Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1609
Peer-review started: July 30, 2021
First decision: October 25, 2021
Revised: November 7, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 16, 2022
Processing time: 196 Days and 1.7 Hours
The prognosis of refractory extranodal natural killer/T-cell lymphoma (ENKTL) is poor. Recent data have indicated that immune checkpoint blockade with a programmed cell death protein-1 (PD-1) antibody in combination with administration of histone deacetylase inhibitors represents a potentially effective treatment strategy. Compared with PD-1 antibodies, programmed death-ligand 1 antibodies have fewer side effects. Here, we present a rare case of a patient with refractory metastatic ENKTL who achieved sustained remission of approximately 10 mo with minor adverse effects after combination therapy with atezolizumab, chidamide, and radiotherapy.
A 56-year-old woman underwent resection of a tumour in her left nasal cavity and was diagnosed with ENKTL (nasal type). Medical examination revealed tumours observed in the bilateral nasal mucosa, the subcutaneous soft tissue of the inner side of the left eye, the soft tissue of the nasopharynx, the bilateral tonsils, and the left preauricular, right hilar, bilateral neck lymph nodes and bone marrow. However, tomography/computed tomography showed increased metabolism of the bilateral nasal mucosa and subcutaneous soft tissue of the inner side of the left eye and newly increased metabolism of the left cervical lymph node after chemotherapy. Therefore, combination therapy with chidamide, atezolizumab, and radiotherapy was performed. Fortunately, the patient achieved a complete response following 10 mo of combination therapy.
The outcome in this case suggests that the combination of atezolizumab, chidamide, and radiotherapy is a promising regimen for treating refractory metastatic ENKTL following chemotherapy treatment failure.
Core Tip: extranodal natural killer/T-cell lymphoma (ENKTL) is a subtype of non-Hodgkin lymphoma with poor outcomes because ENKTL cells express high levels of P-glycoprotein that mediate tumour multidrug resistance. Furthermore, the standard treatment modality for chemotherapy-resistant ENKTL remains debated. We have experienced a patient with refractory metastatic ENKTL who was resistant to conventional DDGP chemotherapy. Following systemic therapy with atezolizumab and chidamide in combination with local radiotherapy, the patient achieved sustained remission of approximately 10 mo with minor adverse effects.
- Citation: Wang J, Gao YS, Xu K, Li XD. Combination of atezolizumab and chidamide to maintain long-term remission in refractory metastatic extranodal natural killer/T-cell lymphoma: A case report. World J Clin Cases 2022; 10(5): 1609-1616
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1609.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1609
Extranodal natural killer (NK)/T-cell lymphoma (ENKTL) is a distinct subtype of mature T-cell and NK-cell lymphoma that is prevalent in regions of East Asia and South America[1-3]. ENKTL progresses rapidly and has a poor prognosis. Although options for therapy continue to evolve, their curative effects remain unsatisfactory. Because ENKTL cells express high levels of P-glycoprotein that mediate tumour multidrug resistance, conventional chemotherapy regimens such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) have poor outcomes. Thus, nonanthracycline-based chemotherapy has become the main therapeutic strategy. However, in patients for whom L-asparaginase-based regimens are ineffective, progression-free survival (PFS) after relapse or first progression was only 4.1 mo[4].
Recently, several studies have reported that HDAC inhibitors (HDACis) combined with anti-death protein-1 (PD-1) immunotherapy showed encouraging efficacy, thus representing a new treatment strategy for relapsed/refractory (r/r) ENKTL[5,6]. However, the combination of death-ligand 1 (PD-L1) antibody and HDACi for r/r ENKTL has not yet been investigated. Here, we report the case of a patient with refractory metastatic ENKTL who achieved a durable response following systemic therapy with PD-L1 antibody and chidamide in combination with local radiotherapy.
A 56-year-old woman had been diagnosed with ENKTL (nasal type) for one month.
The patient underwent resection of a tumour in her left nasal cavity and was diagnosed with ENKTL (nasal type). Before being transferred to our hospital, she accepted her first cycle chemotherapy with CHOPE (cyclophosphamide 1000 mg Day 1 + vincristine 2 mg Day 1 + epirubicin 100 mg Day 1 + etoposide 100 mg Days 1-3 + prednisone acetate 100 mg Days 1-5) and developed grade IV myelosuppression.
The patient had a free previous medical history.
Personal and family history was non-contributory.
The patient’s temperature was 36.4 °C, heart rate was 102 beats/min, respiratory rate was 25 breaths/min, and blood pressure was 122/95 mmHg. The clinical examination revealed facial strut and pain.
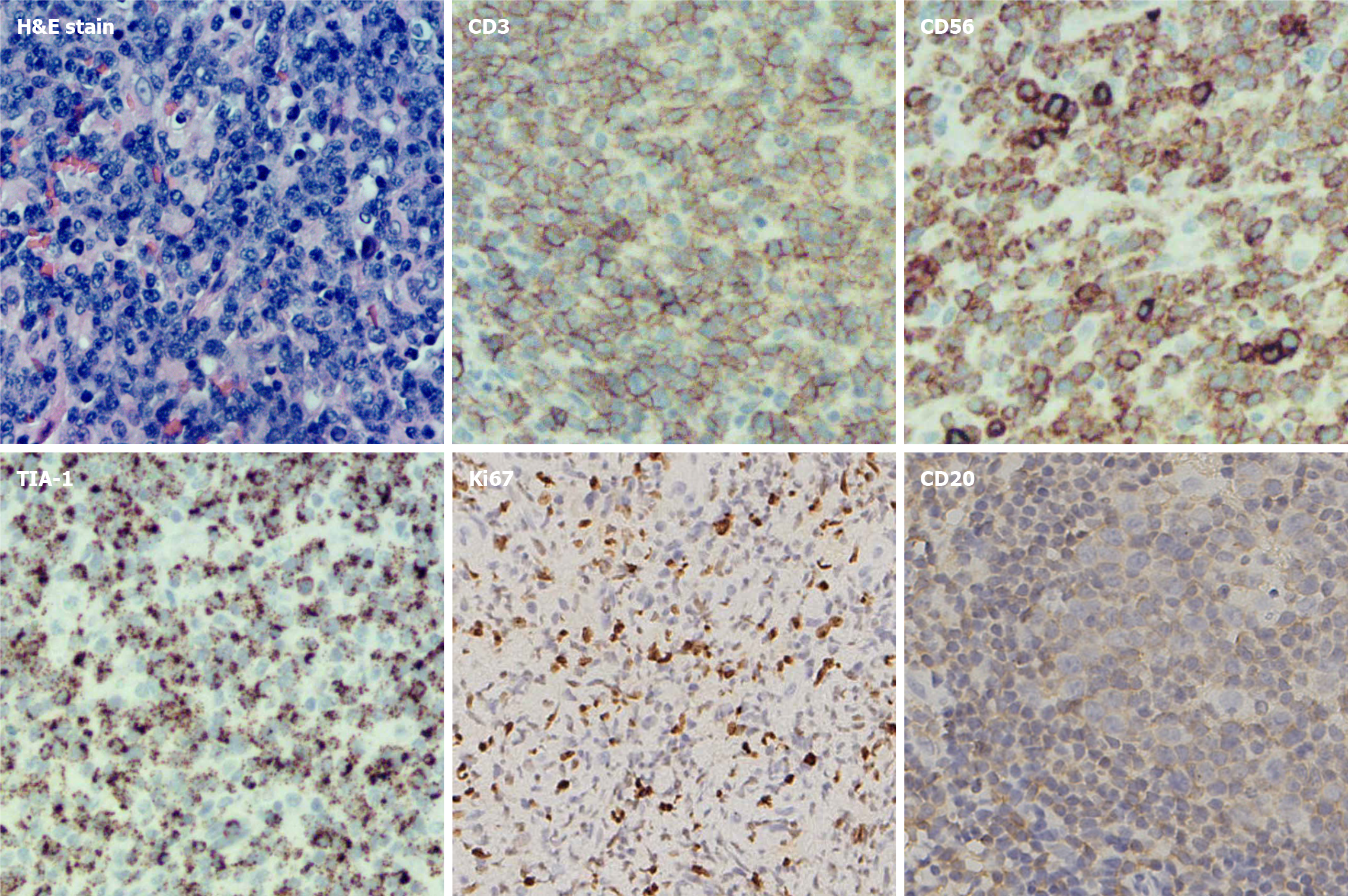
The tumour cells stained positive for CD3, CD56, TIA-1, and Ki-67 (approximately 40%) but were negative for CD20 (Figure 1). Bone marrow examination was performed. Flow cytometry revealed 0.71% NK cells with the following abnormal immunophenotypes: CD2+, CD7+, CD56+, CD94+, CD161+, CD5-, CD16-, and CD8+/-.
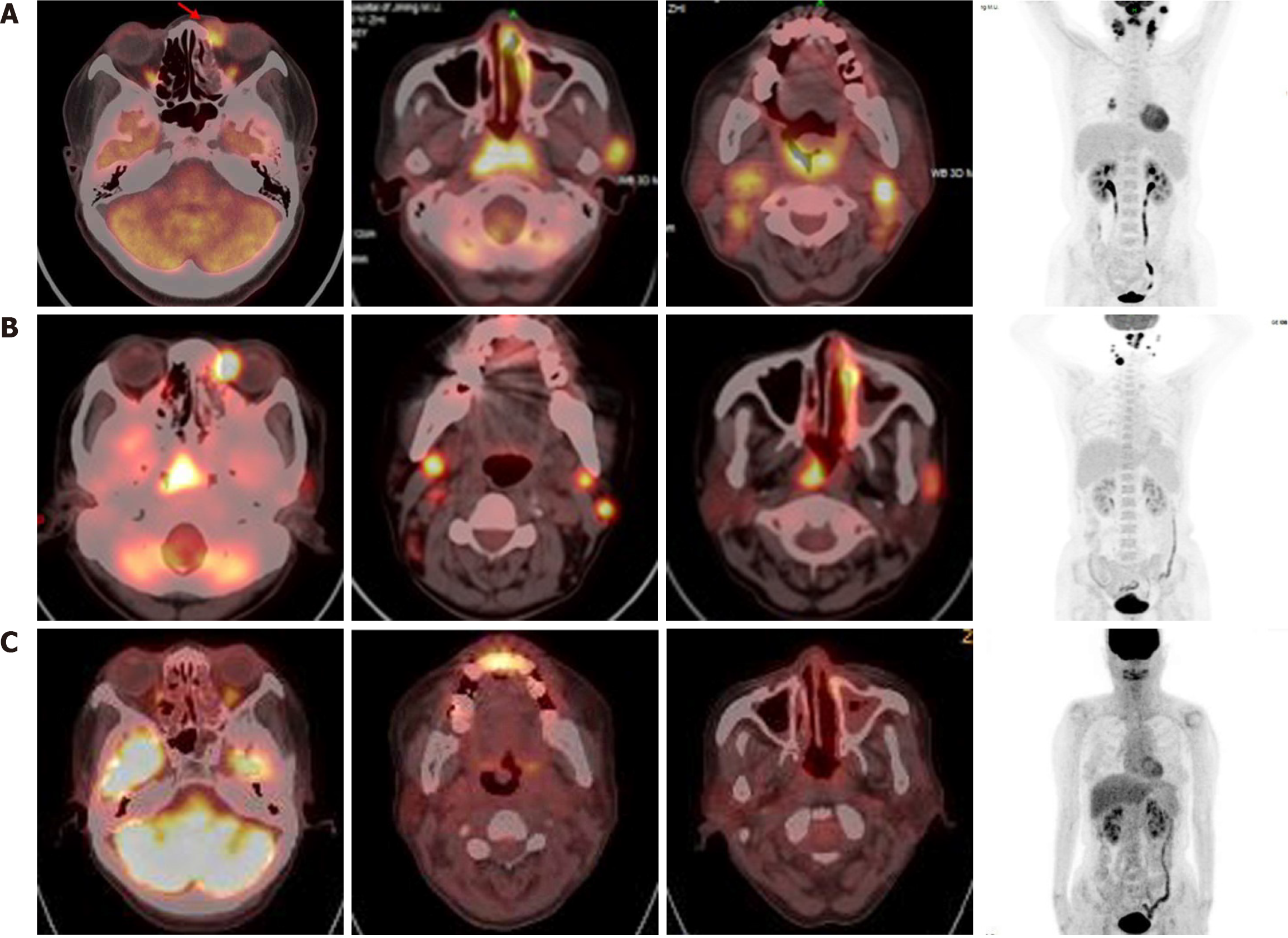
Positron emission tomography/computed tomography (PET/CT) was performed for staging, and increased 18F-fluorodeoxyglucose (FDG) uptake was observed in the bilateral nasal mucosa, the subcutaneous soft tissue of the inner side of the left eye, the soft tissue of nasopharynx, the bilateral tonsils, and the left preauricular, right hilar, and bilateral neck lymph nodes. These patterns were consistent with the infiltration of malignant lymphoma (Figure 2).
The patient was diagnosed with ENKTL (nasal type). Disease was evaluated as Ann Arbor stage IVE A, the prognostic index for NK/T-cell lymphoma, including Epstein-Barr virus DNA load (PINK-E), was calculated as 3, and disease was classified as high risk.
Radiotherapy, chidamide, and nivolumab were concurrently administered. The target volume included the partial frontal sinus, the right maxillary sinus, all ethmoid sinuses, the sphenoid sinus, the left orbit and eye contents, the left maxillary sinus, the nasopharynx, the left preauricular lymphoid drainage area, and the bilateral neck level Ib, 2, 3, 4, and 5 Lymphatic drainage areas. The radiation dose was 50 Gy/25 fractions. The patient developed transient rash on the third day after nivolumab treatment and grade 4 thrombocytopenia following the first cycle of combination therapy. Therefore, the PD-1/PD-L1 inhibitor was changed to atezolizumab for subsequent immunotherapy after her haemogram recovered.
After four cycles of chidamide and atezolizumab, PET/CT showed slightly higher metabolism of the nasal cavity. Treatment was continued as planned. Fortunately, PET/CT showed no obvious FDG uptake after 11 cycles of combination therapy with chidamide and atezolizumab (Figure 2). Grade 3 adverse events, including neutropenia and thrombocytopenia, were manageable and resolved during maintenance treatment.
ENKTL is a subtype of non-Hodgkin lymphoma with poor outcome. The standard treatment modality for refractory ENKTL is still debated, especially for chemotherapy-resistant tumours[4]. Here, we present the case of a patient with refractory metastatic ENKTL who was resistant to conventional DDGP chemotherapy. Following systemic therapy with a PD-L1 inhibitor and chidamide in combination with local radiotherapy, the patient achieved sustained remission of approximately 10 mo with minor adverse effects.
Previous studies suggested that NKTL was resistant to anthracycline[7]. Thus, pegaspargase, gemcitabine, or other non-anthracycline-based chemotherapy regimens are generally used for the first-line treatment of patients with newly diagnosed refractory NKTL[8,9]. Additionally, as described in previous reports, allogeneic stem cell transplantation (allo-SCT) may be beneficial for patients with ENKTL[10,11]. However, PFS in the subset of patients who maintained remission following allo-SCT was only approximately 10.0 mo. There has been no randomized, prospective study to evaluate the safety and efficacy of allo-SCT in ENKTL[12].
Recently, radiotherapy, PD-1 inhibitors, and HDACis (alone or in combination) have shown promising efficacy in treating r/r ENKTL. Chidamide is a novel benzamide-type HDACi that can selectively block HDAC1, 2, 3, and 10[13]. Recent data demonstrated that chidamide induced growth inhibition and apoptosis in NK/T lymphoma cells[14]. A phase II clinical trial of chidamide for r/r peripheral T-cell lymphoma showed median PFS and overall survival of 2.1 and 21.4 mo, respectively[15]. In this study, 16 ENKTL patients were enrolled and showed lower response rates compared with other studies: one patient achieved a complete response (CR), and two patients achieved partial responses (PRs).
PD-1/PD-L1 inhibitors are additional new agents for the treatment of r/r ENKTL. In previous reports (Table 1), combining PD-1 antibody with chemotherapy or chidamide obtained satisfactory results, and most of the cases achieved complete response and sustained curative effects[16-21]. The anti-PD-1 antibody (sintilimab) plus chidamide regimen was evaluated in a phase 1b/II clinical trial[22], where the CR rate was 44.4% in 41 r/r-NKTCL patients. A previous study demonstrated that anti-PD-L1 antibodies have better efficacy and fewer adverse effects[23]. In particular, an open-label phase 2 study demonstrated that a PD-L1 antibody as a single agent induced tumour remission in a subset of patients. CRs were observed in 24% of patients, and the overall response rate was 38%; the study was terminated because of a lower than expected response rate[24]. Five responders in this study continued to show sustained responses, and the only adverse events observed were grades 1 or 2. However, to our knowledge, there have been no case reports evaluating the effects of PD-L1 antibody for r/r-ENKTL patients who could not tolerate previous treatment with PD-1 antibody.
| Ref. | Number of cases | Age mean year (range) | Gender | Treatment | Stage | Response | OS or PFS |
| McGehee et al[16] 2021 | 1 | 72 | 1 M | Pembrolizumab plus RT | IV | CR | 33 mo, alive |
| Du et al[17] 2020 | 3 | 52 (51-54) | 3 M | PD-1 antibody, plus Chidamide, etoposide, and thalidomide | 1 (33.3%) IV; 1 (33.3%) III; 1 (33.3%) II | 2 (66.7%) CR; 1 (33.3%) PD | - |
| Kwong et al[18] 2017 | 7 | 49 (31-68) | 7 M | Pembrolizumab | 5 (71.4%) IV; 2 (28.6%) IE | 5 (71.4%) C; 2 (28.6%) PR | - |
| Li et al[19] 2018 | 7 | 47 (17-61) | 4 M; 3 F | Pembrolizumab | 2 (28.6%) IV; 3 (42.9%) II; 1 (14.3%) IIIE; 1 (14.3%) IE | 2 (28.6%) CR; 2 (28.6%) PR | 5 mo OS; 4.8 mo PFS |
| Diab et al[20] 2021 | 1 | 82 | M | Pembrolizumab | IV | CR | 21 mo, alive |
| Lai et al[21] 2017 | 1 | 37 | F | Pembrolizumab | IV | CR | - |
| Gao et al[22] 2020 | 41 | 48 (20-72) | 27 M; 14 F | Sintilimab plus chidamide | 26 (70.3%) IV; 15 (29.7%) Non-IV | 16 (44.4%) CR; 5 (13.9%) PR | - |
| Kim et al[24] 2020 | 21 | ≤ 60 16; > 60 5 | 13 M; 8F | Avelumab | - | 5 (23.8%) CR; 3 (14.3%) PR | - |
Recently, multiple lines of evidence have demonstrated that HDACis could enhance the therapeutic effects of PD-1 antibodies[25,26]. Epigenetic modification could regulate T cell trafficking and reactivation, thus enhancing the efficacy of the PD-1 antibody. A few case reports suggested that the combination of PD-1 antibodies and HDACis might be effective in patients with refractory ENKTL[5,6]. However, the antitumour effect of combination therapy with PD-L1 antibody and chidamide has not been demonstrated for refractory ENKTL. The patient described here was successfully treated with local radiotherapy and systemic therapy with chidamide and PD-L1 antibodies. Evaluation 10 mofollowing the end of radiation therapy showed a sustained CR. We presume that the sustained therapeutic efficacy observed in this patient may result from synergistic effects of PD-L1 antibody, chidamide, and local radiotherapy. Studies with larger numbers of patients are needed to evaluate the efficacy and safety of this combination therapy regimen for refractory ENKTL.
We present a rare case of a patient with refractory ENKTL who was successfully treated with a combination of radiotherapy, chidamide, and PD-L1 antibody. Additional evidence is needed to evaluate the potential activity and safety of this regimen.
We thank the patient and her family for providing signed informed consent.
Provenance and peer review: Unsolicited manuscript; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shekouhi R S-Editor: Wang LYT L-Editor: A P-Editor: Wang LYT
| 1. | Shi Y. Current status and progress of lymphoma management in China. Int J Hematol. 2018;107:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, Otto B, Barrionuevo C, Baiocchi RA, Rochford R, Porcu P. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTL-NT): An Update on Epidemiology, Clinical Presentation, and Natural History in North American and European Cases. Curr Hematol Malig Rep. 2016;11:514-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Fox CP, Civallero M, Ko YH, Manni M, Skrypets T, Pileri S, Kim SJ, Cabrera ME, Shustov AR, Chiattone CS, Horwitz SM, Dlouhy I, Spina M, Hitz F, Montoto S, Nagler A, Martinez V, De Souza CA, Fernandez-Alvarez R, Ballova V, Gabús R, Inghirami G, Federico M, Kim WS. Survival outcomes of patients with extranodal natural-killer T-cell lymphoma: a prospective cohort study from the international T-cell Project. Lancet Haematol. 2020;7:e284-e294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 4. | Lim SH, Hong JY, Lim ST, Hong H, Arnoud J, Zhao W, Yoon DH, Tang T, Cho J, Park S, Ko YH, Kim SJ, Suh C, Lin T, Kim WS. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28:2199-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Xu J, Xu X, Chen J, Wang J, Jiang C, Lv C, Chen B. Sustained remission of multi-line relapsed extranodal NK/T-cell lymphoma, nasal type, following sintilimab and chidamide: A case report. Medicine (Baltimore). 2021;100:e24824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Yan Z, Yao S, Liu Y, Zhang J, Li P, Wang H, Chu J, Zhao S, Yao Z. Durable Response to Sintilimab and Chidamide in a Patient With Pegaspargase- and Immunotherapy-Resistant NK/T-Cell Lymphoma: Case Report and Literature Review. Front Oncol. 2020;10:608304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410-4416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 464] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 8. | Zhang L, Wang Y, Li X, Li L, Wang X, Sun Z, Wu J, Fu X, Zhang X, Yu H, Wang G, Chang Y, Yan J, Zhou Z, Wu X, Nan F, Li W, Zhang M. Radiotherapy vs sequential pegaspargase, gemcitabine, cisplatin and dexamethasone and radiotherapy in newly diagnosed early natural killer/T-cell lymphoma: A randomized, controlled, open-label, multicenter study. Int J Cancer. 2021;148:1470-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Wang JH, Wang L, Liu CC, Xia ZJ, Huang HQ, Lin TY, Jiang WQ, Lu Y. Efficacy of combined gemcitabine, oxaliplatin and pegaspargase (P-gemox regimen) in patients with newly diagnosed advanced-stage or relapsed/refractory extranodal NK/T-cell lymphoma. Oncotarget. 2016;7:29092-29101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Tse E, Chan TS, Koh LP, Chng WJ, Kim WS, Tang T, Lim ST, Lie AK, Kwong YL. Allogeneic haematopoietic SCT for natural killer/T-cell lymphoma: a multicentre analysis from the Asia Lymphoma Study Group. Bone Marrow Transplant. 2014;49:902-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ennishi D, Maeda Y, Fujii N, Kondo E, Shinagawa K, Ikeda K, Ichimura K, Yoshino T, Tanimoto M. Allogeneic hematopoietic stem cell transplantation for advanced extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma. 2011;52:1255-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Jeong SH, Song HN, Park JS, Yang DH, Koh Y, Yoon SS, Lee HW, Eom HS, Won JH, Kim WS, Kim SJ. Allogeneic Stem Cell Transplantation for Patients with Natural Killer/T Cell Lymphoid Malignancy: A Multicenter Analysis Comparing Upfront and Salvage Transplantation. Biol Blood Marrow Transplant. 2018;24:2471-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Ning ZQ, Li ZB, Newman MJ, Shan S, Wang XH, Pan DS, Zhang J, Dong M, Du X, Lu XP. Chidamide (CS055/HBI-8000): a new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother Pharmacol. 2012;69:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Zhang C, Sui X, Cao S, Tang F, Sun S, Wang S, Chen B. Histone deacetylase inhibitor chidamide induces growth inhibition and apoptosis in NK/T lymphoma cells through ATM-Chk2-p53-p21 signalling pathway. Invest New Drugs. 2018;36:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, Yu L, Ke X, Huang H, Shen Z, Fan Y, Li W, Zhao X, Qi J, Zhou D, Ning Z, Lu X. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol. 2015;26:1766-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 16. | McGehee E, Patel H, Pearson C, Clements K, Jaso JM, Chen W, Callan A, Desai N, Ramakrishnan Geethakumari P. Combined immune checkpoint blockade and radiotherapy induces durable remission in relapsed natural killer/T-cell lymphoma: a case report and review of theliterature. J Med Case Rep. 2021;15:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Du L, Zhang L, Li L, Li X, Yan J, Wang X, Fu X, Sun Z, Zhang X, Li Z, Wu J, Yu H, Chang Y, Zhou Z, Nan F, Wu X, Tian L, Zhang M. Effective Treatment with PD-1 Antibody, Chidamide, Etoposide, and Thalidomide (PCET) for Relapsed/Refractory Natural Killer/T-Cell Lymphoma: A Report of Three Cases. Onco Targets Ther. 2020;13:7189-7197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kwong YL, Chan TSY, Tan D, Kim SJ, Poon LM, Mow B, Khong PL, Loong F, Au-Yeung R, Iqbal J, Phipps C, Tse E. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437-2442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 19. | Li X, Cheng Y, Zhang M, Yan J, Li L, Fu X, Zhang X, Chang Y, Sun Z, Yu H, Zhang L, Wang X, Wu J, Li Z, Nan F, Tian L, Li W, Young KH. Activity of pembrolizumab in relapsed/refractory NK/T-cell lymphoma. J Hematol Oncol. 2018;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Diab R, Kamran S, Adcock B, Choucair K, Truong QV. Extra-Nodal, Nasal, Natural Killer T-Cell Lymphoma Treated With a Checkpoint Inhibitor: A Case Report of a Sustained Complete Response. Cureus. 2021;13:e14654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lai J, Xu P, Jiang X, Zhou S, Liu A. Successful treatment with anti-programmed-death-1 antibody in a relapsed natural killer/T-cell lymphoma patient with multi-line resistance: a case report. BMC Cancer. 2017;17:507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Gao Y, Huang H, Wang X, Bai B, Zhang L, Xiao Y, Liu X, Li W, Xu W, Feng R, Chen Y, Wu H, Li J, Wu X. Anti-PD-1 Antibody (Sintilimab) Plus Histone Deacetylase Inhibitor (Chidamide) for the Treatment of Refractory or Relapsed Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (r/r-ENKTL): Preliminary Results from a Prospective, Multicenter, Single-Arm, Phase Ib/II Trial (SCENT). Blood. 2020;136:39-40. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Feng D, Guan Y, Liu M, He S, Zhao W, Yin B, Liang J, Li Y, Wang J. Excellent Response to Atezolizumab After Clinically Defined Hyperprogression Upon Previous Treatment With Pembrolizumab in Metastatic Triple-Negative Breast Cancer: A Case Report and Review of the Literature. Front Immunol. 2021;12:608292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Kim SJ, Lim JQ, Laurensia Y, Cho J, Yoon SE, Lee JY, Ryu KJ, Ko YH, Koh Y, Cho D, Lim ST, Enemark MB, D'Amore F, Bjerre M, Ong CK, Kim WS. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: an open-label phase 2 study. Blood. 2020;136:2754-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Burke B, Eden C, Perez C, Belshoff A, Hart S, Plaza-Rojas L, Delos Reyes M, Prajapati K, Voelkel-Johnson C, Henry E, Gupta G, Guevara-Patiño J. Inhibition of Histone Deacetylase (HDAC) Enhances Checkpoint Blockade Efficacy by Rendering Bladder Cancer Cells Visible for T Cell-Mediated Destruction. Front Oncol. 2020;10:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Chen X, Pan X, Zhang W, Guo H, Cheng S, He Q, Yang B, Ding L. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10:723-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |