Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1702
Peer-review started: September 21, 2021
First decision: December 2, 2021
Revised: December 7, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 16, 2022
Processing time: 143 Days and 5 Hours
Infectious abscesses in the abdominal wall can be secondary to retained foreign bodies (e.g., stones, use of artificial mesh, use of silk yarn in surgical suture), inflammatory diseases (e.g., acute appendicitis), and perforated malignancies of the digestive tract (particularly the colon). Aseptic abscesses (AAs) are relatively rare. To the best of our knowledge, this is the first report of an AA in the abdominal wall accompanied by monoclonal gammopathy of undetermined significance (MGUS) at 5 years after laparoscopic proctectomy.
A 72-year-old female patient presented with an enlarged painless mass in the lower abdomen for 1 year. She had a history of obesity, diabetes, and MGUS. Her surgical history was laparoscopic resection for rectal cancer 6 years prior, followed by chemotherapy. She was afebrile. Abdominal examination revealed a smooth abdomen with a clinically palpable solid mass under a laparotomy scar in the left lower quadrant. No obvious tenderness or skin redness was spotted. Laboratory data were not remarkable. Computed tomography scan revealed a low-density mass of 4.8 cm in diameter in the lower abdominal wall, which showed high uptake on positron emission tomography. The preoperative diagnosis was an abscess or tumor, and surgical resection was recommended. The mass was confirmed to be an AA by microbiological and pathological examinations. The patient recovered well after surgery. There was no evidence of recurrence 2 years later.
It is important to consider underlying conditions (diabetes, chemotherapy, MGUS) which may contribute to AA formation in the surgical wound.
Core Tip: We report a case of aseptic abscess (AA) in the abdominal wall accompanied by monoclonal gammopathy of undetermined significance (MGUS) at 5 years after laparoscopic proctectomy. This case report describes the clinical characteristics, laboratory findings, computed tomography images, and treatment, and discusses the possible relationship between AAs and a medical history that includes past surgery, MGUS, diabetes, or chemotherapy.
- Citation: Yu Y, Feng YD, Zhang C, Li R, Tian DA, Huang HJ. Aseptic abscess in the abdominal wall accompanied by monoclonal gammopathy simulating the local recurrence of rectal cancer: A case report. World J Clin Cases 2022; 10(5): 1702-1708
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1702.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1702
Infectious abscesses in the abdominal wall can be secondary to retained foreign bodies (e.g., stones, use of artificial mesh, use of silk yarn in surgical suture)[1], inflammatory diseases (e.g., acute appendicitis[2]), and perforated malignancies of the digestive tract (particularly in the colon)[3]. Patients often present with a painful anterior abdominal wall mass, sometimes with purulent discharge and systemic symptoms (e.g., fever)[2]. A diagnosis is made based on abdominal computed tomography (CT) and is confirmed by surgical pathology[3]. Aseptic abscesses (AAs) are relatively rare. To the best of our knowledge, this is the first report of a sterile AAin the abdominal wall accompanied by monoclonal gammopathy of undetermined significance (MGUS) at 5 years after laparoscopic resection for rectal cancer. The atypical symptoms and imaging findings of an AA mimic a tumor, posing a diagnostic dilemma. The underlying diseases contributing to AA formation and treatments are discussed.
A 72-year-old woman presented to the outpatient department of our hospital complaining of a painless mass in the left lower quadrant of the abdomen.
The patient’s symptoms began 10 mo prior and had worsened in the last 1 mo. She denied any changes in bowel habits. She was systemically well, with a good appetite and no fever.
The patient had a history of obesity, hypertension, coronary heart disease, and poorly controlled type 2 diabetes. She had been diagnosed with MGUS 1 year prior. Regular medications included ramipril, amlodipine, aspirin, and gliclazide. Her surgical history included percutaneous coronary intervention in 2009 and laparoscopic radical resection for rectal cancer approximately 6 years and 4 mo prior to her present admission. Postoperative histopathological examination revealed moderately differentiated adenocarcinoma of the rectum with direct invasion to the deep muscular layer of the intestinal wall. All surgical margins were free of disease, and four lymph nodes were retrieved and found to be non-malignant. The pathological staging was pT3N0M0 stage II, according to American Joint Committee on Cancer Staging. The postoperative course was uneventful. The patient received seven cycles of chemotherapy (capecitabine 3000 mg/d) after surgery with curative intent. She was followed up and free of cancer recurrence at 56 mo after surgery.
The patient was afebrile (36.3 °C). Her body mass index (BMI) was 30 kg/m2, and her blood pressure and pulse were 127/88 mmHg and 80 beats per min, respectively. Abdominal examination at presentation revealed a smooth abdomen with a clinically palpable solid mass (approximately 4 cm in diameter) under a laparotomy scar in the left lower quadrant. No obvious tenderness, skin redness, swelling, or increased skin temperature was observed around the mass. Abdominal auscultation revealed normal bowel sounds.
Serum levels of glycosylated hemoglobin [8.9%, normal range (NR): 4%–6%], triglycerides (6.79 mmoL/L, NR: 0.9-1.7 mmoL/L), and glucose (12.6 mmoL/L, NR: 4.1-6.0 mmoL/L) were elevated. Hemoglobin levels (114 g/L, NR: 115-150 g/L) were decreased. Serum immunofixation electrophoresis revealed the presence of M-protein (11%, NR: 0%) and elevation of monoclonal immunoglobulin G (IgG) lambda (2.36 g/L, NR: 0.9-2.1 g/L). Other laboratory tests were within NR. The laboratory data were not either indicative of acute inflammation (white blood cell count of 5400 cells/μL; neutrophil bands of 66%, serum C-reactive protein level of 0.7 mg/dL) or tumor recurrence (carcinoembryonic antigen level of 2.8 ng/mL).
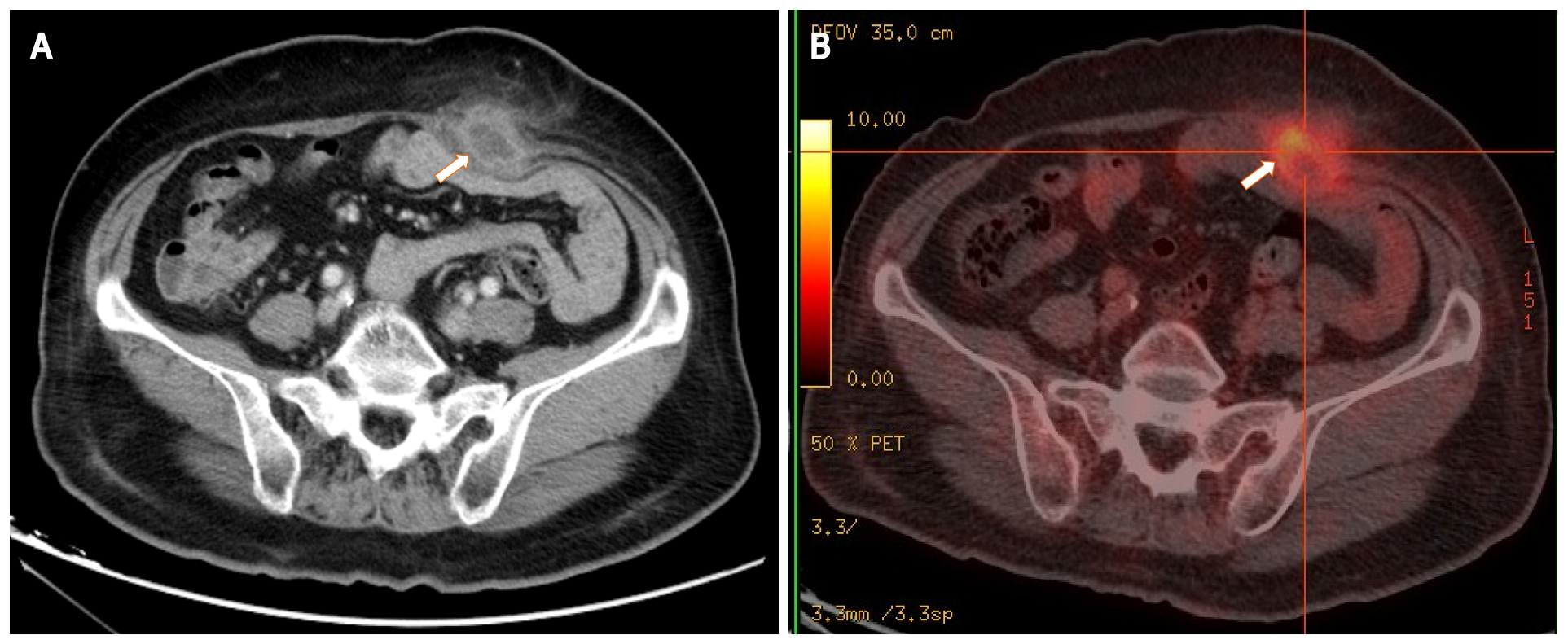
Ultrasonography of the left lower quadrant of the abdominal wall demonstrated a relatively well-demarcated, oval-shaped mass with mixed echogenicity (relatively more hypoechoic) and dimensions of 4.8 cm × 2.2 cm. Blood flow signals were seen in the hypoechoic area. Contrast-enhanced abdominal CT showed a low-density mass with rim enhancement adjacent to the rectus abdominisin the lower abdominal wall (Figure 1A), and 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET)/CT revealed high uptake of fluorodeoxyglucose, with a maximum standardized uptake value of 6.0 (Figure 1B). Colonoscopy showed no cancer recurrence. These findings suggested the possibility of either delayed abscess formation or abdominal wall recurrence of rectal cancer with central necrosis.
AA in the abdominal wall.
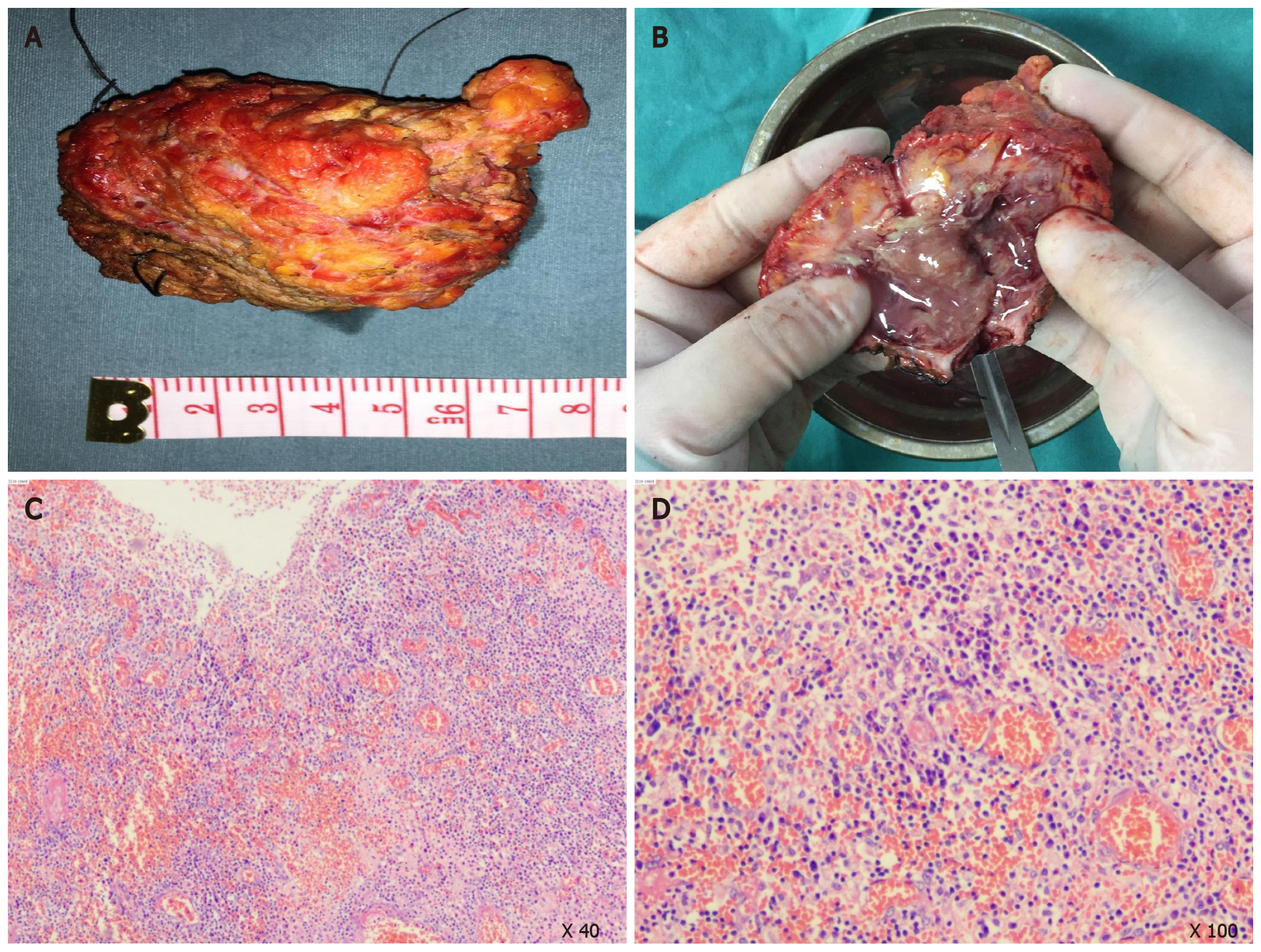
Complete resection of the mass for therapeutic and diagnostic purposes was proposed. The patient consented to surgery. She subsequently underwent exploratory laparotomy through a midline incision in an elliptical fashion to include the affected abdominal wall part in the specimen. The lesion was confirmed to be an abscess. Upon exploration, a grey white and irregular-shaped mass with central purulent necrosis in the center was found within the abdominal wall (Figure 2). Postoperative transvenous cefoperazone (2.0 g, twice daily) was administered for 3 d. Pathological examination of the specimen revealed a large number of infiltrated neutrophils, lymphocytes, and macrophages in the adipose and connective tissues, accompanied by focal abscess, inflammatory granulation tissue formation, and interstitial fibrosis (Figure 2C and D). Giant epithelioid cells, granuloma, amyloid substance, un-absorbable yarn, or a foreign body were not found in the specimen. A pus culture produced no bacterial or fungal growth after 7 d. The patient was discharged on the fourth postoperative day.
The patient was in good health at the 2-year follow-up.
AA is an inflammatory condition characterized by deep sterile collections of neutrophils, clinically mimicking bacterial abscess[4]. The diagnosis is established by excluding other diseases in different ialdiagnosis[4]. AA can arise in many parts of the body, including the abdominal cavity[5], liver[6], spleen[6], brain[7], lung[8], and extremities[9].
Although the causes of AA are not completely clear, it is known to be accompanied by some conditions such as inflammatory bowel disease (IBD)[6], surgery[5], drug usage (trastuzumab, crizotinib, vaccine)[7,10,11], and MGUS[8], with IBD being by far the most frequently associated condition[6]. AA can antedate, be concomitant with, or follow the diagnosis of IBD[6]; however, AA does not appear to be strongly associated with the disease activity[6]. In our case, ileocolonoscopy was performed, and no IBD was found. Cholecystectomy has been mentioned in intra-abdominal AA, secondary to foreign body reaction to dropped gallstones in gallbladder leakage[5,12,13]. The time of AA presentation canrange from 4 years to 8 years after surgery[5,13]. For example, Hawasli et al[12] reported a sterile abscess in the abdominal wall containing gallstones at 4 years and 4 mo after an elective laparoscopic cholecystectomy. In the present case, the AA appeared 5 years after laparoscopic resection for rectal cancer, and was located in the laparoscopic wound. Although no foreign body was retained in the wound, high BMI, diabetes mellitus, and chemotherapy might prevent wound healing, causing local inflammation[14]. Moreover, drug usage is suspected to be one cause as well, as an intracranial AA was formed after the first cycle of trastuzumab in a breast cancer patient[7]. Our patient had received seven cycles of capecitabine and developed AA approximately 4 years after her last chemotherapy treatment. To date, no paper has reportedAA as a side effect of capecitabine.
There is evidence suggesting an association between AA and MGUS. MGUS is a condition characterized by the presence of a monoclonal gammopathy in which the clonal mass has not reached a predefined state in which the condition is considered malignant[15]. It is a precursor to conditions such asmultiple myelomaor lymphoma at a rate of approximately 1% per year[15]. MGUS is associated with infections, fractures, peripheral neuropathy, and thromboembolism[16,17]. Only 4 cases of aseptic organ abscesses (spleen, liver, lung, and pancreas) occurring with MGUS have been reported[6,8]. Neutrophilicdermatoses, which are characterized by neutrophil infiltration in the skin, have been reported in monoclonal gammopathy[18,19]. The strongest association is between IgA monoclonal gammopathies and pyodermagangrenosum, a non-infectious neutrophilicdermatosis[18,20,21]. Interestingly, an abnormal neutrophil increase has also been observed in MGUS patients. A leukemoid reaction presenting as neutrophilic leukocytosis can occur with MGUS, which is attributable to cytokine release by neoplastic plasma cells[22,23]. The prompt and long-lasting regression of neutrophilia observed after short-term chemotherapy suggests that the present case should also be considered as a case of plasma-cell dyscrasia-associated neutrophilia[22]. In our case, one could postulate that the poor healing of the surgical wound may have served as an inducing factor and was enhanced by MGUS-associated neutrophil abnormality, ultimately leading to AA formation.
Pain is the most common symptom in patients with AA[13]. Symptoms vary among the organs involved[8,9]. Weight loss, abdominal pain, nausea, and fatigue suggest involvement of the digestive system[9]. In patients with MGUS, the prevalence of symptomatic neuropathy is 8% to 36%[18]. Most patients with neuropathy and IgG monoclonal gammopathy have IgG MGUS[18]. Both MGUS and diabetes can cause neuropathy, which might explain why the AA was painless in our patient. Multiple lesions in the spleen, liver, and skin are present in IBD and prone to relapse[6,9]. Anti-tumor drugs can cause multiple[10] or single[7] AAs. Surgery or a foreign body can cause a single AA and no recurrence has been reported[13]. Recurrences have occurred with anti-tumor drug usage and vaccination[10,11]. With MGUS, multipleAA lesions have been found in the lung and relapse[8]. In some cases, high fever, weight loss, and pain are the most frequent clinical manifestations associated with severe inflammatory response and elevated polymorphonuclear leukocyte count[6]. Elevated inflammatory markers are mostly seen in patients with IBD[4]. Conversely, the absence of fever or abdominal pain or lack of a raised leukocyte count does not exclude the possibility of AA[6]. Acute inflammatory symptoms are not obvious and have been observed in patients after surgery[13], anti-tumor drug usage[10], and vaccination[11]. Laboratory tests are unremarkable in patients with AA due to surgery or foreign body[13]. The absence of remarkable inflammatory indicators and pain make it difficult to distinguish AAs from the recurrence of cancer.
Abdominal CT is a very valuable tool for detecting locally advanced colon cancer and its invasion along the tissue planes, which may result in the formation of abdominal wall abscess (AWA)[24]. However, CT is not adequate in distinguishing inflammation and carcinoma in the abdominal wall, especially when the original tumor is absent. Although 18F-FDG-PET/CT is a powerful tool for detecting cancer, 18FDG uptake is not tumor-specific[25]. Our case highlights that sterile AWA-complicating MGUS post-surgerymay pose a diagnostic dilemma mimicking tumors due to their similar radiologic and laboratory appearance.
AA does not respond to antibiotic therapy[9]; however, its response to steroids is excellent[8]. In some cases, AA completely resolves with the combination of cyclophosphamide and prednisone or anti-tumor necrosis factor-alpha therapy in patients with IBD[6]. For surgery-associated AA, en bloc resection is also a treatment[5,12,13]. However, for relapsing patients, special attention should be paid to pathologic changes to avoid iterative surgical procedures. The limitation of our case was that corticosteroids were not applied, because the patient had a history of rectal cancer and the lesion could not be preoperatively excluded from a tumor. Therefore, we lack experience on steroid usage in patients with AA. However, our case suggests that AA associated with surgical wound and MGUS can be treated by en bloc resection without the aid of corticosteroids.
In conclusion, if local recurrence is suspected by symptoms and imaging modalities in the postoperative period for colorectal cancer patients, although rare, the possibility of AA formation should be considered. Likewise, it is important to consider underlying diseases such as diabetes, chemotherapy, and MGUS, which may contribute to AA formation in the surgical wound, regardless of the time elapsed from surgery.The atypical manifestations of AA lead to difficulties in differentiating from cancer based on the results of imaging modalities. AA associated with surgical wound and MGUS can be treated by en bloc resection without the aid of corticosteroids. Greater knowledge of AA among physicians will promote early diagnosis and effective treatment.
We reported a rare case of an aseptic AWA with MGUS at 5 years after laparoscopic radical resection for rectal cancer. This case study described the clinical characteristics, laboratory results, imaging findings, and treatment. We also discussed the possible relationship among aseptic abscess, MGUS, and laparoscopic surgery. We believe that this case provides a foundation for further studies on the relationship between MGUS, neutrophils and AA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shiryajev YN, Tsimogiannis K S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Kawai K, Sunami E, Nishikawa T, Tanaka J, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Kazama S, Ishihara S, Yamaguchi H, Kitayama J, Watanabe T. Delayed abdominal wall abscess after abdomino-perineal resection simulating local recurrence of rectal cancer. Springerplus. 2014;3:681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Souza IMAG, Nunes DAA, Massuqueto CMG, Veiga MAM, Tamada H. Complicated acute appendicitis presenting as an abscess in the abdominal wall in an elderly patient: A case report. Int J Surg Case Rep. 2017;41:5-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Amer E, Er A, Cengiz F, Karaisli S, Peskersoy M. Colon Cancer Presenting as Abdominal Wall Abscess. Cyprus J Med Sci. 2018;3:202-203. |
| 4. | Snast I, Ostfeld I, Pavlovsky L, Hodak E, Gafter-Gvili A. Pyoderma Gangrenosum and Extensive Aseptic Chest Wall Abscess in a Patient with Inflammatory Bowel Disease. Isr Med Assoc J. 2018;20:712-713. [PubMed] |
| 5. | Kakaty D, Gosztonyi J, Anthamatten C, Zengaffinen R. Sterile abscess mimicking an abdominal tumor 8 years after laparoscopic cholecystectomy. J Surg Case Rep. 2017;2017:rjx176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | André MFJ, Piette JC, Kémény JL, Ninet J, Jego P, Delèvaux I, Wechsler B, Weiller PJ, Francès C, Blétry O, Wismans PJ, Rousset H, Colombel JF, Aumaître O; and the French Study Group on Aseptic Abscesses. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore). 2007;86:145-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Mezei T, Hajdu M, Czigléczki G, Lotz G, Kocsis J, Kulka J, Horváth A. Sterile, abscess-like cerebral lesion during trastuzumab therapy after HER2 status switch in a triple negative breast cancer patient: a case report and literature review. BMC Cancer. 2020;20:615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Mitrevski M, Granata M, Sedati P, Rota F, De Santis A, Remotti D, Callea F, Visentini M. Sterile abscesses complicating monoclonal gammopathy of undetermined significance. Eur J Haematol. 2008;81:246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Agirgol S, Ustaoglu E, Demir FT, Akbulut TO, Turkoglu Z, Kaya H, Pehlivanoğlu F. Aseptic Abscess Syndrome with Severe Skin Involvement: Case Report. Indian J Dermatol. 2020;65:434-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Weber D, Decker M, Schuster M, Folz S, Stürmer CJ, Lutz MP. Crizotinib: aseptic abscesses in multiple organs during treatment of EML4-ALK-positive NSCLC. J Cancer Res Clin Oncol. 2021;147:3769-3771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kaya A, Kaya SY. A case of recurrent sterile abscesses following tetanus-diphtheria vaccination treated with corticosteroids. BMC Infect Dis. 2021;21:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hawasli A, Schroder D, Rizzo J, Thusay M, Takach TJ, Thao U, Goncharova I. Remote complications of spilled gallstones during laparoscopic cholecystectomy: causes, prevention, and management. J Laparoendosc Adv Surg Tech A. 2002;12:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Bartels AK, Murali AR, Zamora JG. Subhepatic Sterile Abscess 10 Years After Laparoscopic Cholecystectomy. ACG Case Rep J. 2015;2:113-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Xu Z, Qu H, Kanani G, Guo Z, Ren Y, Chen X. Update on risk factors of surgical site infection in colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:2147-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Glavey SV, Leung N. Monoclonal gammopathy: The good, the bad and the ugly. Blood Rev. 2016;30:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Tete SM, Bijl M, Sahota SS, Bos NA. Immune defects in the risk of infection and response to vaccination in monoclonal gammopathy of undetermined significance and multiple myeloma. Front Immunol. 2014;5:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Mouhieddine TH, Weeks LD, Ghobrial IM. Monoclonal gammopathy of undetermined significance. Blood. 2019;133:2484-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Decaux O, Laurat E, Perlat A, Cazalets C, Jego P, Grosbois B. Systemic manifestations of monoclonal gammopathy. Eur J Intern Med. 2009;20:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Gusdorf L, Lipsker D. Schnitzler Syndrome: the paradigm of an acquired adult-onset auto-inflammatory disease. G Ital Dermatol Venereol. 2020;155:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Montagnon CM, Fracica EA, Patel AA, Camilleri MJ, Murad MH, Dingli D, Wetter DA, Tolkachjov SN. Pyoderma gangrenosum in hematologic malignancies: A systematic review. J Am Acad Dermatol. 2020;82:1346-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Velasco-Tamariz V, Carreño-Tarragona G, Tous-Romero F, Gil-de la Cruz E, Martín-Clavero E, Rivera-Díaz R. Dramatic resolution of disseminated pyoderma gangrenosum associated with monoclonal gammopathy after therapy with bortezomib and dexamethasone. Int Wound J. 2017;14:1382-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Gnerre P, Ottonello L, Montecucco F, Boero M, Dallegri F. Nephrotic syndrome in a patient with IgM myeloma with associated neutrophilia. Eur J Haematol. 2007;79:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Bain BJ, Ahmad S. Chronic neutrophilic leukaemia and plasma cell-related neutrophilic leukaemoid reactions. Br J Haematol. 2015;171:400-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kim SW, Shin HC, Kim IY, Kim YT, Kim CJ. CT findings of colonic complications associated with colon cancer. Korean J Radiol. 2010;11:211-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Flaus A, Longo MG, Dematons M, Granjon D, Prevot N. 18F-FDG PET/CT in Urachal Abscess. Clin Nucl Med. 2019;44:e349-e350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |