Published online Feb 16, 2022. doi: 10.12998/wjcc.v10.i5.1498
Peer-review started: September 18, 2021
First decision: October 27, 2021
Revised: November 19, 2021
Accepted: January 11, 2022
Article in press: January 11, 2022
Published online: February 16, 2022
Processing time: 145 Days and 23.2 Hours
Almost all elderly patients with peritoneal metastatic gastric cancer (PGC) are unlikely to tolerate cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) and adjuvant chemotherapy. However, determining how to optimize the treatment strategy for such patients has always been a clinical problem. Both HIPEC and palliative adjuvant chemotherapy can benefit patients with PGC. Therefore, optimizing HIPEC and chemotherapy regimens has potential clinical value in reducing side effects, and improving treatment tolerance and clinical effectiveness.
To explore the effect of HIPEC containing elemene, which is an anti-cancer component extracted in traditional Chinese herbal medicine, combined with reduced capecitabine and oxaliplatin (CapeOx) chemotherapy regimens, in elderly patients with PGC.
In the present study, 39 of 52 elderly PGC patients were included and assigned to different HIPEC treatment groups [lobaplatin group (group L) and mixed group (group M)] for analysis. Lobaplatin was used for all three HIPECs in group L. In group M, lobaplatin was used in the middle of the three HIPECs, and elemene was used for the first and third HIPEC. After HIPEC, patients received CapeOx chemotherapy. The incidence of complications (abdominal infection, lung infection, and urinary tract infection), myelosuppression, immune function (CD4/CD8 ratio), average length of hospital stay, and prognosis were compared between these two groups.
There was no significant difference in the incidence of complications between the two groups during hospitalization (P > 0.05). Compared to patients in group M, patients in group L exhibited severe myelosuppression (P = 0.027) and increased length of hospital stay (P = 0.045). However, no overall survival benefit was observed in group M. Furthermore, the immune function of patients in group M was less affected (P < 0.001), when compared to that of patients in group L. The multivariate analysis suggested that the cycles of chemotherapy after perfusion significantly affected the prognosis of patients in both groups.
Compared to the lobaplatin-based HIPEC regimen, the administration of elemene reduced the myelosuppression incidence in elderly PGC patients. The present study sheds light on the implementation of this therapeutic strategy for this set of patients.
Core Tip: Elderly patients with advanced gastric cancer (GC) cannot tolerate high-intensity treatment. In addition, intraperitoneal hyperthermic perfusion chemotherapy (HIPEC) and capecitabine and oxaliplatin (CapeOx) regimens have limited therapeutic effects on advanced GC. On the other hand, the extracted Chinese herbal medicine, elemene, has anti-cancer effects and negligible side effects. In the present study, the use of elemene-containing HIPEC combined with a CapeOx chemotherapy regimen was proven to be more tolerant for elderly patients with peritoneal metastatic gastric cancer, making this a clinical choice for such patients.
- Citation: Chen ZX, Li J, Liu WB, Zhang SR, Sun H. Elemene-containing hyperthermic intraperitoneal chemotherapy combined with chemotherapy for elderly patients with peritoneal metastatic advanced gastric cancer. World J Clin Cases 2022; 10(5): 1498-1507
- URL: https://www.wjgnet.com/2307-8960/full/v10/i5/1498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i5.1498
In 2020, it was reported that there were approximately 1089103 new gastric cancer (GC) cases and 789793 gastric cancer-related deaths worldwide[1]. The lack of typical clinical symptoms at the early stage has made GC one of the malignancies with the highest incidence in the gastroenterological tract in China. Furthermore, a recent study demonstrated that more than half of patients were diagnosed at the advanced stage[2]. In addition, nearly 20%-30% of diagnosed GC patients were found to develop peritoneal metastasis[3]. Despite the advances achieved by treatment approaches, the median survival time still ranges within 3-4 mo[3,4]. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) has been shown to be an effective therapeutic strategy for prolonging survival in a variety of peritoneal malignancies[5-7]. Most recent evidence has shown that CRS-HIPEC can improve the prognosis of GC patients with peritoneal metastasis[8-10]. However, it has been reported that elderly patients have an increased risk for postoperative morbidity[11]. Therefore, this group of patients may not be able to tolerate regular CRS-HIPEC. Thus, developing an effective therapeutic strategy for elderly GC patients with peritoneal metastasis is urgently important.
An increasing body of evidence has revealed that HIPEC combined with systemic chemotherapy using capecitabine and oxaliplatin (CapeOx) can significantly improve the outcome of GC patients with peritoneal metastasis[12,13]. It is noteworthy that CapeOx has been accepted as a regimen with acceptable toxicity for metastatic or advanced GC[14,15]. Furthermore, it has been reported that elderly patients with advanced GC can tolerate and benefit from CapeOx treatment, even at lower doses[16]. However, the use of HIPEC combined with systemic chemotherapy for elderly patients with advanced GC has not been described to date. Furthermore, a recommended treatment paradigm to achieve optimum therapeutic effects and minimum side-effects for patients with advanced GC has not been established.
Accumulating evidence has revealed that β-elemene, an active ingredient in the ginger family of Chinese herbal medicine Wenyujin, has anti-cancer activity. Importantly, it has been shown that β-elemene can increase the susceptibility of multidrug-resistant cancer cells and exhibit synergistic anti-cancer effects[17] with neglectable side effects. Furthermore, it has been reported that elemene can be utilized to treat a variety of cancers, including lung cancer[18], liver cancer[19], brain cancer[20], breast cancer[21], and GC[22,23]. Moreover, elemene has been shown to increase the susceptibility of cancer cells to chemotherapy and radiotherapy[17,24]. In addition, it has fewer side effects, and can inhibit M2 macrophage-mediated immunosuppressive effects[24]. Its extremely high penetrating potential[23] also enables elemene to penetrate the blood-brain barrier and prolong the survival of patients with glioma[25]. The therapeutic efficacy of elemene for malignant pleural and ascites has been well-documented[26-28]. Therefore, it was speculated that the β-elemene-containing HIPEC paradigm might be a promising therapeutic strategy for elderly patients with advanced GC. In line with this, the present study evaluated the efficacy and safety of the elemene-supplemented HIPEC combined with CapeOx regimen in elderly GC patients with peritoneal metastasis.
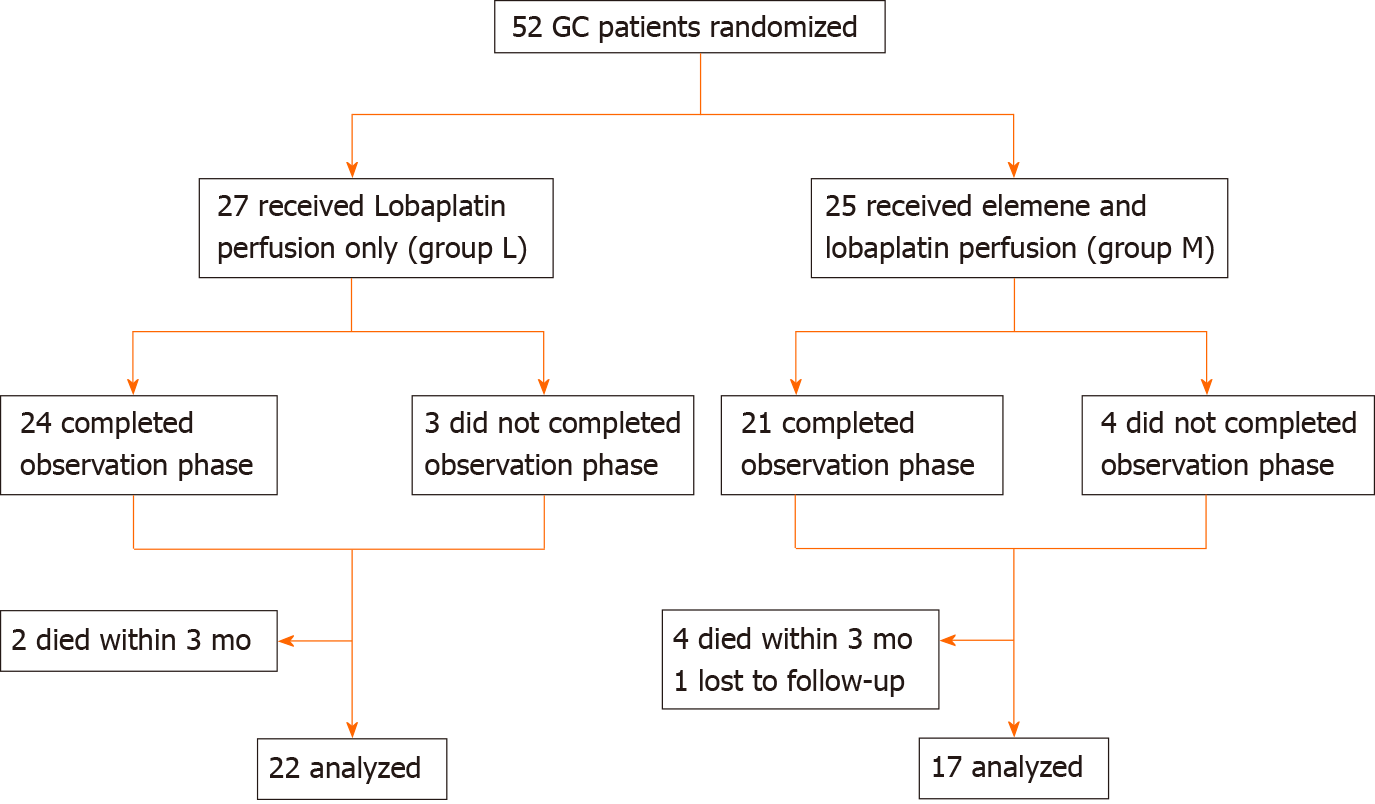
A single-center research was conducted for patients recruited at the Gastrointestinal Cancer Center of Chongqing University Cancer Hospital (CUCH) between July 2016 and April 2020. All patients (n = 52) who participated in the present study provided a signed informed consent. The inclusion criteria were, as follows: (1) Age ≥ 65 years old; (2) Diagnosed with advanced GC and peritoneal metastasis; (3) Eastern Cooperative Oncology group (ECOG) score ≤ 2; (4) No severe organ or metabolic dysfunction; (5) No hypertension; (6) Mentally capable to adapt to intervention; and (7) > 3 mo of expected survival time. Finally, 39 patients who met the inclusion criteria were analyzed for the present study (Figure 1), and none of these cases were HER2-positive.
Groupings: A total of 52 recruited elderly peritoneal metastatic gastric cancer (PGC) patients were randomly assigned to two groups: lobaplatin group (group L), in which lobaplatin was used during HIPEC; mixed group (group M), in which both elemene and lobaplatin were used during HIPEC.
Before HIPEC, patients underwent preventive fasting, gastric intubation, intravenous fluid replacement, nebulization, and sputum suction. Then, patients received laparoscopic puncture and catheterization, and no postoperative ICU admission was needed. Three cycles of HIPEC were performed for each patient. The first cycle was administered upon completion of the catheterization, and while the patient was still under anesthesia. The remaining cycles were performed when the patient was transferred to the ward (within 36, 72, or 120 h after surgery). In general, the HIPEC system (BR-TRG-I, BRM, Guangzhou, China) was perfused with 3000 mL of 0.9% sodium chloride, and pre-heated for 15 minutes until the ambiance temperature reached 43 °C. Then, the chemotherapy agents were delivered into the system in different groups. In particular, for patients in group L, lobaplatin [State Drug Administration (SDA) approval number: H20050308, 50 mg/m2] supplemented in 250 mL of 5% glucose was added into the system for all three HIPEC cycles. For patients in group M, elemene (SDA approval number: H20110114, 0.6 g) supplemented in 250 mL of 5% glucose was administered at the first and third HIPEC cycles. For the second HIPEC cycle, these patients were administered with the same HIPEC chemotherapy reagent as that administered for patients in group L. The perfusion machine was operated according to manufacturer’s instructions. The perfusion time ranged within 30-90 min, based on the tolerance of each recipient (patients who failed to achieve a perfusion speed higher than 350 mL/min were excluded from the present study). Morphine and tramadol were used to relieve the pain before and after the perfusion. Oxygen was given to patients during the perfusion. In group M, 1.5 g of lidocaine was administered into the HIPEC system at five minutes before the administration of elemene to reduce peritoneal irritation. After the HIPEC treatment, case-specific supportive care was provided based on the complications (analgesia, acid suppression, antiemetic, maintenance of electrolyte balance, albumin infusion, and blood transfusion; subcutaneous injection of granulocyte stimulating factor, thrombopoietin [300 µg, once], and/or interleukin-11 (IL-11; 3 mg, per day, 5-7 d, according to the manual; Qilu Pharmaceutical Co., Ltd.); partial parenteral nutrition support therapy). Within two weeks after discharge, these patients received CapeOx chemotherapy (60% or 80%, or the standard CapeOx dose). The dose was gradually reduced to 60% from 80% for some patients. Each cycle was repeated every 21 d.
The vital signs of each patient were collected during hospitalization, and these patients were followed up monthly. The average follow-up time for the entire cohort was 9.04 mo (range: 3.0-17.9 mo), and the follow-up of all patients recruited for the present study was concluded in April 2020. After perfusion, the abdominal drainage was sent to the laboratory for bacterial test. In addition, during the CapeOx chemotherapy, the results for the routine blood test, liver and kidney function evaluation, immune function analysis (CD4/CD8 ratio), and serum markers for gastric cancer (CEA, CA-125 and CA-199; any serum marker level that increases to two or becomes more than two times of the normal threshold was defined as high; otherwise, the level was defined as normal) were recorded for each patient.
The data were analyzed using the SPSS software package (version 22.0). The measurement data were presented as mean ± SD, categorical variables were analyzed by Chi-square test or Fisher’s exact test, and ordinal data were analyzed by nonparametric Mann-Whitney U-test. Furthermore, the survival differences were analyzed using the Kaplan-Meier survival curve and Log-rank test. In addition, univariate analysis and Cox multivariate risk ratio regression analysis were performed to identify the potential factors related to the prognosis. A P value of < 0.05 in the two-sided test was considered statistically significant.
A total of 39 GC patients were analyzed (Figure 1). The clinical characteristics of these patients are summarized in Table 1. There were 22 GC patients in group L (n = 22, 69.9 ± 3.6 years old), and 17 GC patients in group M (n = 17, 68.6 ± 3.0 years old). There were no significant differences between the two groups, in terms of gender, ECOG score, age, tumor site composition ratio, tumor size, and occurrence of complications during the first hospitalization (except for myelosuppression). However, patients in group L developed severe myelosuppression during the first hospitalization, when compared to patients in group M (P = 0.027). In addition, the average length of hospital stay was 18.7 d for patients in group L. This was a slightly longer than that for patients in group M, which was merely 16.1 d (P = 0.045).
| Clinical parameters | Group L | Group M | P value |
| Gender | 0.531 | ||
| Male | 14 | 9 | |
| Female | 8 | 8 | |
| Age group (yr) | 0.748 | ||
| 65-69 | 11 | 10 | |
| 70-75 | 11 | 7 | |
| ECOG score | 0.561 | ||
| 0 | 14 | 8 | |
| 1 | 5 | 5 | |
| 2 | 3 | 4 | |
| Tumor location | 0.667 | ||
| Proximal | 2 | 3 | |
| Middle | 4 | 2 | |
| Distal | 16 | 12 | |
| Tumor stage | 0.192 | ||
| cT3 | 12 | 5 | |
| cT4a | 9 | 9 | |
| cT4b | 1 | 3 | |
| PCI scores | 0.508 | ||
| < 20 | 15 | 9 | |
| ≥ 20 | 7 | 8 | |
| Abdominal infection (cases) | 2 | 0 | 0.495 |
| pulmonary infection (cases) | 1 | 1 | 1.000 |
| Urinary tract infection (cases) | 0 | 1 | 0.436 |
| Myelosuppression system (grade 3/4, cases) | 6 | 0 | 0.027 |
| Blood transfusion therapy (cases) | 4 | 0 | 0.118 |
| Average length of stay (d) | 18.7 | 16.1 | 0.045 |
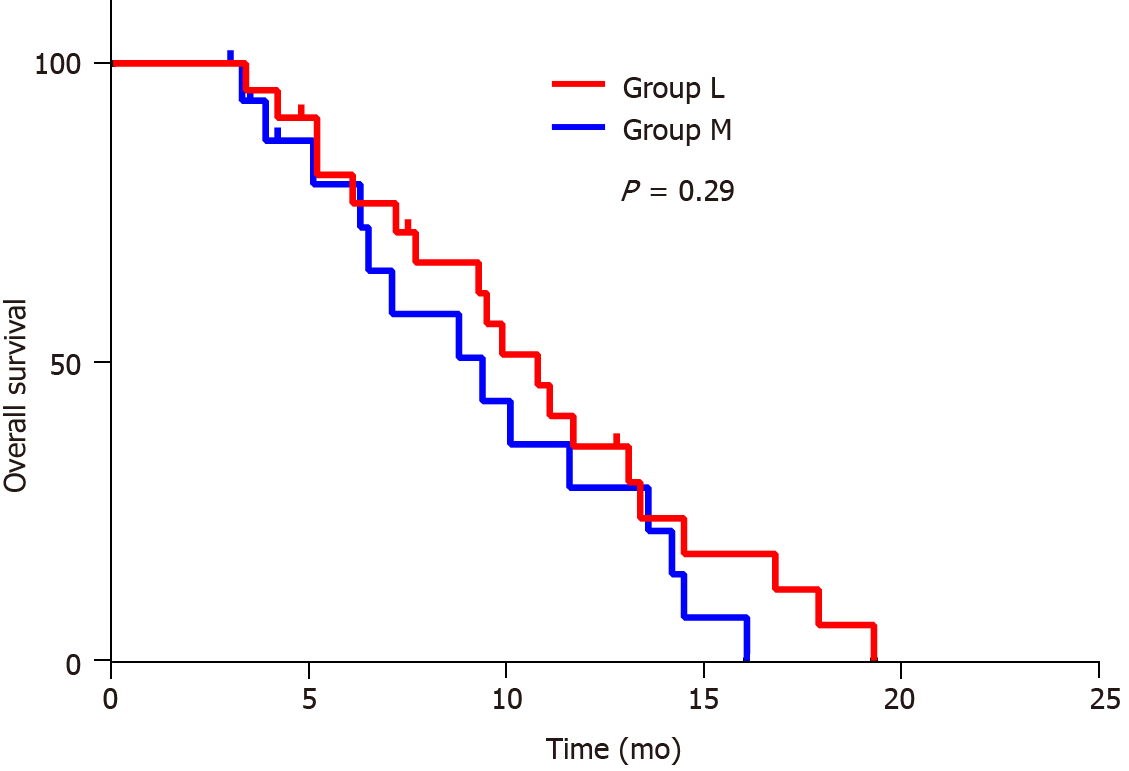
The average follow-up duration for all patients was 9.04 ± 4.30 mo (3.00-17.90 mo). The follow-up duration was 9.60 ± 4.20 mo (3.40-17.90 mo) for patients in group L, and 8.30 ± 4.40 mo (3.00-16.10 mo) for patients in group M. There was no significant difference in follow-up duration between these two groups (9.60 ± 4.20 mo (3.40-17.90 mo) vs 8.30 ± 4.40 mo (3.00-16.10 mo), P = 0.292). Three patients were lost to follow-up during the follow-up period, and 33 patients became deceased, while three patients survived at the end of the follow-up period. There were no significant differences in survival performance, in terms of HIPEC regimen (P = 0.29, Figure 1). Furthermore, both groups' median overall survival (OS) was 9.90 mo (95%CI: 5.30-13.50). The median survival was 10.80 mo (95%CI: 8.50-13.10) and 9.40 mo (95%CI: 5.30-13.50) for patients in groups L and M, respectively.
In order to identify the potential prognostic factors, independent variables, including gender, age, treatment regimen, serum tumor marker levels, immune function (CD4/CD8 ratio), myelosuppression status, cycles of chemotherapy (CapeOx chemotherapy) after HIPEC, peritoneal cancer index (PCI), and observation/presence of malignant ascites during catheterization, were analyzed using univariate analysis, followed by multivariate analysis. The results revealed that high-level serum tumor markers, low CD4/CD8 ratio (< 1), fewer cycles of chemotherapy (reduced CapeOx regimen) after HIPEC (< 6 times), high PCI (not < 20), and the appearance of ascites during laparoscopic catheter placement indicates poor prognosis (Table 2).
| Clinical parameters | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male vs female) | 0.858 (0.424-1.734) | 0.670 | ||
| Age (< 70 vs ≥ 70) | 1.139 (0.564-2.301) | 0.717 | ||
| Group (L vs M) | 1.467 (0.714-3.016) | 0.297 | ||
| T stage (T3 vsT4 or above) | 2.403 (1.118-5.163) | 0.025 | 1.457 (0.554-3.832) | 0.445 |
| Tumor marker (normal vs high) | 1.009 (0.473-2.153) | 0.981 | ||
| CD4/CD8 (< 1 vs ≥ 1) | 2.505 (1.147-5.467) | 0.021 | 2.051 (0.8534.933) | 0.109 |
| Myelosuppression (1/2 vs 3/4) | 3.570 (1.291-9.874) | 0.014 | 3.220 (0.985-10.530) | 0.053 |
| Times of chemotherapy after HIPEC (< 6 vs ≥ 6) | 0.143 (0.060-0.343) | 0.000 | 0.210 (0.070-0.634) | 0.006 |
| PCI (< 20 vs ≥ 20) | 2.444 (1.105-5.404) | 0.027 | 1.247 (0.417-3.725) | 0.693 |
| Ascites (exist1 vs none) | 4.106 (1.746-9.658) | 0.001 | 3.084 (0.948-10.027) | 0.061 |
Compared to the OS of simple systemic palliative chemotherapy, the present study revealed that the elemene-containing HIPEC combined with CapeOx regimen can extend the OS (the median OS was approximately 9.40 mo) of elderly PGC patients, which is consistent with the report of Blum Murphy et al[29] (the OS was approximately 12 mo). It is noteworthy that clinical studies have revealed that multiple chemotherapy regimens, including fluorouracil plus leucovorin, oxaliplatin (FLOT4), and docetaxel and docetaxel, cisplatin, and 5-fluorouracil (DCF), are poorly tolerated by elderly patients[30,31]. The present study provides the possibility that the tolerance of these regimens by elderly PGC patients could be potentially elevated. However, further investigations are needed to determine whether implementing elemene to lobaplatin-based HIPEC can improve the chemotherapy efficacy of FLOT4 or DCF to be as potent as that of CapeOx. The present finding revealed that the implementation of the lobaplatin-based HIPEC combined with CapeOx regimen resulted in a high occurrence of myelosuppression in elderly GC patients (27.3%). Although the addition of elemene in the lobaplatin-based HIPEC combined with CapeOx regimen did not improve the OS of these patients, when compared to that in group L (Figure 2), this significantly reduced the incidence of myelosuppression in the recipients (P = 0.027). It is noteworthy that elderly patients with advanced cancer, such as GC, often undergo myelosuppression after receiving high-intensity chemotherapies, including lobaplatin-based regimens[32]. Notably, such complications would lead to treatment delay, and some can be life-threatening[33]. Although the OS of patients was not directly influenced, this complication increased the cost of treatment and length of hospital stay of these patients. In addition, the subgroup analysis revealed that the cycles of CapeOx treatment after HIPEC, PCI, and the CD4/CD8 ratio are prognostic factors in elderly patients. The finding that a low CD4/CD8 ratio indicates poor prognosis is in line with the findings reported by previous studies, suggesting that GC patients with impaired immune function have a poor prognosis[34,35].
The present study has the following limitations. First, the sample size was small, and a control group that only used elemene HIPEC was not added. Hence, a larger cohort is needed in future studies. Second, it has been reported that reduced CapeOx doses can affect the clinical outcomes of elderly patients with advanced GC[16]. In the present study, the reduced dose of CapeOx was utilized during the treatment for part of the patients, and this may have led to biased results. Third, further exploration is needed to determine whether patients can benefit from the intravenous administration of elemene combined with CapeOx after HIPEC. Lastly, a control group for systemic chemotherapy should also be considered. Finally, it has been reported that NCT03333967[36], TAGS[37] and EPOC1201[38] can improve the prognosis of patients with advanced GC. Therefore, in future studies, it should be determined whether elemene-containing HIPEC can improve the therapeutic effects of these drugs.
The elemene-containing HIPEC combined with CapeOX regimen has fewer side effects, and is safe for elderly PGC patients. However, further exploration is needed to determine how to further optimize the dose and combine this with other chemotherapy regimens.
Elderly patients with peritoneal metastatic gastric cancer (PGC) have poor tolerance to intensive treatment, such as cytoreductive surgery plus hyperthermic perfusion chemotherapy (HIPEC). To date, no guidelines or consensus has standardized the HIPEC composition. However, elemene, a Chinese herbal extract with anti-cancer activity and low toxicity, turns out to be a promising ingredient for HIPEC.
The study aims to determine whether implementing elemene in lobaplatin-based HIPEC benefits elderly PGC patients during chemotherapy.
The study aims to explore the clinical effectiveness and potential side effects of elemene-containing lobaplatin-based HIPEC in elderly PGC patients.
The included patients were assigned into two groups: patients who received elemene-containing lobaplatin-based HIPEC plus oxaliplatin and capecitabine (CapeOx) treatment (group M) and patients who received elemene-free lobaplatin-based HIPEC plus CapeOx treatment (group L). The incidence of complications such as myelosuppression, immune function (CD4/CD8 ratio), average length of hospital stay, and prognosis were compared between these two groups.
There was no significant difference in the incidence of complications and overall survival between the two groups during hospitalization. In addition, supplementing elemene in HIPEC lessened the myelosuppression (P = 0.027) and shortened the length of hospital stay (P = 0.045) of elderly PGC patients.
The administration of elemene led to the amelioration of myelosuppression in elderly PGC patients.
The present study sheds light on the implemention of an elemene-containing HIPEC therapeutic strategy for elderly patients with PGC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawabata H, Mohamed SY S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Nguyen HV, Nguyen HV, Nguyen LT, Pham NQ, Nguyen HX, Nguyen VT. A Case of Advanced Gastric Cancer with Folfiri as a Preoperative Chemotherapy. Case Rep Oncol Med. 2019;2019:1352173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, Lemmens VE, de Hingh IH. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Sarela AI, Miner TJ, Karpeh MS, Coit DG, Jaques DP, Brennan MF. Clinical outcomes with laparoscopic stage M1, unresected gastric adenocarcinoma. Ann Surg. 2006;243:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Gilly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol. 2009;27:6237-6242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 488] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 780] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 7. | Goéré D, Malka D, Tzanis D, Gava V, Boige V, Eveno C, Maggiori L, Dumont F, Ducreux M, Elias D. Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg. 2013;257:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 8. | Bonnot PE, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O; FREGAT and BIG-RENAPE Networks. Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J Clin Oncol. 2019;37:2028-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Ellison LM, Man Y, Stojadinovic A, Xin H, Avital I. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis. Chin J Cancer Res. 2017;29:86-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Chia CS, Seshadri RA, Kepenekian V, Vaudoyer D, Passot G, Glehen O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: a systematic review. Pleura Peritoneum. 2016;1:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Votanopoulos KI, Shen P, Stewart JH, Levine EA; Wake Forest HIPEC Group. Outcomes of Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Patients Older than 70 Years; Survival Benefit at Considerable Morbidity and Mortality: A Reply. Ann Surg Oncol. 2017;24:602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Yu P, Ye Z, Dai G, Zhang Y, Huang L, Du Y, Cheng X. Neoadjuvant systemic and hyperthermic intraperitoneal chemotherapy combined with cytoreductive surgery for gastric cancer patients with limited peritoneal metastasis: a prospective cohort study. BMC Cancer. 2020;20:1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Bittoni A, Faloppi L, Giampieri R, Cascinu S. Selecting the best treatment for an individual patient. Recent Results Cancer Res. 2012;196:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Xie BW, Zang L, Ma JJ, Sun J, Yang X, Wang ML, Lu AG, Hu WG, Zheng MH. [Safety and effectiveness of oxaliplatin combined with capecitabine or oxaliplatin combined with S-1 neoadjuvant chemotherapy in the treatment of advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Satake H, Yasui H, Kotake T, Okita Y, Hatachi Y, Kotaka M, Kato T, Tsuji A. First-line chemotherapy with capecitabine/oxaliplatin for advanced gastric cancer: A phase I study. Mol Clin Oncol. 2017;7:347-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Hwang IG, Ji JH, Kang JH, Lee HR, Lee HY, Chi KC, Park SW, Lee SJ, Kim ST, Lee J, Park SH, Park JO, Park YS, Lim HY, Kang WK. A multi-center, open-label, randomized phase III trial of first-line chemotherapy with capecitabine monotherapy vs capecitabine plus oxaliplatin in elderly patients with advanced gastric cancer. J Geriatr Oncol. 2017;8:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Jiang Z, Jacob JA, Loganathachetti DS, Nainangu P, Chen B. β-Elemene: Mechanistic Studies on Cancer Cell Interaction and Its Chemosensitization Effect. Front Pharmacol. 2017;8:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Zhou K, Wang L, Cheng R, Liu X, Mao S, Yan Y. Elemene Increases Autophagic Apoptosis and Drug Sensitivity in Human Cisplatin (DDP)-Resistant Lung Cancer Cell Line SPC-A-1/DDP By Inducing Beclin-1 Expression. Oncol Res. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Mao Y, Zhang J, Hou L, Cui X. The effect of beta-elemene on alpha-tubulin polymerization in human hepatoma HepG2 cells. Chin J Cancer Res. 2013;25:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 20. | Zhu T, Li X, Luo L, Wang X, Li Z, Xie P, Gao X, Song Z, Su J, Liang G. Reversion of malignant phenotypes of human glioblastoma cells by β-elemene through β-catenin-mediated regulation of stemness-, differentiation- and epithelial-to-mesenchymal transition-related molecules. J Transl Med. 2015;13:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Zhang J, Zhang Hd, Chen L, Sun DW, Mao Cf, Chen W, Wu JZ, Zhong SL, Zhao JH, Tang JH. β-elemene reverses chemoresistance of breast cancer via regulating MDR-related microRNA expression. Cell Physiol Biochem. 2014;34:2027-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Zhai B, Zeng Y, Zeng Z, Zhang N, Li C, You Y, Wang S, Chen X, Sui X, Xie T. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. Int J Nanomedicine. 2018;13:6279-6296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Mu XD, Li EZ, Luo Y, Song N, Qu XJ, Hu XJ, Liu YP. The role of E3 ubiquitin ligase Cbl proteins in β-elemene reversing multi-drug resistance of human gastric adenocarcinoma cells. Int J Mol Sci. 2013;14:10075-10089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Yu X, Xu M, Li N, Li Z, Li H, Shao S, Zou K, Zou L. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem Biophys Res Commun. 2017;490:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Ma C, Zhou W, Yan Z, Qu M, Bu X. β-Elemene treatment of glioblastoma: a single-center retrospective study. Onco Targets Ther. 2016;9:7521-7526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Wang QT, Zhang ZL, Xiong H, Zhou DS, Li J, Liang J, Wang YF. Evaluation of the efficacy and safety of elemene in treating malignant pleural effusion caused by tumors: A PRISMA guided meta-analysis. Medicine (Baltimore). 2018;97:e12542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Wang X, Wang H, Li L. A meta-analysis of elemene vs DDP intrapleural injection in the treatment of malignant pleural effusion caused by lung cancer. J Cancer Res Ther. 2016;12:C244-C247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Jiang ZY, Qin SK, Yin XJ, Chen YL, Zhu L. Synergistic effects of Endostar combined with β-elemene on malignant ascites in a mouse model. Exp Ther Med. 2012;4:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Blum Murphy M, Ikoma N, Wang X, Estrella J, Roy-Chowdhuri S, Das P, Minsky BD, Song S, Mansfield P, Ajani J, Badgwell B. Phase I Trial of Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) with Cisplatin, Mitomycin, and Paclitaxel in Patients with Gastric Adenocarcinoma and Associated Carcinomatosis or Positive Cytology. Ann Surg Oncol. 2020;27:2806-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel vs fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1643] [Article Influence: 273.8] [Reference Citation Analysis (0)] |
| 31. | Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 421] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 32. | Wang Z, Xu L, Wang H, Li Z, Lu L, Li X, Zhang Q. Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment. Saudi J Biol Sci. 2018;25:909-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Pan L, Zhang T, Cao H, Sun H, Liu G. Ginsenoside Rg3 for Chemotherapy-Induced Myelosuppression: A Meta-Analysis and Systematic Review. Front Pharmacol. 2020;11:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J, Zhang TT, Wang XA, Zhang FM, Ge HL, Shen LS, Xu D. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Li F, Sun Y, Huang J, Xu W, Liu J, Yuan Z. CD4/CD8 + T cells, DC subsets, Foxp3, and IDO expression are predictive indictors of gastric cancer prognosis. Cancer Med. 2019;8:7330-7344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 36. | Du Y, Cao Q, Jiang C, Liang H, Ning Z, Ji C, Wang J, Zhou C, Jiang Z, Yu C, Li L, Zhao Y, Xu Y, Xu T, Hu W, Wang D, Cheng H, Wang G, Zhou J, Wang S, Zhang Y, Hu Z, Li X, Lu D, Zhang J, Xie H, Sun G. Effectiveness and safety of low-dose apatinib in advanced gastric cancer: A real-world study. Cancer Med. 2020;9:5008-5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Shitara K, Doi T, Dvorkin M, Mansoor W, Arkenau HT, Prokharau A, Alsina M, Ghidini M, Faustino C, Gorbunova V, Zhavrid E, Nishikawa K, Hosokawa A, Yalçın Ş, Fujitani K, Beretta GD, Cutsem EV, Winkler RE, Makris L, Ilson DH, Tabernero J. Trifluridine/tipiracil vs placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 38. | Bando H, Doi T, Muro K, Yasui H, Nishina T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, Sato A, Ohtsu A. A multicenter phase II study of TAS-102 monotherapy in patients with pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer. 2016;62:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |