Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1684
Peer-review started: August 26, 2021
First decision: October 22, 2021
Revised: October 24, 2021
Accepted: January 5, 2022
Article in press: January 5, 2022
Published online: February 16, 2022
Processing time: 168 Days and 17.4 Hours
Downgrading target treatment and laparoscopic partial nephrectomy have become increasingly popular in patients with renal cell carcinomas. Rare as it is, pneumothorax is one of the most severe intraoperative complications which needs immediate recognition. On the other hand, as a rheumatological disease, lupus nephritis requires a long period of hormone therapy. Cases of pneumothorax in hormone-consuming renal cancer patients are even fewer.
A 39-year-old woman was admitted to our department to take a laparoscopic partial nephrectomy. The patient had a medical history of lupus nephritis and renal clear cell carcinoma with hormone and target treatment. Her blood oxygen saturation dropped to 92% during the operation, and pneumothorax was detected by ultrasound. O2 inhalation and lung dilation were performed. Her vital signs were monitored closely throughout the operation. The operation was accomplished, and she regained consciousness smoothly. A postoperative bedside chest X-ray was conducted after she was transferred to the urosurgery ward, while no evidence of further pneumothorax or lib injury was observed.
Pneumothorax is a severe complication in laparoscopic or robotic-assisted laparoscopic operations, especially in retroperitoneal ones. It is easily neglected unless the injury of the diaphragm is found. Low insufflation pressure and shorter operation time are necessary for patients with a history of long-term hormone consumption or chronic immune system disease.
Core Tip: Controlling the gas pressure in the abdomen or retroperitoneum is an essential issue in laparoscopic operations. High gas pressure may lead to the injury of the diaphragm and, thereafter, pneumothorax. This article presents a case with accidental pneumothorax during the operation. The novelties are: First, this patient may be at high risk of pneumothorax due to long-term hormone application, and this article could arouse everyone's attention to this issue by sharing a clinical example; second, our early recognition and quick reaction to the pneumothorax could provide precious data for peers. Overall, this case should have enlightening significance for managing surgical patients with long-term application of hormones.
- Citation: Zhao Y, Xue XQ, Xia D, Xu WF, Liu GH, Xie Y, Ji ZG. Pneumothorax during retroperitoneal laparoscopic partial nephrectomy in a lupus nephritis patient: A case report. World J Clin Cases 2022; 10(5): 1684-1688
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1684.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1684
Laparoscopic partial nephrectomy and robotic-assisted laparoscopic partial nephrectomy have been widely used with features of less invasiveness and quicker recovery. However, some complications such as pneumothorax or potential bleeding are uncommon but lethal. The approximate incidence rate of pneumothorax was 1%. Moreover, it could be detected in almost all laparoscopic or robotic operations and calls for prompt treatment[1]. There are many reasons for pneumothorax, amongst which spontaneous diaphragm injury is one of the most difficult to discover. We present a lupus nephritis case with a long-term target treatment and hormone therapy who developed a pneumothorax during the operation.
A 39-year-old woman demanded the resection of her primary renal carcinoma after receiving 6-mo target therapy.
The patient was diagnosed with renal clear cell carcinoma with bone metastasis half a year ago. After receiving 6-mo target therapy, manifestations of bone lesions disappeared while the size of the renal mass increased. A partial nephrectomy was recommended for the treatment of her primary renal tumor.
The patient had been on hormone for lupus nephritis treatment for 17 years.
The patient had no markable personal and family history.
On arrival at the urosurgery ward, the patient’s blood pressure (BP) was 128/76 mmHg, and her pulse rate was 77 beats per minute (bpm). No percussion pain was detected alongside her urinary system.
Nothing abnormal was shown in the laboratory examinations.
A mass with mixing density was found on her left kidney on the enhanced computed tomography (CT). Its size was about 5.6 × 4.4 × 4.8 cm.
Hydrocortisone was prescribed as a premedication preoperatively. The patient's electrocardiogram, blood oxygen saturation (SpO2), heart rate (HR), BP, body temperature, and end-tidal carbon dioxide (EtCO2) were monitored. Anesthesia was induced with 150 mg of propofol, 10 mg of oxycodone, and 40 mg of rocuronium. The endotracheal intubation depth was 21 cm from the incisors. The anesthesia was maintained by inhaled sevoflurane and intravenous remifentanil. Ventilation parameters were set to volume-controlled mode (tidal volume at 6-8 mL/kg, respiratory rate at 10-12 times/min). As a result, the EtCO2 partial pressure was about 31-44 mmHg, and airway pressure was maintained between 20-25 mmHg.
The patient was set to the right lateral position for laparoscopic partial neph
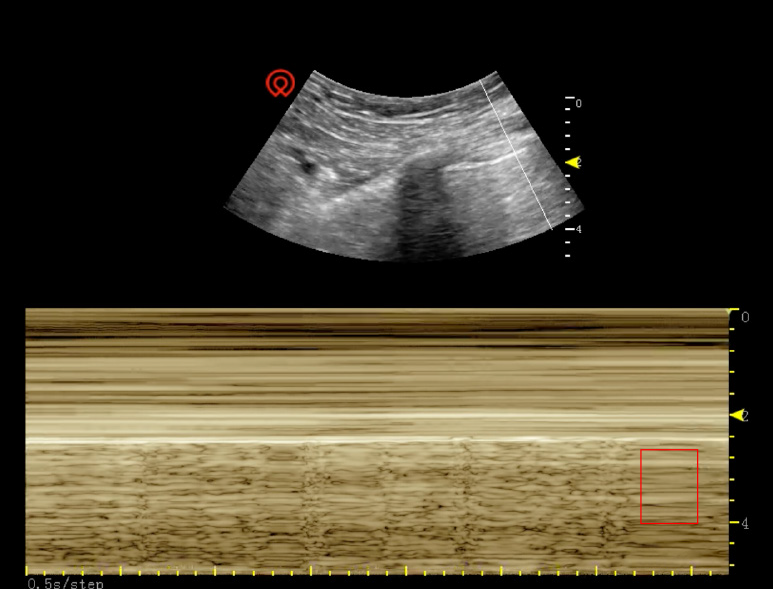
The operation had to be ceased temporarily to set the patient's body position to supine. The anesthetist withdrew all intravenous medications and maximized the O2 supply to 100%. Lung dilation was also conducted. However, after a 5-min resuscitation, her highest SpO2 could only reach 92%. Arterial blood gas analysis showed that her partial CO2 pressure was 47.5 mmHg, and blood lactic acid was 1.0 mmol/L. Due to the shielding of the metal part of the operating table, intraoperative X-ray was not feasible in this case. Intraoperative ultrasound of the right lung was conducted, and the result showed an advection requisition on M-mode (Figure 1). With this evidence, intraoperative pneumothorax was diagnosed. Residual retroperitoneal CO2 was released immediately. The patient's SpO2 could be maintained at 94%-98%, and intraarterial pressure was controlled at 90-100/60-70 mmHg after 30 min of pure O2 flow and lung dilation. Finally, her breath sound of the right lung could be heard again.
After careful evaluation, the patient was again set to the right lateral position, and the retroperitoneal CO2 pressure was lowered to 10-12 mmHg. Laparoscopic exploration proved the integrity of the diaphragm: No injury or damage was found. The surgery was finished within the next hour, and the patient's SpO2 could still be around 90%-94% after turning her back to a supine position. An extra 30-min pure O2 insufflation and lung dilation were performed until her SpO2 reached 100%. We transferred the patient to the post-anesthesia care unit and monitored her vital signs for more than 1 h. The patient claimed mild pain in her right chest during the monitoring. She was sent back to the urosurgery ward after her SpO2 was sustained at 100% and all other vital signs were steady.
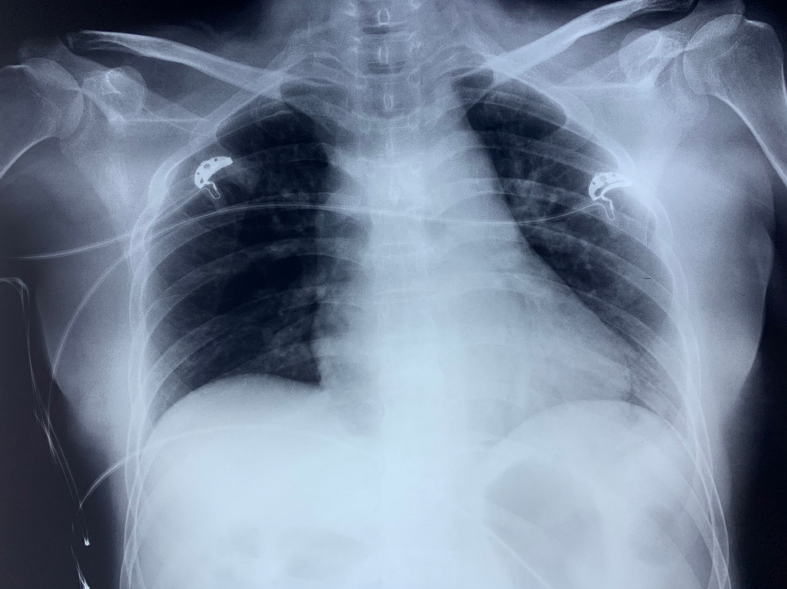
As soon as she entered the ward, she received low-flow oxygen support with a nasal cannula, and her SpO2 was maintained at 100%. A bedside chest X-ray (Figure 2) was ordered, whereas no evidence of pneumothorax or lib injury was found. Her spontaneous breathing SpO2 was about 96%-98% on the first day after surgery. There were no other complications till the patient was discharged.
The final diagnosis of the presented case was left renal cell carcinoma with a history of bone metastasis. Intraoperative pneumothorax was diagnosed in this patient.
After pure O2 flowing, lung dilation, and lowering the retroperitoneal CO2 pressure, the patient's breath sound of the right lung could be heard again. Laparoscopic partial nephrectomy was performed successfully for this patient.
The patient reported no shortness of breath or chest pain at her first and second postoperative outpatient visits.
Pneumothorax is one of the most dangerous complications during laparoscopic or robot-assisted laparoscopic operations. The literature review showed 11 cases of intraoperative pneumothorax that happened[2-3]. The diagnosis of a pneumothorax always depends on ultrasound, X-ray, or chest CT scanning. Chest X-ray is usually the initial tool to detect potential cases. However, its application is limited in the operation room due to the inconvenience of fetching the equipment and low accuracy.
In contrast, transthoracic ultrasound has been reported to be a cheaper, more efficient, and more accurate source of evidence than chest X-ray with an 81% sensitivity and 100% specificity[4]. The typical manifestation of pneumothorax under ultrasound is the multiple advection levels on M-mode, and the pleura moves without line B. X-ray or CT scan reveals the compression or atrophy of the lung. Intraoperative pneumothorax may result from: (1) Intraoperative diaphragm injury, including sharp instrument puncturing or thermal burning; (2) the congenital defection of the diaphragm, which was not discovered before the operation[5]; and (3) high insufflation pressure and long surgery time[6].
Pneumothorax is sometimes misdiagnosed or confused with pulmonary embolism. In this case, we performed the arterial blood gas analysis to assess the patient's condition and exclude pulmonary embolism. An intraoperative ultrasound examination of the lung was also performed to seek more evidence of the pneumothorax. In this case, no injury or damage to the diaphragm was found before or during the operation. However, intraoperative exploration showed that her connective tissues and vessels were extremely fragile. Considering that cases had been sporadically reported, a 12-15 mmHg pneumoperitoneum pressure might cause intraoperative pneumothorax[3,7], while the insufflation pressure was set to 14 mmHg in this case. Given that this patient had a medical history of lupus nephritis and had been on hormone therapy for more than 10 years, we supposed that this might be the reason for the development of the pneumothorax, as CO2 could transfer from the retroperitoneal space to the chest under a higher insufflation pressure and a longer operation time.
The treatment of the pneumothorax should be adjusted dynamically according to its cause and severity. Regardless, the CO2 insufflation should be discontinued, whereas endotracheal intubation, hyperventilation, and higher positive end-expiratory pressure should be maintained for lung dilation[8]. In this patient, an insufflation pressure of 10-12 mmHg might be feasible as her renal tumor was located in the middle to lower kidney, where there was a relatively wider retroperitoneal space. However, in those where the tumors are particularly close to the diaphragm, lowering the retroperitoneal CO2 pressure might interfere with the operation.
Pneumothorax is a rare but severe complication in laparoscopic or robot-assisted laparoscopic operations. It is sometimes neglected unless the injury or damage of the diaphragm is found. Intraoperative ultrasound is a convenient method for diagnosis. Low insufflation pressure and shorter operation time might be necessary for patients with a long-term hormone treatment history or chronic immune systematic disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Surani S S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Del Pizzo JJ, Jacobs SC, Bishoff JT, Kavoussi LR, Jarrett TW. Pleural injury during laparoscopic renal surgery: early recognition and management. J Urol. 2003;169:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Wu Q, Zhang H. Carbon dioxide pneumothorax following retroperitoneal laparoscopic partial nephrectomy: a case report and literature review. BMC Anesthesiol. 2018;18:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Mamić I, Danolić D, Puljiz M, Kasum M, Alvir I, Kostić L, Milas I, Šoštar A, Pedišić I, Bečejac T. Pneumothorax and Pneumomediastinum as a Rare Complication of Laparoscopic Surgery. Acta Clin Croat. 2016;55:501-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1192] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 5. | Park HJ, Kim DK, Yang MK, Seo JE, Kwon JH. Carbon dioxide pneumothorax occurring during laparoscopy-assisted gastrectomy due to a congenital diaphragmatic defect: a case report. Korean J Anesthesiol. 2016;69:88-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Murdock CM, Wolff AJ, Van Geem T. Risk factors for hypercarbia, subcutaneous emphysema, pneumothorax, and pneumomediastinum during laparoscopy. Obstet Gynecol. 2000;95:704-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Aravind A, Shroff P, Dewoolkar L. Epigastric pain caused by pneumothorax following extubation in a case of transperitoneal laparoscopic nephrectomy. Intern J Anesthesiol. 2006;13. [DOI] [Full Text] |
| 8. | Joris JL, Chiche JD, Lamy ML. Pneumothorax during laparoscopic fundoplication: diagnosis and treatment with positive end-expiratory pressure. Anesth Analg. 1995;81:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |