovascular events (MACEs) included cardiac death, MI, stroke and revascularization during a maximum 5-year follow-up.
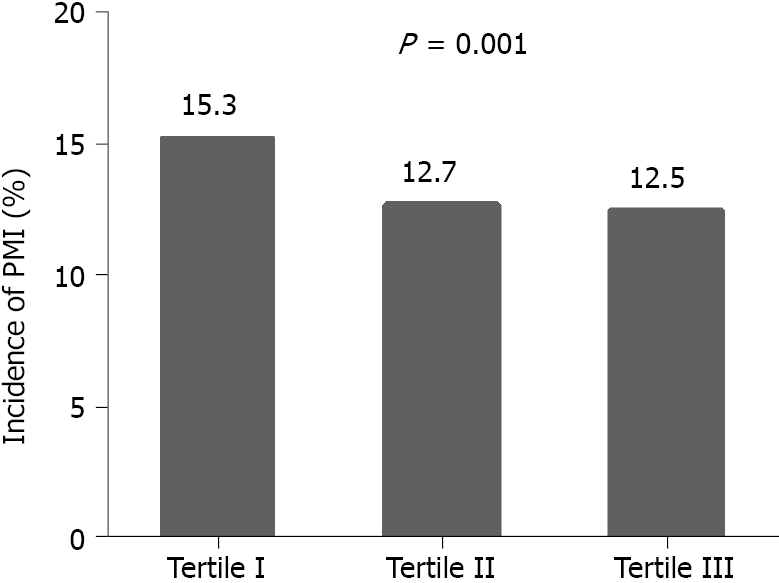
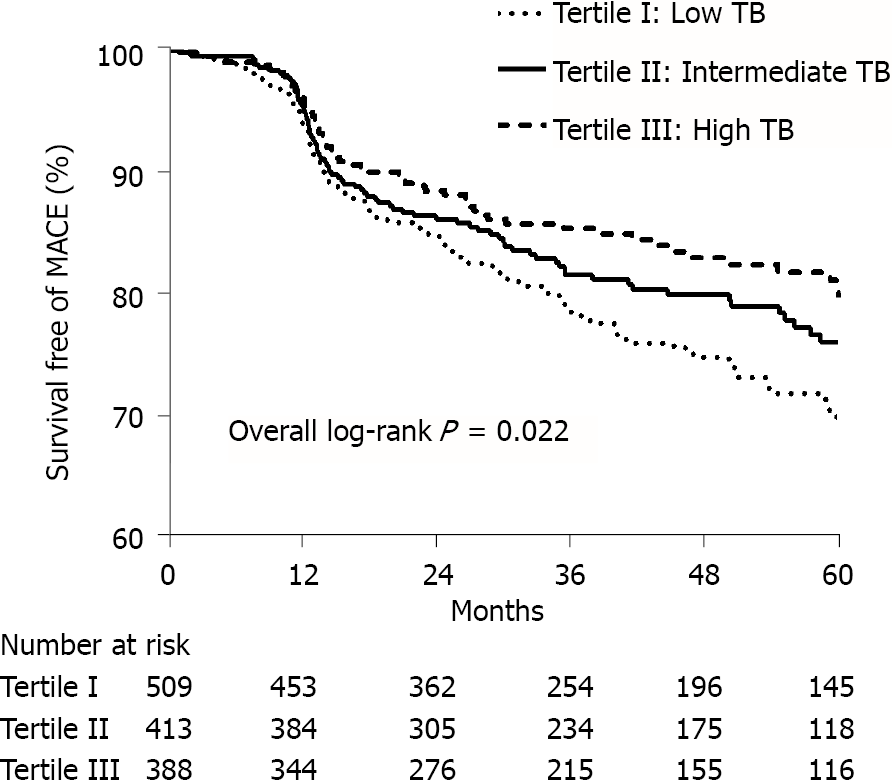
PMI was detected in 526 (15.3%), 431 (12.7%) and 424 (12.5%) of patients with pre-PCI TB levels of < 10.2, 10.2-14.4 and > 14.4 μmol/L (P = 0.001), respectively. Multivariate logistical analysis indicated that patients with TB 10.2-14.4 and > 14.4 μmol/L had a lower incidence of PMI [TB 10.2-14.4 μmol/L: Odds ratio (OR): 0.854; 95% confidence interval (CI): 0.739-0.987; P = 0.032; TB > 14.4 μmol/L: OR: 0.846; 95%CI: 0.735-0.975; P = 0.021] compared with patients with TB < 10.2 μmol/L. Construction of a Kaplan-Meier curve demonstrated a higher MACE-free survival time for patients with higher TB than for those with lower TB (log-rank P = 0.022). After adjustment for cardiovascular risk factors and angiographic characteristics, multivariate Cox analysis showed that a TB level > 14.4 μmol/L was associated with a reduced risk of MACEs compared with a TB level < 10.2 μmol/L (hazard ratio 0. 667; 95%CI: 0.485-0.918; P = 0.013).
Bilirubin was a protective factor in PMI prediction. For post-PMI patients, elevated bilirubin levels were independently associated with a reduced risk of MACEs during long-term follow-up.
Core Tip: Perioperative myocardial infarction (PMI) is a frequent complication of percutaneous coronary intervention, with an adverse long-term outcome. Previous studies have sought to identify potential targets for PMI avoidance. The current study was designed to explore the effect of bilirubin on PMI and its utility for long-term prognosis. Bilirubin has a protective effect making it a suitable predictor of PMI. Furthermore, elevated levels of bilirubin are associated with a reduced risk of major adverse cardiovascular events during long-term follow-up of post-PMI patients. We present evidence of the suitability of bilirubin as a therapeutic target for PMI prevention and other oxidative diseases.
- Citation: Li Y, Li DB, Zhao LD, Lv QB, Wang Y, Ren YF, Zhang WB. Effects of bilirubin on perioperative myocardial infarction and its long-term prognosis in patients undergoing percutaneous coronary intervention. World J Clin Cases 2022; 10(6): 1775-1786
- URL: https://www.wjgnet.com/2307-8960/full/v10/i6/1775.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i6.1775
Perioperative myocardial infarction (PMI) is a frequent complication of percutaneous coronary intervention (PCI)[1,2]. Despite technological advances over the past two decades, the frequency of PMI remains between 5% and 30% with higher rates in patients with complex lesions. PMI may occur via several mechanisms, including side branch occlusion, distal embolization, inflammation and endothelial injury, all of which may contribute to myocardial damage[3]. Many other causative factors affecting PMI risk remain controversial.
Traditionally, the end product of heme catabolism, bilirubin, has been considered a cytotoxic waste product. More recently, an appreciation of its anti-oxidant and anti-inflammatory effects, involving scavenging of ROS to improve vascular and microvascular dysfunction, has emerged[4-6]. Indeed, it is more than 20 years since the antioxidant role of bilirubin in in vivo ischemia-reperfusion was established[7] and, more recently, Bösch et al[8] also found an ameliorating effect on ischemia reperfusion damage in mice[8]. A number of recent clinical studies have reported a protective role of bilirubin in coronary artery disease (CAD)[4-6], although there are also some contradictory reports[9,10]. Indeed, elevated bilirubin has been associated with increased in-hospital mortality in acute coronary syndrome[11] and positively correlated with SYNTAX score[10]. Thus, the relationship between bilirubin and CAD remains controversial. No previous study has investigated its effect on PMI and its utility for long-term prognosis.
The current study was designed to explore the relationship between bilirubin and PMI in patients undergoing PCI and its utility for predicting long-term outcomes. The following article is presented in accordance with the STROBE reporting checklist.
The current retrospective study enrolled 10263 patients who had been diagnosed with CAD without pre-PCI elevation of cardiac troponin I (cTnI) between January 2014 and September 2018. All patients had elected to have single-vessel PCI. Patients were excluded for the following reasons: (1) Acute or chronic liver injury, biliary tract disease, hematological disease, vitamin B12 deficiency, heart failure or other factors leading to elevated bilirubin; (2) Acute myocardial infarction (MI) in the previous 4 wk; (3) Elective PCI for chronic total occlusion; and (4) Intraoperative factors leading to elevated cTnI, including side-branch occlusion during the procedure, severely calcified lesions with a rotablator or dissection, to enable evaluation of bilirubin effects with less confounding intraoperative factors. Acute liver injury was screened with an acute elevation of transaminases. Chronic liver injury was screened mainly by chronic elevation of transaminases with the case history, such as viral hepatitis, fatty liver disease, alcoholic hepatitis, autoimmune liver disease, biliopancreatic disease, drug-induced liver injury, liver cancer, liver cirrhosis and so on. Heart failure was defined according to the 2021 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure[12].
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of Sir Run Run Shaw Hospital.
Stent implantation was performed by experienced cardiac surgeons using the radial artery approach, according to current clinical practice. Patients were treated with aspirin (100 mg/night) and P2Y12 inhibitor (clopidogrel: 75 mg/d or ticlopidine: 180 mg twice daily) for three days before PCI. In the absence of pre-treatment, patients received 300 mg aspirin plus 300 mg clopidogrel or 180 mg ticlopidine before the operation as a loading dose. CTnI levels were measured by immunoassay pre-PCI and at 8, 16 and 24 h post-PCI. The peak value of cTnI over 24 h was used for analysis [upper limit of normal (ULN): 0.011 ng/mL]. Serum bilirubin level was measured before PCI.
PMI was defined as a post-procedural cTnI > 5 × ULN (revised diagnosis criteria from the third or fourth version of the universal MI definition published in 2012 and 2018[13,14]. End points were defined as major adverse cardiovascular events (MACEs), a composite of cardiac death, MI, stroke and revascularization. PMI is not a composition of MACEs.
Statistical analyses were performed by the SPSS 22.0 statistical package (Chicago, Illinois, United States). Continuous variables were reported as mean ± SD or as median with interquartile range. Continuous variables were compared by the t-test (normal distribution) or Kruskal–Wallis test (non-normal distribution). Comparisons of continuous variables among three groups were performed by ANOVA. Categorical variables were expressed as frequencies and compared by chi-square test.
Multivariate logistical analysis was performed to determine independent predictors of PMI after adjustment for significant variables by univariate analysis (P < 0.05). Events rates were calculated using the Kaplan–Meier method. Analysis of factors relative to reported events was performed by multivariate Cox proportional hazards modeling. Hazard ratios (HRs) were presented with 95%CIs. A value of P < 0.05 was considered to show statistical significance.
The design of the present study is shown in Figure 1. Baseline clinical and procedural characteristics of the 10236 participants, grouped by pre-operative serum TB concentrations (< 10.2; 10.2-14.4; > 14.4 μmol/L), are shown in Table 1. Patients with lower TB were more likely to be older, female and to have a prevalence of unstable angina, hypertension, diabetes, and renal failure (estimated glomerular filtration rate: < 60 mL/min/1.73 m2). Patients in the lower TB group were also more likely to be taking angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blocker (ARB), calcium-channel blocker (CCB), receiving more stents and greater balloon pre-dilation.
| Variable | Tertile I < 10.2 μmol/L, n = 3430 | Tertile II 10.2-14.4 μmol/L, n = 3405 | Tertile III > 14.4 μmol/L, n = 3401 | P value (All) | Tertile I vs Tertile II | Tertile I vs Tertile III | Tertile II vs Tertile III |
| Age (years) | 66.9 ± 10.4 | 66.3 ± 10.3 | 65.6 ± 10.0 | < 0.001 | 0.020 | < 0.001 | 0.006 |
| Female, n (%) | 1232 (36.0) | 1017 (29.9) | 676 (19.9) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| BMI (kg/m2) | 24.6 ± 4.9 | 24.9 ± 11.2 | 24.9 ± 10.1 | 0.314 | 0.063 | 0.091 | 0.651 |
| Current smoking, n (%) | 774 (22.6) | 731 (21.5) | 735 (21.6) | 0.491 | 0.401 | 0.288 | 0.821 |
| Diabetes, n (%) | 1015 (29.6) | 848 (24.9) | 782 (23.0) | < 0.001 | < 0.001 | < 0.001 | 0.028 |
| Hypertension, n (%) | 2413 (70.4) | 2327 (68.3) | 2276 (66.9) | 0.008 | 0.021 | < 0.001 | 0.122 |
| Hyperlipidemia, n (%) | 532 (15.5) | 557 (16.4) | 522 (15.3) | 0.470 | 0.449 | 0.726 | 0.266 |
| Prior stroke, n (%) | 353 (10.3) | 300 (8.8) | 253 (7.4) | < 0.001 | 0.037 | < 0.001 | 0.110 |
| eGFR < 60 (mL/min/1.73 m2), n (%) | 488 (14.2) | 345 (10.1) | 269 (7.9) | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| LVEF (%) | 66.8 ± 8.0 | 67.2 ± 7.8 | 67.0 ± 9.0 | 0.096 | 0.031 | 0.575 | 0.008 |
| Prior MI, n (%) | 395 (11.5) | 360 (10.6) | 399 (11.7) | 0.271 | 0.054 | 0.861 | 0.071 |
| Prior PCI, n (%) | 876 (25.5) | 800 (23.5) | 806 (23.7) | 0.094 | 0.052 | 0.056 | 0.978 |
| Unstable angina, n (%) | 1850 (54.0) | 1740 (51.1) | 1708 (50.3) | 0.006 | 0.002 | < 0.001 | 0.528 |
| Perioperative medications, n (%) | |||||||
| ACEI/ARB | 1979 (57.7) | 1916 (56.3) | 1825 (53.7) | 0.003 | 0.613 | < 0.001 | 0.021 |
| Beta-blocker | 1746 (50.9) | 1746 (51.3) | 1691 (49.7) | 0.407 | 0.835 | 0.129 | 0.082 |
| Calcium-channel blocker | 1270 (37.0) | 190 (34.9) | 1114 (32.8) | 0.001 | 0.004 | < 0.001 | 0.034 |
| LDL-C (mmol/L) | 2.05 ± 0.88 | 2.09 ± 0.86 | 2.04 ± 0.86 | 0.053 | 0.117 | 0.843 | 0.076 |
| Lesions in vessels, n (%) | |||||||
| Left main | 197 (5.7) | 186 (5.5) | 180 (5.3) | 0.711 | 0.613 | 0.578 | 0.959 |
| Left anterior descending | 1828 (53.3) | 1848 (54.3) | 1837 (54.0) | 0.702 | 0.187 | 0.321 | 0.926 |
| Left circumflex | 579 (16.9) | 524 (15.4) | 578 (17.0) | 0.136 | 0.068 | 0.902 | 0.051 |
| Right coronary artery | 983 (28.7) | 994 (29.2) | 955 (28.1) | 0.597 | 0.960 | 0.196 | 0.184 |
| AHA/ACC classification | 1228 (35.8) | 1256 (36.8) | 1356 (39.9) | 0.002 | 0.487 | < 0.001 | 0.006 |
| Calcification, n (%) | 406 (11.8) | 391 (11.5) | 390 (11.5) | 0.864 | 0.204 | 0.485 | 0.567 |
| FFR/IVUS/OCT, n (%) | 357 (10.4) | 372 (10.9) | 402 (11.8) | 0.170 | 0.496 | 0.050 | 0.210 |
| Number of implanted stents, median (IQR) | 1 (1-2) | 1 (1-2) | 1 (1-2) | < 0.001 | 0.005 | 0.005 | 0.133 |
| Mean stent size > 2.5 mm, n (%) | 3107 (90.6) | 3077 (90.4) | 3056 (89.9) | 0.606 | 0.755 | 0.282 | 0.464 |
| Balloon pre-dilation, n (%) | 3031 (88.4) | 2970 (87.2) | 2921 (85.9) | 0.009 | 0.039 | 0.001 | 0.183 |
| Balloon post-dilation, n (%) | 3175 (92.6) | 3145 (92.4) | 3156 (92.8) | 0.793 | 0.999 | 0.828 | 0.827 |
PMI was detected in 526 (15.3%), 431 (12.7%) and 424 (12.5%) of patients with pre-PCI TB levels of < 10.2, 10.2-14.4 and > 14.4 μmol/L (P = 0.001), respectively (Figure 2). Recorded rates of PMI were lower in patient groups with the two higher TB levels [TB 10.2-14.4 μmol/L: Odds ratio (OR): 0.854; 95% confidence interval (CI): 0.739-0.987; P = 0.032; TB > 14.4 μmol/L: OR: 0.846; 95%CI: 0.735-0.975; P = 0.021; Table 2] compared with the lowest level group after adjustment for age, gender, smoking, hypertension, renal function, left ventricular ejection fraction (LVEF), prior MI, the use of ACEI or ARB, American Heart Association/American College of Cardiology (AHA/ACC) classification, calcification, the use of fractional flow reserve (FFR)/intravascular ultrasound (IVUS)/optical coherence tomography (OCT) and number of implanted stents.
| Variable | Univariate model | Multivariate model | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age > 65 yr | 1.428 (1.268, 1.607) | < 0.001 | 1.272 (1.110, 1.457) | 0.001 |
| Female | 1.225 (1.084, 1.384) | 0.001 | 1.200 (1.034, 1.393) | 0.017 |
| BMI | 0.983 (0.966, 1.000) | 0.052 | ||
| Current smoking | 0.832 (0.721, 0.959) | 0.011 | 0.943 (0.797, 1.117) | 0.499 |
| Diabetes | 1.134 (0.999, 1.287) | 0.052 | ||
| Hypertension | 1.271 (1.120, 1.443) | < 0.001 | 1.054 (0.901, 1.233) | 0.509 |
| Prior stroke | 1.181 (0.976, 1.428) | 0.087 | ||
| Prior MI | 1.309 (1.108, 1.547) | 0.002 | 1.200 (0.991, 1.454) | 0.062 |
| eGFR < 60 (mL/min/1.73 m2) | 1.645 (1.400, 1.933) | < 0.001 | 1.369 (1.135, 1.651) | 0.001 |
| LVEF | 0.975 (0.968, 0.982) | < 0.001 | 0.980 (0.972, 0.988) | < 0.001 |
| Unstable angina | 1.116 (0.996, 1.250) | 0.059 | ||
| Perioperative medications | ||||
| ACEI/ARB | 1.243 (1.107, 1.395) | < 0.001 | 1.112 (0.965, 1.281) | 0.144 |
| Beta-blocker | 0.992 (0.886, 1.112) | 0.896 | ||
| Calcium-channel blocker | 1.067 (0.948, 1.201) | 0.280 | ||
| LDL-C > 1.8 mmol/L | 0.930 (0.808, 1.070) | 0.311 | ||
| AHA/ACC classification B2/C | 1.167 (1.040, 1.311) | 0.009 | 1.363 (1.192, 1.558) | < 0.001 |
| Calcification | 1.767 (1.514, 2.063) | < 0.001 | 1.303 (1.091, 1.556) | 0.004 |
| FFR/IVUS/OCT | 1.391 (1.178, 1.642) | < 0.001 | 1.275 (1.056, 1.539) | 0.011 |
| Number of implanted stents | 1.868 (1.741, 2.006) | < 0.001 | 1.882 (1.738, 2.038) | < 0.001 |
| Mean stent size > 2.5 mm | 1.117 (0.917, 1.362) | 0.272 | ||
| Balloon pre-dilation | 1.116 (0.937, 1.330) | 0.217 | ||
| Balloon post-dilation | 1.330 (0.926, 1.912) | 0.123 | ||
| Total bilirubin | ||||
| Tertile I | 1 (ref) | 1 (ref) | ||
| Tertile II | 0.800 (0.698, 0.918) | 0.001 | 0.854 (0.739, 0.987) | 0.032 |
| Tertile III | 0.786 (0.685, 0.902) | 0.001 | 0.846 (0.735, 0.975) | 0.021 |
A total of 1310 post-PMI patients were followed up long-term. The median follow-up period was 3.2 years (interquartile range: 1.8-5.0). During follow-up, 258 (19.7%) cases of MACE were identified, including 53 (4.0%) cardiac deaths, 31 (2.4%) non-fatal MIs, 6 (0.5%) non-fatal strokes and 182 (13.9%) revascularizations. Kaplan-Meier curves were used to demonstrate that the cumulative incidence of MACEs decreased with the higher tertile of TB level (log-rank test; P = 0.022; Figure 3). The data indicated that better outcomes were correlated with higher TB levels.
Cox proportional hazard analysis was performed after adjustment for age, diabetes, unstable angina, low-density lipoprotein cholesterol (LDL-C) and number of stents implanted. The results demonstrated that patients with TB > 14.4 μmol/L had a reduced risk of long-term MACEs with an adjusted HR of 0.667 (95%CI: 0.485-0.918; P = 0.013; Table 3) compared with patients with TB < 10.2 μmol/L. Multivariate Cox models were constructed for further analysis of the relationships between TB levels and MACE component events. Patients with TB > 14.4 μmol/L were at decreased risk of revascularization (HR: 0.633; 95%CI: 0.458-0.875; P = 0.006; Table 4) compared to those with TB < 10.2 μmol/L. Adjusted HRs for different TB tertiles did not differ significantly with regard to cardiac death, non-fatal MI and non-fatal stroke (Table 4).
| Variable | Univariate model | Multivariate model | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age > 65 yr | 1.199 (1.080, 1.332) | 0.001 | 1.123 (1.001, 1.260) | 0.048 |
| Male | 1.213 (0.921, 1.598) | 0.170 | ||
| BMI | 1.005 (0.969, 1.042) | 0.782 | ||
| Current smoking | 0.982 (0.733, 1.316) | 0.905 | ||
| Diabetes | 1.135 (1.098, 1.173) | < 0.001 | 1.119 (1.079, 1.161) | < 0.001 |
| Hypertension | 1.122 (0.848, 1.484) | 0.420 | ||
| eGFR < 60 (mL/min/1.73 m2) | 1.291 (0.936, 1.780) | 0.120 | ||
| LVEF | 0.996 (0.984, 1.008) | 0.540 | ||
| Unstable angina | 1.284 (1.003, 1.644) | 0.048 | 1.381 (1.071, 1.781) | 0.013 |
| LDL-C > 1.8 mmol/L | 1.160 (1.650, 1.264) | 0.001 | 1.094 (0.996, 1.201) | 0.060 |
| Perioperative medications | ||||
| ACEI/ARB | 1.077 (0.825, 1.405) | 0.586 | ||
| Beta-blocker | 1.044 (0.810, 1.345) | 0.739 | ||
| Calcium-channel blocker | 1.197 (0.872, 1.644) | 0.266 | ||
| AHA/ACC classification B2/C | 0.848 (0.638, 1.128) | 0.258 | ||
| Calcification | 1.197 (0.872, 1.644) | 0.266 | ||
| Number of stents implanted | 1.186 (1.031, 1.365) | 0.017 | 1.171 (1.012, 1.354) | 0.034 |
| Total bilirubin | ||||
| Tertile I | 1 (ref) | 1 (ref) | ||
| Tertile II | 0.801 (0.604, 1.063) | 0.125 | 0.837 (0.627, 1.119) | 0.229 |
| Tertile III | 0.640 (0.469, 0.875) | 0.005 | 0.667 (0.485, 0.918) | 0.013 |
| Events | Tertile I | Tertile II | Tertile III |
| Composite MACE1 | |||
| Number events/participants | 118 (23.2) | 81 (19.6) | 59 (15.3) |
| Adjust HR and 95%CI | 1.0 (ref) | 0.837 (0.627, 1.119) | 0.667 (0.485, 0.918)a |
| Cardiac death | |||
| Number events/participants | 27 (5.3) | 13 (3.1) | 13 (3.4) |
| Adjust HR and 95%CI | 1.0 (ref) | 0.546 (0.28, 1.065) | 0.588 (0.295, 1.171) |
| Non-fatal myocardial infarction | |||
| Number events/participants | 13 (2.6) | 11 (2.7) | 7 (1.8) |
| Adjust HR and 95%CI | 1.0 (ref) | 1.113 (0.487, 2.547) | 0.736 (0.286, 1.898) |
| Non-fatal stroke | |||
| Number events/participants | 2 (0.4) | 2(0.5) | 2 (0.5) |
| Adjust HR and 95%CI | 1.0 (ref) | 1.534 (0.213, 11.067) | 1.888 (0.251, 14.211) |
| Revascularization2 | |||
| Number events/participants | 84 (16.5) | 59 (14.3) | 39 (10.1) |
| Adjust HR and 95%CI | 1.0 (ref) | 0.814 (0.608, 1.089) | 0.633 (0.458, 0.875)a |
The current study presents data to demonstrate an independent association between higher preoperative TB levels and a lower incidence of PMI in patients receiving PCI. Furthermore, a high TB level is a protective factor producing a better long-term prognosis in post-PMI patients.
Persistent high rates of PMI, which are of particular concern among patients with complex lesions, are thought to be largely due to oxidative stress causing free radical and inflammatory damage to vascular endothelial cells[3,15,16]. There is an adverse impact on long-term morbidity for patients with PMI[17,18] which has stimulated the search for potential targets or risk factors to avoid development of the condition. Patients, lesion and procedure-related factors are all implicated[3]. Consistent with previous studies, current findings also indicate that age, gender, renal impairment, complexity of lesions and the number of stents implanted are all predictors of PMI development. Interestingly, the present study also suggests that use of FFR, OCT or IVUS may increase the likelihood of PMI. All these would not only increase additional procedures, but also prolong the operating time and increase the dose of contrast agent, which may aggravate myocardial damage.
Antioxidant properties have been attributed to bilirubin[19] and this breakdown product of heme may directly scavenge ROS[20] and inhibit NADPH oxidase[21]. Furthermore, bilirubin has been shown to inhibit peroxidation of lipids and lipoproteins, especially low-density lipoprotein[22-24], indirectly improving microvascular dysfunction. Any resulting improvement in endothelial function will be instrumental in inhibiting the development of atherosclerosis and reducing cardiovascular complications.
Several clinical studies have demonstrated a negative correlation between serum bilirubin concentrations and cardiovascular disease risk. Schwertner et al[4] were the first to report serum bilirubin as an inverse risk factor for CAD and several other studies supported this protective role[4-6,25]. Interestingly, patients with Gilbert's syndrome, a hereditary disorder resulting in mild hyperbilirubinemia, have lower rates of ischemic heart disease than the general population (2% vs 12%)[19]. In contrast to previous studies, the present study focused on PMI, finding a negative association between the incidence of PMI and TB levels. After adjustment for age, gender, body mass index, hypertension, diabetes and LDL-C, plasma TB levels were inversely correlated with C-reactive protein (r = -0.023; P = 0.019) and white blood cell count (r = -0.062; P < 0.001), suggesting an anti-inflammatory effect of elevated bilirubin. Peyton et al[26] demonstrated that bilirubin blocks the proliferation and migration of vascular smooth muscle cells, thus reducing post-PCI stenosis. The present study also found a lower risk of post-PMI revascularization in patients with elevated TB levels.
However, a number of studies found contradictory results. Kaya et al[10] demonstrated a positive association between high TB levels and the severity of CAD in non-ST-elevation MI. Similarly, Gul et al[27] and Celik et al[28] found an association between high TB level and increased in-hospital adverse outcomes in patients with ST-elevation MI. Contrary findings among these studies may be attributed to differences in study populations. Previous trials included AMI patients while the present study focused on patients with normal pre-PCI cTnI. Heme oxygenase 1 (HO-1), a rate-limiting enzyme in bilirubin breakdown, can be activated by cellular stresses due to MI, resulting in elevated bilirubin levels[29,30]. Xu et al[31] reported a positive correlation between TB levels and C-reactive protein in AMI patients, reflecting inflammatory activation. Thus, upregulated HO-1 activity and bilirubin would seem to be a defense mechanism to protect the myocardium via antioxidant activity. Previous experiments found that exogenous bilirubin decreased infarct size and ameliorated left ventricular function in the post-ischemic rat heart[32,33].
The findings of the present study assist our understanding of bilirubin actions and our search for therapeutic targets for the management of PMI and other oxidative diseases. A number of drugs are known to induce HO-1, including aspirin and statins[34]. Inhibition of bilirubin UDP-glucuronosyl transferase (the key enzyme responsible for bilirubin conjugation) or prevention of bilirubin oxidation may be other routes to elevated bilirubin concentrations[35]. Moreover, synthetic materials or naturally occurring tetrapyrrolic molecules structurally related to bilirubin may act as mimetics[36].
We acknowledge several limitations in the present study. Firstly, due to its retrospective nature, data regarding ischemic symptoms and electrocardiographs were difficult to collect. PMI in our study was alternatively defined as an isolated rise in cTnI, which did not fulfill the requirement of the revised diagnosis criteria published in 2012 and 2018[13,14]. In addition, patients with abnormal pre-PCI cTnI levels were excluded since AMI may affect pre-PCI bilirubin. Secondly, PMI is known to be associated with surgical factors, such as branch occlusion and distal embolism. Patients with intraoperative factors, including side-branch occlusion and severely calcified lesions with a rotablator or dissection, were excluded to reduce the influence of surgical complications. However, although adjustment for many known predictors of PMI was made, confounding factors may not have been completely eliminated. For example, plaque characteristics, such as via IVUS/OCT, were only available for a proportion of patients. Thirdly, since HO-1 enzyme activity was not measured, the association between HO-1 level, bilirubin and the risk of PMI could not be assessed. Lastly, our findings show that indirect bilirubin, rather than direct bilirubin, has the predictive value for PMI. Although a previous study has shown that patients with mildly elevated indirect serum bilirubin have a much lower incidence of CAD[37], any difference in mechanism between the two forms remains unconfirmed. Due to potential bias, results regarding the different effects of the two forms on PMI and its long-term outcome are not shown.
Bilirubin was an inverse predictor of PMI and has a protective effect. In patients who experienced PMI, elevated levels of bilirubin were independently associated with a reduced risk of MACEs during long-term follow-up.
As a frequent complication of percutaneous coronary intervention (PCI), the rate of perioperative myocardial infarction (PMI) remains high and patients suffering from PMI have poor outcomes.
To identify whether bilirubin could be a potential target for PMI avoidance.
To explore the impact of bilirubin levels on PMI and long-term prognosis in post-PMI patients.
Logistic regression and Cox regression analyses were used to explore the association between bilirubin, PMI and its long-term prognosis.
Higher bilirubin was associated with a reduced rate of PMI and major adverse cardiovascular events.
Bilirubin was a protective factor in PMI prediction and produced a better long-term prognosis in post-PMI patients.
The study provides evidence of bilirubin as a therapeutic target in PMI prevention.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Teragawa H S-Editor: Fan JR L-Editor: Webster JR P-Editor: Fan JR
| 1. | Kizer JR, Muttrej MR, Matthai WH, McConnell J, Nardone H, Sonel AF, Keane MG, Wilensky RL. Role of cardiac troponin T in the long-term risk stratification of patients undergoing percutaneous coronary intervention. Eur Heart J. 2003;24:1314-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 2. | Feldman DN, Kim L, Rene AG, Minutello RM, Bergman G, Wong SC. Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv. 2011;77:1020-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J. 2005;26:2493-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18-23. [PubMed] |
| 5. | Chang CC, Hsu CY, Huang PH, Chiang CH, Huang SS, Leu HB, Huang CC, Chen JW, Lin SJ. Association of Serum Bilirubin with SYNTAX Score and Future Cardiovascular Events in Patients Undergoing Coronary Intervention. Acta Cardiol Sin. 2016;32:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Akboga MK, Canpolat U, Sahinarslan A, Alsancak Y, Nurkoc S, Aras D, Aydogdu S, Abaci A. Association of serum total bilirubin level with severity of coronary atherosclerosis is linked to systemic inflammation. Atherosclerosis. 2015;240:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi T, Terakado M, Horio F, Aoki K, Tanaka M, Nakajima H. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun. 1996;223:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Bösch F, Thomas M, Kogler P, Oberhuber R, Sucher R, Aigner F, Semsroth S, Wiedemann D, Yamashita K, Troppmair J, Kotsch K, Pratschke J, Öllinger R. Bilirubin rinse of the graft ameliorates ischemia reperfusion injury in heart transplantation. Transpl Int. 2014;27:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Breimer LH, Wannamethee G, Ebrahim S, Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem. 1995;41:1504-1508. [PubMed] |
| 10. | Kaya MG, Sahin O, Akpek M, Duran M, Uysal OK, Karadavut S, Cosgun MS, Savas G, Baktir AO, Sarli B, Lam YY. Relation between serum total bilirubin levels and severity of coronary artery disease in patients with non-ST-segment elevation myocardial infarction. Angiology. 2014;65:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Baumann S, Huseynov A, Koepp J, Jabbour C, Behnes M, Becher T, Renker M, Lang S, Borggrefe M, Lehmann R, Akin I. Comparison of Serum Uric Acid, Bilirubin, and C-Reactive Protein as Prognostic Biomarkers of In-Hospital MACE Between Women and Men With ST-Segment Elevation Myocardial Infarction. Angiology. 2016;67:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 7268] [Article Influence: 1817.0] [Reference Citation Analysis (0)] |
| 13. | Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; Joint ESC/ACCF/AHA/WHF Task Force for Universal Definition of Myocardial Infarction; Authors/Task Force Members Chairpersons, Thygesen K, Alpert JS, White HD; Biomarker Subcommittee, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA; ECG Subcommittee, Chaitman BR, Clemmensen PM, Johanson P, Hod H; Imaging Subcommittee, Underwood R, Bax JJ, Bonow JJ, Pinto F, Gibbons RJ; Classification Subcommittee, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW; Intervention Subcommittee, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J; Trials & Registries Subcommittee, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML; Trials & Registries Subcommittee, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G; Trials & Registries Subcommittee, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D; Trials & Registries Subcommittee, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S; ESC Committee for Practice Guidelines (CPG), Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S; Document Reviewers, Morais J, Aguiar C, Almahmeed W, Arnar DO, Barili F, Bloch KD, Bolger AF, Botker HE, Bozkurt B, Bugiardini R, Cannon C, de Lemos J, Eberli FR, Escobar E, Hlatky M, James S, Kern KB, Moliterno DJ, Mueller C, Neskovic AN, Pieske BM, Schulman SP, Storey RF, Taubert KA, Vranckx P, Wagner DR. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2076] [Cited by in RCA: 2312] [Article Influence: 177.8] [Reference Citation Analysis (0)] |
| 14. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618-e651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 2133] [Article Influence: 355.5] [Reference Citation Analysis (0)] |
| 15. | Gaspardone A, Crea F, Versaci F, Tomai F, Pellegrino A, Chiariello L, Gioffrè PA. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol. 1998;82:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Bonz AW, Lengenfelder B, Jacobs M, Strotmann J, Held S, Ertl G, Voelker W. Cytokine response after percutaneous coronary intervention in stable angina: effect of selective glycoprotein IIb/IIIa receptor antagonism. Am Heart J. 2003;145:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, Morrow DA. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation. 2012;125:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Milani RV, Fitzgerald R, Milani JN, Lavie CJ. The impact of micro troponin leak on long-term outcomes following elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2009;74:819-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Vítek L, Jirsa M, Brodanová M, Kalab M, Marecek Z, Danzig V, Novotný L, Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis. 2002;160:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Neuzil J, Stocker R. Bilirubin attenuates radical-mediated damage to serum albumin. FEBS Lett. 1993;331:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Lanone S, Bloc S, Foresti R, Almolki A, Taillé C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Yesilova Z, Serdar M, Ercin CN, Gunay A, Kilciler G, Hasimi A, Uygun A, Kurt I, Erbil MK, Dagalp K. Decreased oxidation susceptibility of plasma low density lipoproteins in patients with Gilbert's syndrome. J Gastroenterol Hepatol. 2008;23:1556-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Wu TW, Fung KP, Wu J, Yang CC, Weisel RD. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol. 1996;51:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem. 1994;269:16712-16719. [PubMed] |
| 25. | Ghem C, Sarmento-Leite RE, de Quadros AS, Rossetto S, Gottschall CA. Serum bilirubin concentration in patients with an established coronary artery disease. Int Heart J. 2010;51:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Peyton KJ, Shebib AR, Azam MA, Liu XM, Tulis DA, Durante W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front Pharmacol. 2012;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Gul M, Uyarel H, Ergelen M, Akgul O, Karaca G, Turen S, Ugur M, Ertürk M, Kul S, Surgit O, Bozbay M, Uslu N. Prognostic value of total bilirubin in patients with ST-segment elevation acute myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2013;111:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Celik T, Kaya MG, Akpek M, Yarlioglues M, Sarli B, Topsakal R, Gibson CM. Does Serum Bilirubin level on admission predict TIMI flow grade and in-hospital MACE in patients with STEMI undergoing primary PCI. Angiology. 2014;65:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1689] [Cited by in RCA: 1801] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 30. | Okuhara K, Kisaka T, Ozono R, Kurisu S, Inoue I, Soga J, Yano Y, Oshima T, Kihara Y, Yoshizumi M. Change in bilirubin level following acute myocardial infarction is an index for heme oxygenase activation. South Med J. 2010;103:876-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Xu C, Dong M, Deng Y, Zhang L, Deng F, Zhou J, Yuan Z. Relation of Direct, Indirect, and Total bilirubin to Adverse Long-term Outcomes Among Patients With Acute Coronary Syndrome. Am J Cardiol. 2019;123:1244-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643-H651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 281] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 33. | Ben-Amotz R, Bonagura J, Velayutham M, Hamlin R, Burns P, Adin C. Intraperitoneal bilirubin administration decreases infarct area in a rat coronary ischemia/reperfusion model. Front Physiol. 2014;5:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Bharucha AE, Choi KM, Saw JJ, Gibbons SJ, Farrugia GF, Carlson DA, Zinsmeister AR. Effects of aspirin & simvastatin and aspirin, simvastatin, & lipoic acid on heme oxygenase-1 in healthy human subjects. Neurogastroenterol Motil. 2014;26:1437-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | McCarty MF. ''Iatrogenic Gilbert syndrome''--a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses. 2007;69:974-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Kim MJ, Lee Y, Jon S, Lee DY. PEGylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials. 2017;133:242-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 37. | Kundur AR, Singh I, Bulmer AC. Bilirubin, platelet activation and heart disease: a missing link to cardiovascular protection in Gilbert's syndrome? Atherosclerosis. 2015;239:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |