Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1580
Peer-review started: September 6, 2021
First decision: December 1, 2021
Revised: December 7, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 16, 2022
Processing time: 157 Days and 17.5 Hours
Cytokine release syndrome (CRS) is defined as systemic inflammation that usually occurs following chimeric antigen receptor T-cell therapy administration; however, it has not been reported in patients with untreated non-small cell lung cancer to date.
A 44-year-old nonsmoking woman presented to the hospital due to fever, palpitation, nausea, and cough for 1 mo and was diagnosed with stage cT3N3M0 (IIIc) adenocarcinoma of the lung. Auxiliary examinations revealed elevated cytokine [tumor necrosis factor-α, interleukin (IL)-1β, and IL-6] and inflammatory factor levels, which decreased after treatment with corticosteroids and immunoglobulin and when tumor growth was controlled following chemotherapy, radiotherapy, and antiangiogenesis therapy. However, tumor recurrence was observed. After administration of nivolumab as third-line treatment, the patient’s condition was transiently controlled; however, CRS-like symptoms suddenly emerged, which led to a resurgence of cytokines and inflammatory factors and rapid death.
CRS can develop in treatment-naïve lung cancer patients. Patients with tumor-related CRS may be at risk of CRS recurrence, aggravation, and onset of immune checkpoint inhibitor-related adverse events.
Core Tip: Cytokine release syndrome (CRS) is defined as systemic inflammation that usually occurs after chimeric antigen receptor T-cell therapy is administered. But the case we report suggests CRS can develop in treatment-naïve lung cancer patient. Patients with tumor-related CRS may be at risk of CRS recurrence, aggravation, and onset of immune checkpoint inhibitor (ICI)-related adverse events when ICIs are administered. Therefore, it is necessary to carefully evaluate whether the patient has CRS prior to the initiation of ICI treatment.
- Citation: Deng PB, Jiang J, Hu CP, Cao LM, Li M. Tumor-related cytokine release syndrome in a treatment-naïve patient with lung adenocarcinoma: A case report . World J Clin Cases 2022; 10(5): 1580-1585
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1580.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1580
Cytokine release syndrome (CRS) is defined as systemic inflammation that usually occurs after the initiation of chimeric antigen receptor (CAR) T-cell therapy[1]. Several case reports have shown that patients treated with immune checkpoint inhibitors (ICIs) such as pembrolizumab[2] and nivolumab[3] anti-programmed cell death-1 (antibody) can develop CRS. To our knowledge, CRS has not been previously reported in treatment-naïve patients with lung cancer. Based on the results of our follow-up on patients with non-small cell lung cancer, the present patient’s primary CRS was attributed to lung cancer, which usually recurs due to the development of tumors and an increase in tumor burden. Moreover, the patient developed CRS after being administered nivolumab, which led to rapid death (Table 1). This finding suggests that tumor-related CRS may be associated with ICI-related adverse events (irAEs) and poor prognosis among patients treated with nivolumab.
| Time | Syndrome or treatment | Oncologic response |
| October 2017 | Fever (maximum 41 °C), palpitation, nausea and cough for 1 mo | |
| October 2017 | Diagnosed as medium differentiated adenocarcinoma lung cancer with EGFR and ALK gene mutations negative by CT-guided puncture biopsy | |
| November 2017 | Considered have primary CRS with related to lung cancer, and treated with DXM, gamma globulin and other supporting treatments. The patient stopped fever soon | |
| December 2017 to February 2018 | Four cycles of chemotherapy with pemetrexed + cisplatin | PR |
| June 11, 2018 | Recurrent fever for 10 d with CT showed tumor progressed again | PD |
| July 2018 to August 2018 | Radiotherapy then stated to take | PR |
| August 2018 | Anlotinib | PR |
| May 2019 | Nivolumab for 5 cycles | PR |
| April 2019 | Died | PD |
A 44-year-old nonsmoking woman visited our hospital (Xiangya Hospital, Central South University, Changsha, Hunan Province, China) in October 2017 due to fever (maximum, 41 °C), palpitation, nausea, and cough for 1 mo.
The patient had fever (maximum, 41 °C), palpitation, nausea, and cough for 1 mo.
No special history of past illness.
No special personal or family history was reported.
The patient had palpable right-sided supraclavicular lymph nodes, low breath sounds on the right lung, and the absence of rales.
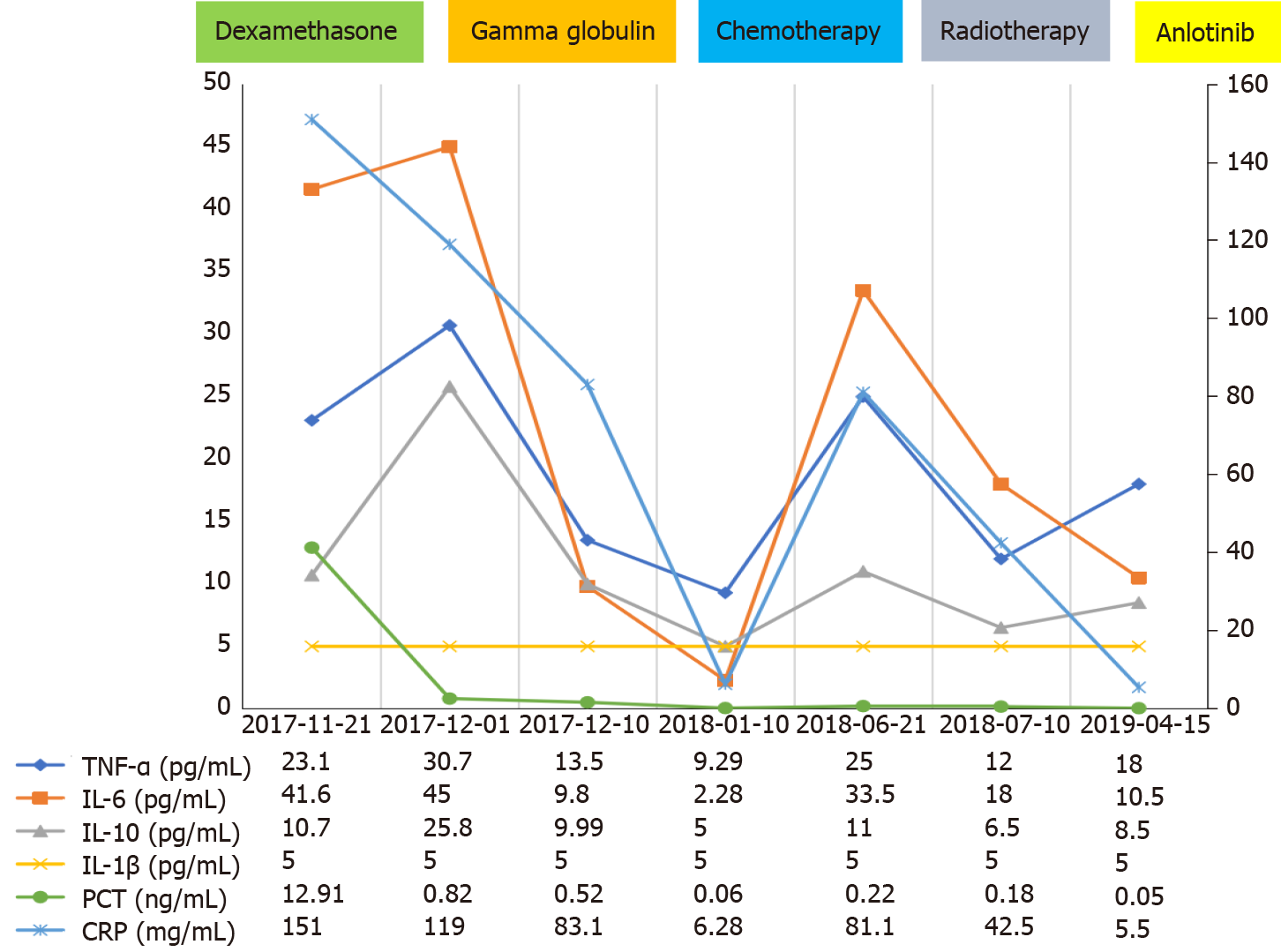
The patient was diagnosed with partially differentiated adenocarcinoma of the lung with negative epidermal growth factor receptor and anaplastic lymphoma kinase gene mutations based on the results of computed tomography (CT)-guided puncture biopsy. The patient exhibited characteristics similar to those of CRS based on her clinical manifestations (high fever, tachycardia, nausea, appetite loss, and malaise) and laboratory examination results (elevated cytokines [tumor necrosis factor α (TNFα) and interleukin (IL)-1β, IL-6, and IL-10 levels (Figure 1), organ dysfunction (liver), and elevated ferritin levels][4]. We excluded other conditions that may have caused similar symptoms, such as tumor lysis syndrome (no hyperkalemia, uric acidemia, etc.), infection, and hemophagocytic syndrome (absence of hematopoietic cells on bone marrow biopsy).
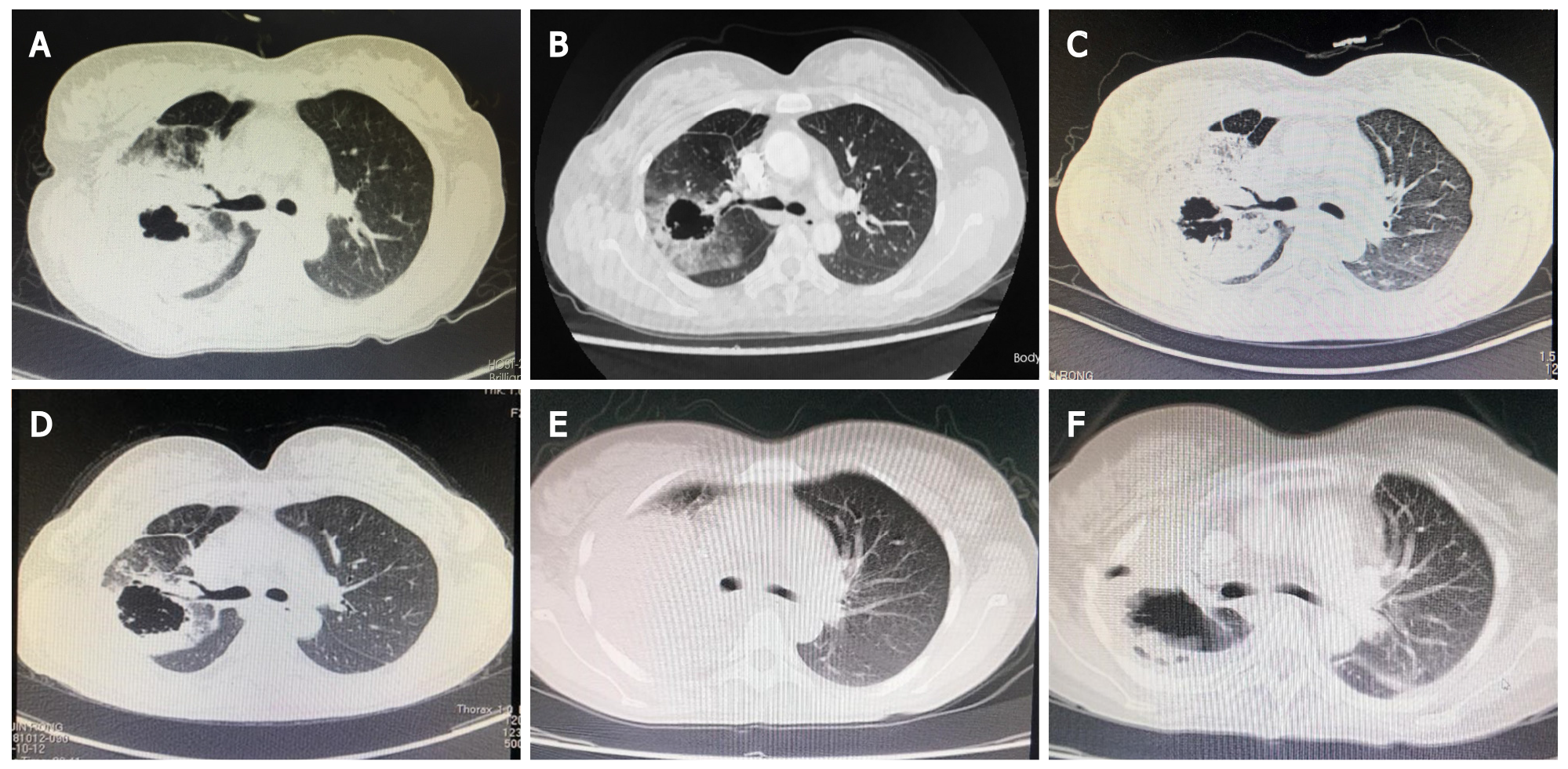
CT on October 9, 2017 revealed a thick-walled cavity in the upper right lobe (Figure 2A). Tumor stage was cT3N3M0 (IIIc).
Lung adenocarcinoma (stage T3N3M) and CRS.
We speculated that the patient may have primary CRS related to lung cancer and administered a 10 mg intravenous infusion of dexamethasone qd for 7 d, 20 g intravenous infusion of gamma globulin for 3 d, and other supportive treatments. The patient’s fever eventually subsided, her general condition improved, the levels of inflammatory factors and cytokines decreased (Figure 1), and the Eastern Cooperative Oncology Group (ECOG) score improved. Four cycles of chemotherapy with pemetrexed and cisplatin were initiated. The patient achieved partial remission (PR) at 1-mo follow-up according to the Response Evaluation Criteria in Solid Tumors, version 1.1 (Figure 2B). The patient had an ECOG score of 1, and her routine blood tests and cytokine and inflammatory factor levels had returned to normal (October 1, 2017) (Figure 1).
On June 11, 2018, she experienced recurrent fever for 10 d, and CT showed tumor progression (Figure 2C). The levels of cytokine and inflammatory factors began to increase (Figure 1), and we excluded the possibility of infectious fever and considered recurrent CRS. As the patient had stage IIIc adenocarcinoma, she was treated with radiotherapy from July 2018 to August 2018, and anlotinib therapy was initiated. The patient did not develop fever during this period. In October 2018, follow-up CT was performed, which revealed that the tumor had shrunk (Figure 2D); however, the size of the tumor started to increase in May 2019 (Figure 2E). Hence, five cycles of nivolumab treatment was administered. CT was performed in August 2019 and showed that the patient had achieved PR (Figure 2F).
Seventeen days after receiving the last dose of nivolumab, the patient was sent to the emergency department due to exacerbation of sudden dyspnea, high fever, respiratory failure, and sudden cardiac arrest. The patient eventually died on September 8, 2019, with laboratory tests showing elevated cytokine and inflammatory factor levels (Figure 1).
The exact mechanism of CRS has not been fully elucidated. Cytokines are released when the tumor interacts with immune effector cells, and they can originate not only from the CAR T cells but also from host immune cells, such as macrophages[5]. Previous studies have shown that lung cancer cells can directly release inflammatory cytokines, including IL-1, IL-6, TNFα, and interferon (IFN)[6]. Tumor necrosis can also release a large number of cytokines, such as TNF[7]. The patient had obvious necrotic cavities in her lungs which may have been the cause of cytokine release.
This clinical experience demonstrates that corticosteroids are an effective treatment for CRS, and steroids can be rapidly tapered within several days without CRS recurring. Another drug, tocilizumab, is a humanized immunoglobulin G1 + (IgG1 +) anti-human IL-6R monoclonal antibody which can usually resolve fever and hypotension within a few hours in patients with CRS and may induce a response more quickly than corticosteroids[8]. In the present case, corticosteroids and immunoglobulin were administered, and a significant therapeutic effect was achieved. With subsequent chemotherapy and other treatments to control lung cancer, CRS also improved, suggesting that antitumor therapy is also an important treatment for tumor-related CRS. Moreover, targeted immunosuppressive agents are also available to inhibit TNFα and IL-1, both of which may contribute to CRS, such as anti-TNFα monoclonal antibodies (infliximab), soluble TNFα receptor (etanercept), and IL-1R-based inhibitors (anakinra).
This patient was administered nivolumab as third-line treatment and experienced exacerbation of CRS-like symptoms and eventually passed away after showing an oncologic response following nivolumab administration. ICI-related CRS can develop 2 d to 4 mo after treatment, and before or after achieving a significant antitumor response to ICI therapy[2,3]; this type of CRS is related to tumor lysis through the induction of pyroptosis in target cells[9]. Based on the patient’s symptoms and results of auxiliary examinations combined with her previous CRS, her disease progression may have been related to nivolumab treatment. A series of recent studies suggest inflammatory cytokines are potential biomarkers for irAEs, and one study found that patients treated with nivolumab who had a high level of soluble IL-2 measured at the initial tumor evaluation had a significantly increased risk of developing grade 3-4 nivolumab-related irAEs[10]. The above phenomena suggest that the use of ICIs in patients with tumor-associated CRS may induce the onset or aggravation of CRS or serious irAEs, which may be life-threatening.
We believe that CRS can occur in treatment-naïve patients with lung cancer. Corticosteroids, immunoglobulins, and subsequent antitumor treatments have played important roles in the control of tumor-related CRS. Patients with tumor-related CRS may be at risk of CRS recurrence, aggravation, and onset of irAEs when treated with ICIs; therefore, it is necessary to carefully evaluate whether the patient has CRS prior to initiating ICI treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: International Association for the Study of Lung Cancer, No. 414957.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brat K S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, Barrett DM, Weiss SL, Fitzgerald JC, Berg RA, Aplenc R, Callahan C, Rheingold SR, Zheng Z, Rose-John S, White JC, Nazimuddin F, Wertheim G, Levine BL, June CH, Porter DL, Grupp SA. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6:664-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 835] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 2. | Kogure Y, Ishii Y, Oki M. Cytokine Release Syndrome with Pseudoprogression in a Patient with Advanced Non-Small Cell Lung Cancer Treated with Pembrolizumab. J Thorac Oncol. 2019;14:e55-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Honjo O, Kubo T, Sugaya F, Nishizaka T, Kato K, Hirohashi Y, Takahashi H, Torigoe T. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Arkader R, Troster EJ, Lopes MR, Júnior RR, Carcillo JA, Leone C, Okay TS. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child. 2006;91:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Gauthier J, Turtle CJ. Insights into cytokine release syndrome and neurotoxicity after CD19-specific CAR-T cell therapy. Curr Res Transl Med. 2018;66:50-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Dinarello CA, Bunn PA Jr. Fever. Semin Oncol. 1997;24:288-298. [PubMed] |
| 7. | Johnson M. Neoplastic fever. Palliat Med. 1996;10:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 2069] [Article Influence: 188.1] [Reference Citation Analysis (0)] |
| 9. | Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, Xie J, Gao Y, Cheng F, Zhou Y, Zhang Z, Hu Y, Zhang X, Gao Q, Zhang Y, Huang B. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 10. | Costantini A, Julie C, Dumenil C, Hélias-Rodzewicz Z, Tisserand J, Dumoulin J, Giraud V, Labrune S, Chinet T, Emile JF, Giroux Leprieur E. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology. 2018;7:e1452581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |