Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1639
Peer-review started: August 7, 2021
First decision: November 6, 2021
Revised: November 14, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 16, 2022
Processing time: 187 Days and 20.8 Hours
Disseminated peritoneal leiomyomatosis (DPL) with myxoid leiomyosarcoma is a rare variant of leiomysosarcoma, and hematuria as a presenting symptom has never been reported. Through this case report, we emphasize the investigation of the etiology, clinical presentation, diagnosis, treatment, and prognosis of DPL with malignant changes mimicking metastatic urinary tract cancer and to help develop further clinical management.
We describe a case of DPL with malignant transformation involving the right ureter after laparoscopic hysterectomy. An exploratory laparotomy was performed and all visible nodules were surgically removed. DPL with focal malignant transformation to myxoid leiomyosarcoma was confirmed based on pathology results.
Professionals who preoperatively diagnose DPL with malignant change to myxoid leiomyosarcoma involving the genitourinary tract should consider symptoms of abdominal pain, hematuria, and imaging of disseminated pelvic tumors in women, especially those with prior history of laparoscopic hysterectomy. Early complete removal of all tumors is the cornerstone to prevent DPL from malignant changes.
Core Tip: Disseminated peritoneal leiomyomatosis (DPL) is a rare disease characterized by the presence of multiple nodules composed of smooth muscle cells located in both peritoneal and extraperitoneal spaces of the abdomen. Malignant changes in DPL correspond to a rare variant of leiomyosarcoma characterized by aggressive behavior. We describe a case of DPL with malignant transformation involving the right ureter after laparoscopic hysterectomy, mimicking urothelial carcinoma with peritoneal carcinomatosis. The aim of our case report is to investigate the etiology, clinical presentation, diagnosis, treatment, and prognosis of DPL and to help develop further clinical management of this disease.
- Citation: Wen CY, Lee HS, Lin JT, Yu CC. Disseminated peritoneal leiomyomatosis with malignant transformation involving right ureter: A case report. World J Clin Cases 2022; 10(5): 1639-1644
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1639.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1639
Disseminated peritoneal leiomyomatosis (DPL) is a rare disease characterized by the presence of multiple nodules composed of smooth muscle cells located in both the peritoneal and extraperitoneal spaces of the abdomen[1]. This disease is usually observed in women of reproductive age. To date, hundreds of cases have been reported[2,3]. However, malignant changes in DPL with myxoid leiomyosarcoma are rare, and hematuria as a presenting symptom has never been reported[4,5]. Herein, we present a case of DPL with malignant transformation involving the right ureter after laparoscopic hysterectomy. The aim of our case report is to investigate the etiology, clinical presentation, diagnosis, treatment, and prognosis of DPL with malignant changes mimicking metastatic urinary tract cancer and to help develop further clinical management.
A 72-year-old woman presented with gross hematuria one month before visiting our hospital.
This patient also noted intermittent abdominal cramping pain for half a year. The patient reported no urinary urgency, dysuria, flank pain, or fever.
The patient had undergone laparoscopic hysterectomy for uterine leiomyoma at another institution 2 years ago prior to her visit. Prior medical histories of hyperten-sion, diabetes mellitus, and gout were noted.
The patient had no relevant personal or family history.
Physical examination revealed multiple painful hard subcutaneous nodules in the lower abdomen.
Laboratory examination revealed an elevated leukocyte count of 15109/mL, hemoglobin count of 12.9 g/dL, and C-reactive protein count of 3.72 mg/dL. Urine cytology, urinalysis, blood coagulation, kidney function, and liver function were all within normal range.
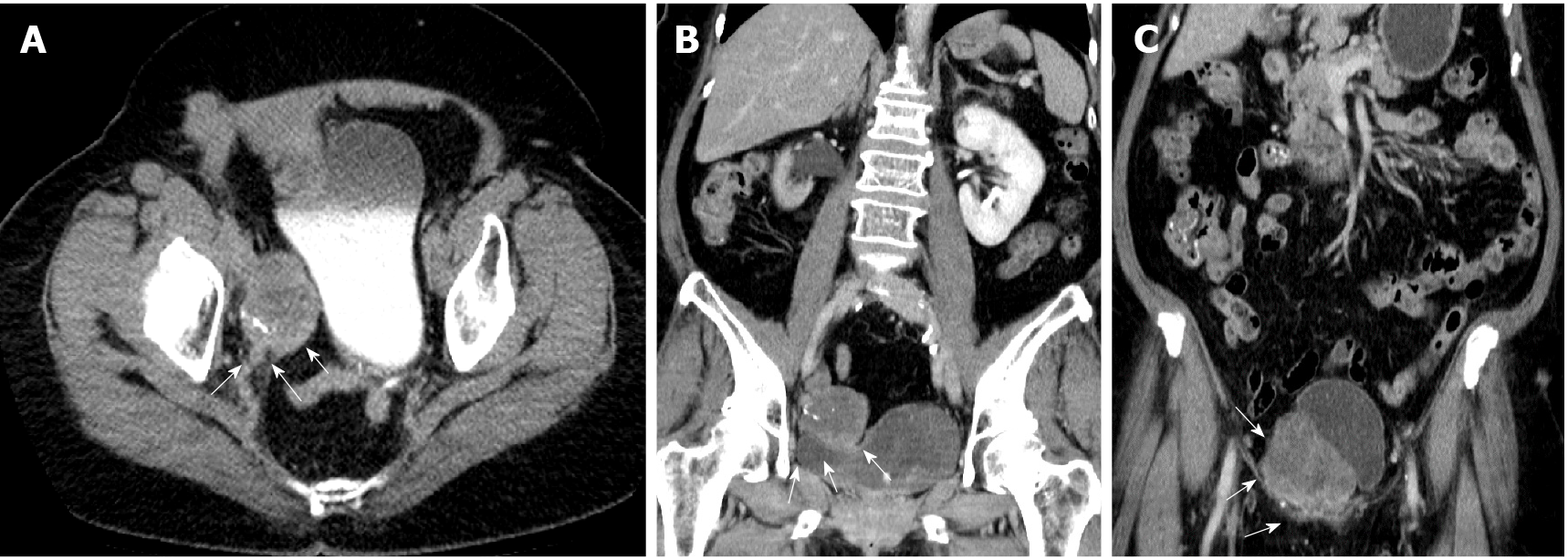
Abdominal computed tomography (CT) suggested urothelial carcinoma of the right lower third ureter with hydronephrosis and multiple seeding lesions at the anterior abdominal wall, subcutaneous fat, and bilateral inguinal areas (Figure 1).
Percutaneous ultrasound-guided biopsy of the most superficial lesion in the right lower quadrant of the abdomen was performed first. The tumor cells showed smooth muscle cell differentiation, which was compatible with leiomyoma as evidenced by pathology results.
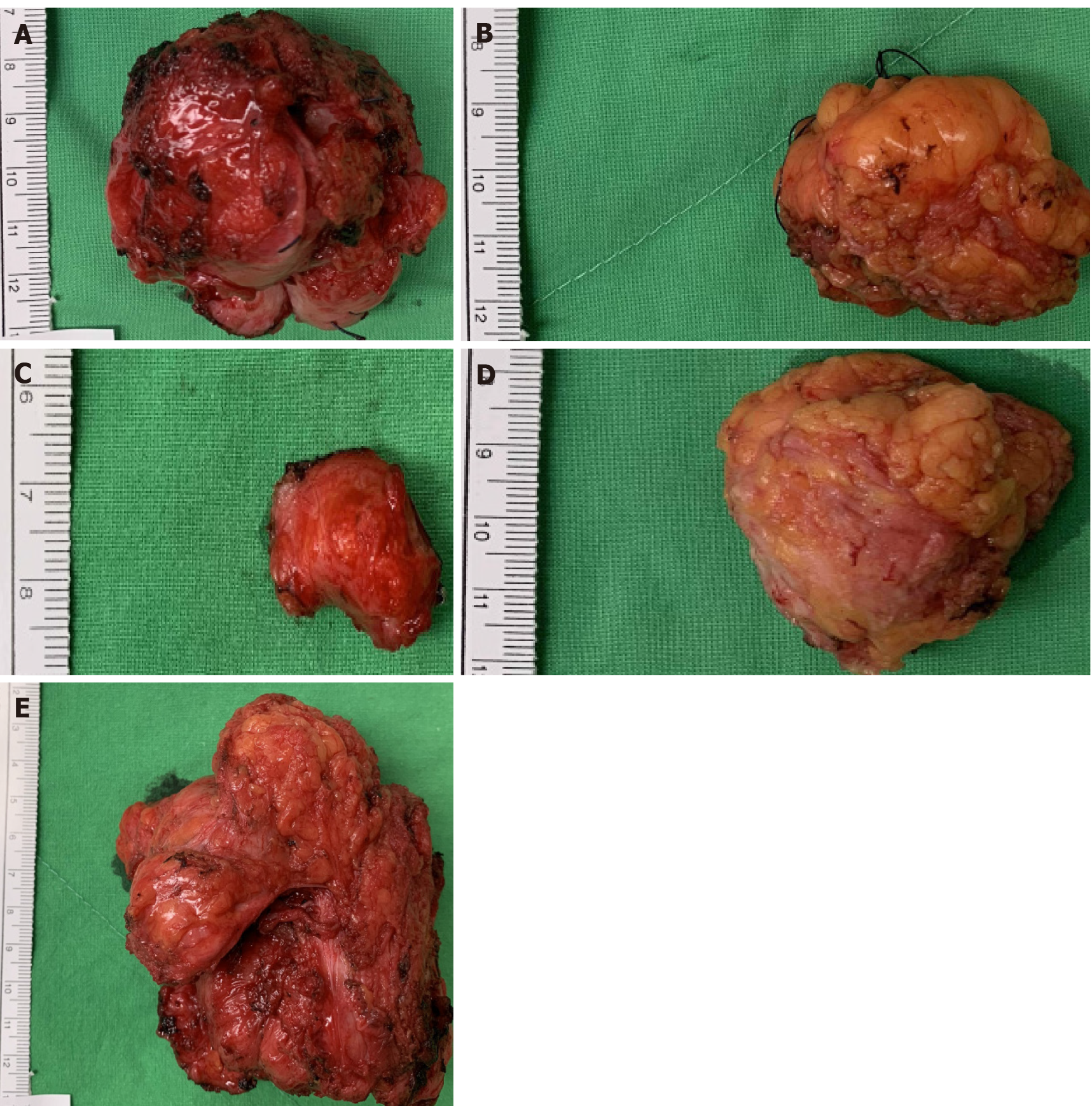
Because of persistent lower abdominal pain, the patient requested all tumors to be removed. An exploratory laparotomy was conducted with a lower midline incision, and multiple tumors of different sizes were found attached to the rectus muscle, bilateral inguinal areas, right ureter, and sigmoid colon (Figure 2). All nodules were meticulously dissected and resected with margins. Segmental resection of the right ureter, ureteroneocystostomy, partial resection of the sigmoid colon wall with primary closure, and transverse colostomy were also performed. The postoperative con
Abdominal discomfort and pain improved significantly postoperatively. The transverse colostomy was closed after 3 mo. Adjuvant systemic chemotherapy was recommended, with periodic follow-up imaging; however, the patient opted for active surveillance only. The patient was doing well without evidence of recurrence 24 mo after the operation.
The etiology and pathophysiology of DPL are not yet well-established. Most reported cases are related to a history of laparoscopic hysterectomy or uterine myomectomy. Iatrogenic contamination after morcellation of myoma during laparoscopic surgery is considered to be a possible cause of DPL[1]. In the current case, the patient underwent laparoscopic hysterectomy 2 years ago; the use of a power morcellator may enhance potential for tumor implantation and dissemination[6,7].
Most patients with DPL are asymptomatic. In these patients, DPL is found incidentally through imaging. Several non-specific symptoms, including abdominal pain, distension, menostaxis, and bleeding from the rectum or vagina have been reported[7-9]. In the present case, the patient reported abdominal pain for one month, which is the most common manifestation of DPL. In addition, she first complained of gross hematuria and was referred to our urology outpatient department. To the best of our knowledge, this is the first report of DPL with hematuria as an initial presentation that could mimic urothelial cancer with peritoneal carcinomatosis[10,11]. Preoperative diagnosis of DPL is challenging, and only histopathologic examination can discriminate DPL from peritoneal metastatic malignancies or benign metastasizing leiomyoma[9,12]. Hence, we performed percutaneous ultrasound-guided biopsy of the abdominal wall lesion to delineate the nature of these tumors, and pathology showed a smooth muscle tumor compatible with leiomyoma.
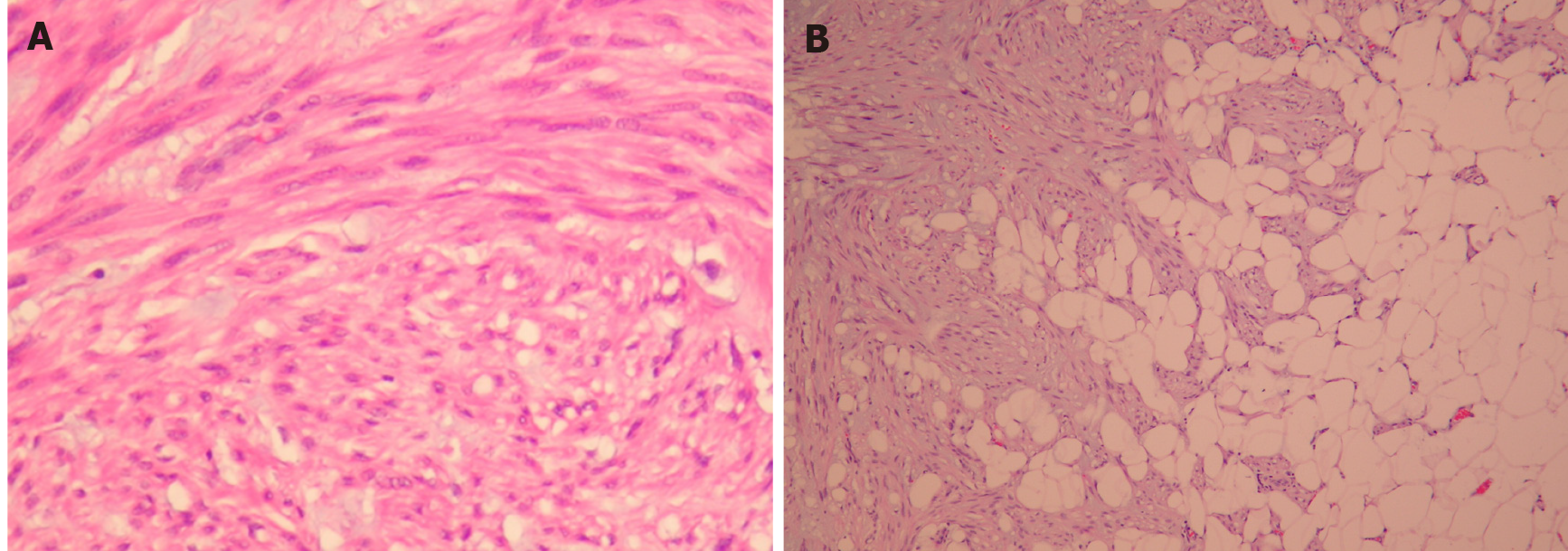
DPL is histologically benign but can transform into a malignant leiomyosarcoma. The duration between the initial diagnosis of DPL and malignant changes varies from 1 mo to 8 years[4,13]. This duration can be under- or overestimated because malignant change may occur focally and insidiously, which makes histological sampling difficult. In the current case, the duration of malignant transformation was speculated to be less than 2 years according to the patient’s operative history. Nevertheless, the focal tumor specimen involving the right ureter revealed a smooth muscle tumor with infiltrative borders, rich myxoid matrix, spindled neoplastic cells arranged in interlacing fascicles, mitotic activity up to 4 mitoses in 10 high-power fields, and foci of tumor necrosis. These findings are compatible with DPL with focal malignant transformation to myxoid leiomyosarcoma.
Standard treatment for DPL is debated. Since most DPLs are found in women of reproductive age, conservative treatment should be considered. Treatment of DPL includes a variety of treatments, such as active surveillance, hormone therapy, debulking surgery, chemotherapy, and radiation therapy, while surgical removal remains the mainstay because of its malignant potential[13,14]. In our case, no adjuvant chemotherapy or radiotherapy was administered.
In conclusion, we present a case of DPL with focal malignant transformation involving the right ureter, mimicking urothelial carcinoma with peritoneal carcinomatosis. Preoperative diagnosis of malignancy is usually challenging. DPL with malignant change to myxoid leiomyosarcoma involving the genitourinary tract should be weighed against differential diagnoses in women presenting with abdominal pain and hematuria with imaging of disseminated pelvic tumors, especially those with prior history of laparoscopic hysterectomy. Early complete surgical resection of all tumors is the most important factor in preventing malignant transformation of DPL, even though it has a relatively favorable outcome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Govindarajan KK, Park SB S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Bekkers RL, Willemsen WN, Schijf CP, Massuger LF, Bulten J, Merkus JM. Leiomyomatosis peritonealis disseminata: does malignant transformation occur? Gynecol Oncol. 1999;75:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Nappi L, Sorrentino F, Angioni S, Pontis A, Barone I, Greco P. Leiomyomatosis Peritonealis Disseminata (LPD) ten years after laparoscopic myomectomy associated with ascites and lymph nodes enlargement: a case report. Int J Surg Case Rep. 2016;25:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Sharma P, Chaturvedi KU, Gupta R, Nigam S. Leiomyomatosis peritonealis disseminata with malignant change in a post-menopausal woman. Gynecol Oncol. 2004;95:742-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Raspagliesi F, Quattrone P, Grosso G, Cobellis L, Di Re E. Malignant degeneration in leiomyomatosis peritonealis disseminata. Gynecol Oncol. 1996;61:272-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Lu B, Xu J, Pan Z. Iatrogenic parasitic leiomyoma and leiomyomatosis peritonealis disseminata following uterine morcellation. J Obstet Gynaecol Res. 2016;42:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Psathas G, Zarokosta M, Zoulamoglou M, Chrysikos D, Thivaios I, Kaklamanos I, Birbas K, Mariolis-Sapsakos T. Leiomyomatosis peritonealis disseminata: A case report and meticulous review of the literature. Int J Surg Case Rep. 2017;40:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Huang SF, Wen CY, Liao CI, Lin JC, Tsai CC. Leiomyomatosis peritonealis disseminata mimicking peritoneal carcinomatosis 13 years after laparoscopic uterine myomectomy: A case report. Int J Surg Case Rep. 2021;81:105745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Li J, Dai S. Leiomyomatosis Peritonealis Disseminata: A Clinical Analysis of 13 Cases and Literature Review. Int J Surg Pathol. 2020;28:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Morita J, Naoe M, Fuji K, Hiramatsu A, Unoki T, Matsui Y, Shimoyama H, Nakasato T, Oshinomi K, Saito K, Maeda Y, Ogawa Y. Indications for ureteropyeloscopy in the detection of upper urinary tract tumors. Urol Sci. 2018;29:186-192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Sharma S, Ksheersagar P, Sharma P. Diagnosis and treatment of bladder cancer. Am Fam Physician. 2009;80:717-723. [PubMed] |
| 12. | Patton KT, Cheng L, Papavero V, Blum MG, Yeldandi AV, Adley BP, Luan C, Diaz LK, Hui P, Yang XJ. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod Pathol. 2006;19:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Yamaguchi T, Imamura Y, Yamamoto T, Fukuda M. Leiomyomatosis peritonealis disseminata with malignant change in a man. Pathol Int. 2003;53:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma. 2010;2010:506182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |