Published online Feb 6, 2022. doi: 10.12998/wjcc.v10.i4.1366
Peer-review started: August 15, 2021
First decision: November 11, 2021
Revised: November 23, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: February 6, 2022
Processing time: 162 Days and 6.5 Hours
Biliary adenofibroma (BF) is a rare benign epithelial tumor with the possibility of malignant transformation. Its main pathological feature is a well-defined cystic or honeycomb mass. BF has no specific clinical manifestations or laboratory and imaging findings; thus, it is easily misdiagnosed before surgery. This report describes a case in which biliary cystadenoma was misdiagnosed preoperatively and BF was diagnosed postoperatively. The imaging features, particularly the magnetic resonance imaging (MRI) features, were analyzed and summarized.
A 68-year-old Chinese man was admitted to our hospital with a 2-mo history of abdominal discomfort. Following admission to our hospital, laboratory examinations showed normal tumor marker concentrations and liver function. Hepatocellular carcinoma was considered after contrast-enhanced ultrasound exami
Our objective is to highlight the imaging diagnostic value of BF, especially on an MRI enhanced scan with gadolinium ethoxybenzyl diethylenetriamine pentaa
Core Tip: The imaging presentation of biliary adenofibroma is complex and diverse, and the clinical history and laboratory examination were not specific. In this case, magnetic resonance imaging characteristics of biliary adenofibroma, especially enhanced scan with Gd-EOB-DTPA and an intravoxel incoherent motion diffusion-weighted imaging sequence were valuable.
- Citation: Li SP, Wang P, Deng KX. Imaging presentation of biliary adenofibroma: A case report. World J Clin Cases 2022; 10(4): 1366-1372
- URL: https://www.wjgnet.com/2307-8960/full/v10/i4/1366.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i4.1366
In 1993, Tsui et al[1] were the first to describe biliary adenofibroma (BF), which is a benign, complex, tubulocystic liver tumor with a bland spindle-cell stromal com
A 68-year-old man reported abdominal discomfort without obvious inducement for 2 mo.
The patient had no other symptoms.
His past medical history indicated hypertension and mild cerebral infarction for more than ten years. After regular drug treatment, these conditions were well controlled.
He had no personal or family history of other diseases.
On physical examination, there was no tenderness or rebound pain in the abdomen, and percussion pain in the liver area was negative. His blood pressure was 121/75 mmHg, pulse rate was 67 bpm and body temperature was 36.4 ℃.
No abnormal laboratory examinations were observed, including liver function, tumor markers, infection markers, coagulation tests and complete blood count.
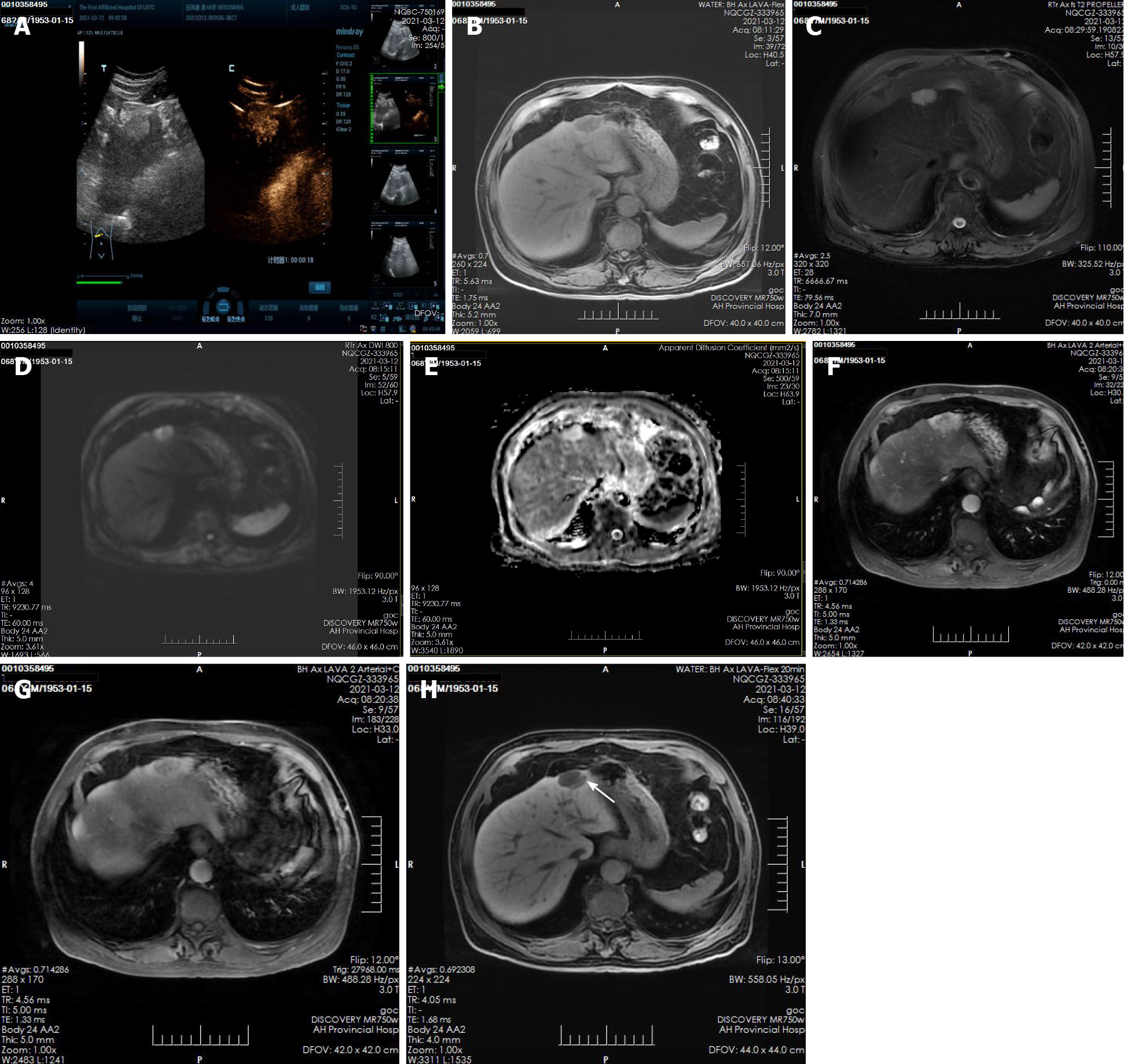
A plain CT scan at a local hospital showed a space occupying lesion in the left lobe of the liver and the patient was admitted to our hospital for further treatment. Ultrasonography showed hyperechoic nodules in the left lobe of the liver. Following injection of contrast agent, early and obvious enhancement was observed (Figure 1A). Plain MRI showed hypointensity on T1-weighted imaging (T1WI) and hyperintensity on T2-weighted imaging (T2WI). In this case, iterative decomposition of water and fat with echo asymmetry and least square estimation-iron quantification (IDEAL-IQ) and intravoxel incoherent motion diffusion-weighted imaging (IVIM-DWI) sequences were performed. The water phase of the IDEAL-IQ sequence showed that the signal of the lesion was higher than that of the liver. DWI showed moderate hyperintensity and isointensity according to the apparent diffusion coefficient (ADC). The ADC and pure diffusion coefficient (D) values were 2.78 and 2.12 × 10-3 mm2/s, respectively, which indicated that there was no obvious limitation in the diffusion of the lesion. There was no significant change in the signal of the lesion in the in-phase and out-phase sequence. The early and late arterial phases showed obvious enhancement of solid components and separation of lesions. The degree of lesion enhancement decreased in the portal phase and delayed scan. In the hepatobiliary phase, enhancement of the bile duct structure was found in the lesion (Figure 1B-H).
Pathology confirmed BF (a rare biliary epithelial tumor) in this patient (Figure 2). Microscopy showed irregular hyperplasia of the bile duct with varying amounts of intervening fibrous stroma and inflammatory cell infiltration. Some bile ducts were dilated and the wall thickened, cholestasis was seen in the bile duct. The cells showed no atypia, and some of them showed apocrine secretion.
The lesion was removed by laparoscopic surgery under general anesthesia.
The patient had no signs of recurrence at the 1-mo postoperative re-examination (Figure 3).
Currently, although BFs are classified as benign bile duct tumors and precursors by the 2019 World Health Organization tumor classification system[5], some cases of BF are characterized by malignant transformation, invasion, and even distant metastasis (Tsui et al[1] first proposed in 1993, postoperative pathology showed malignant transformation in 3 cases[6-8], 3 cases of biliary adenofibroma with invasive carcinoma[9-11], 3 cases were complicated with liver tissue invasion[12-14], 2 cases were complicated with lymph node metastasis[15], local recurrence occurred in 2 cases[16]). The clinical manifestations and imaging characteristics of BF are nonspecific. A definitive diagnosis is difficult to achieve by preoperative imaging. The diagnosis mainly depends on postoperative pathological examination. Pathologically, the lesions are gray or dark red irregular masses that can show cystic, honeycomb, or solid changes and have a relatively clear boundary and no capsule.
Including the present case, 25 cases of BF have been reported to date (Tsui et al[1] in 1993, Parada et al[17] in 1997, Haberal et al[12] in 2001, Akin et al[18] and Garduño-López et al[19] in 2002, Gurrera et al[20] in 2010, Kai et al[6] and Nguyen et al[7] in 2012, Jacobs et al[13] in 2015, Godambe et al[9]; Thai et al[10] and Thompson et al[15] in 2016, Kaminsky et al[8] and Arnason et al[16] in 2017, Esteban et al[11] in 2018, Sturm et al[14] in 2019), and no differences in sex or age at onset are evident. The mean age at onset is 57.2 ± 17.79 years. Of the 25 cases reported 12 were male and 13 were female. The mean diameter of the lesions was 8.22 ± 4.65 cm. Eight patients presented due to upper abdominal discomfort and pain, one patient had jaundice due to an intrahepatic bile duct lesion, and the remaining 16 patients had no reported abdominal pain and were diagnosed by related examinations. The results of laboratory tests were generally nonspecific. One patient had an elevated carbohydrate antigen 19-9 (CA19-9) concentration[19], and all of the remaining patients had normal concentrations of tumor markers including alpha fetoprotein, carcinoembryonic antigen, CA19-9, and CA125. Liver function indices were within normal limits. One patient had a history of hepatitis B[21], but the hepatitis status was not mentioned in the remaining patients. The location of the lesion was the left lobe in 11 patients and the right lobe in 13 patients (the specific location of the liver lesion was not mentioned in the remaining patient). One patient had three lesions[16], and the remaining patients had single lesions. We carefully reviewed the previous literature (including the imaging findings and intraoperative descriptions of the lesion locations) and found that the most common location of BF was under the liver capsule, as was true in the present case. Thus, the lesion location has a certain particularity.
On plain CT and MRI scans, BF is mainly a cystic lesion containing septal and varied solid components. Only three lesions were described as solid tumors. In the present case, the lesion was small and mainly composed of solid components, and ultrasound showed that it was slightly hyperechoic. Plain MRI showed hypointensity on T1WI and hyperintensity on T2WI. In this case, IDEAL-IQ and IVIM-DWI sequences were performed. The water phase of the IDEAL-IQ sequence showed that the signal of the lesion was higher than that of the liver. DWI showed moderate hyperintensity and isointensity according to the ADC. The ADC and D values were 2.78 and 2.12 × 10-3 mm2/s, respectively, which indicated that it was more likely a benign lesion (there was no obvious limitation in the diffusion of the lesion). There was no significant change in the signal of the lesion in the in-phase and out-phase sequence. In previous cases, the bile duct epithelial components of the lesions had no secretory function, but some lesions showed a tendency to enlarge and develop cystic components during the follow-up period[21]. The diameter of the solid lesions in three cases[8,10,20] and the solid lesions in the present case were smaller (4.08 ± 1.16 cm) than the mean diameter of the cystic and solid lesions (7.00 ± 3.03 cm). Therefore, whether the bile duct epithelial components in BF have a secretory function requires further study. The intrahepatic bile duct was dilated in only one case (the lesion was located within the bile duct). Enhanced CT and MRI scans showed enhancement of the solid components and septa of the lesions; one report described a wash-in and wash-out enhancement pattern in the literature[8]. Gd-EOB-DTPA (Primovist, Bayer Schering, Pharma AG, Berlin), a hepatocyte-specific contrast agent, was used in this case. The early and late arterial phases showed obvious enhancement of solid components and separation of lesions. Areas of abnormal perfusion could be seen around the lesion. The degree of lesion enhancement decreased in the portal phase and delayed scan, and the areas of abnormal perfusion disappeared. A similar enhan
Pathological examination revealed that the fibrous tissue matrix of the tumor showed the histological pattern of a partially cystic bile duct. The bile pigment component in the tumor duct indicated direct continuity between the lesion and the biliary system, although postoperative pathology showed that the lesion was not clearly connected with the bile duct[6,21]. Primovist is a hepatobiliary-specific contrast agent that can show the biliary system in the hepatobiliary phase. In this case, enhancement of the bile duct structure was found in the lesion in the hepatobiliary phase, suggesting that the lesion was closely related to the intrahepatic bile duct system; postoperative pathology also showed a bile duct structure and intrabiliary cholestasis. In a previous report, MRI failed to show a relationship between biliary duct adenofibroma and the intrahepatic bile duct. The author speculated that because most of the lesions were located under the liver capsule and the terminal bile duct was slender, it was difficult to show the relationship between the lesion and the bile duct by conventional MRI scanning sequences. Primovist-enhanced MRI, especially hepatobiliary phase images, was a good supplement.
BF is a rare biliary tumor that exhibits malignant transformation and usually occurs under the liver capsule. The lesions are mostly cystic and solid. On enhanced scans, the solid part can show a wash-in and wash-out enhancement pattern, and enha
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pop TL, Wang J S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Tsui WM, Loo KT, Chow LT, Tse CC. Biliary adenofibroma. A heretofore unrecognized benign biliary tumor of the liver. Am J Surg Pathol. 1993;17:186-192. [PubMed] |
| 2. | Lewin M, Mourra N, Honigman I, Fléjou JF, Parc R, Arrivé L, Tubiana JM. Assessment of MRI and MRCP in diagnosis of biliary cystadenoma and cystadenocarcinoma. Eur Radiol. 2006;16:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, Sasaki M. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? Int J Hepatol. 2014;2014:805973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Zucchetti BM, Shimada A, Siqueira LT. Peliosis Hepatis Simulates Liver Metastases. J Glob Oncol. 2018;4:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 6. | Kai K, Yakabe T, Kohya N, Miyoshi A, Iwane S, Mizuta T, Miyazaki K, Tokunaga O. A case of unclassified multicystic biliary tumor with biliary adenofibroma features. Pathol Int. 2012;62:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Nguyen NT, Harring TR, Holley L, Goss JA, O'Mahony CA. Biliary adenofibroma with carcinoma in situ: a rare case report. Case Reports Hepatol. 2012;2012:793963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Kaminsky P, Preiss J, Sasatomi E, Gerber DA. Biliary adenofibroma: A rare hepatic lesion with malignant features. Hepatology. 2017;65:380-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Godambe A, Brunt EM, Fulling KH, Reza Kermanshahi T. Biliary Adenofibroma with Invasive Carcinoma: Case Report and Review of the Literature. Case Rep Pathol. 2016;2016:8068513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Thai E, Dalla Valle R, Evaristi F, Silini EM. A case of biliary adenofibroma with malignant transformation. Pathol Res Pract. 2016;212:468-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Esteban M, Amin J, Hertl M, Jakate S, Singh A. Double Trouble: A Rare Case of Concurrent Biliary Adenofibroma and Hepatobiliary Mucinous Cystic Neoplasm. ACG Case Rep J. 2018;5:e72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Haberal AN, Bilezikci IB, Demirhan B, Karakayali H, Haberal M. Malignant transformation of biliary adenofibroma: a case report. Turk J Gastroenterol. 2001;12:149-153. |
| 13. | Jacobs MA, Lanciault C, Weinstein S. Incidental biliary adenofibroma with dysplastic features. BJR Case Rep. 2015;1:20150100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sturm AK, Welsch T, Meissner C, Aust DE, Baretton G. A case of biliary adenofibroma of the liver with malignant transformation: a morphomolecular case report and review of the literature. Surg Case Rep. 2019;5:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Thompson SM, Zendejas-Mummert B, Hartgers ML, Venkatesh SK, Smyrk TC, Mahipal A, Smoot RL. Malignant transformation of biliary adenofibroma: a rare biliary cystic tumor. J Gastrointest Oncol. 2016;7:E107-E112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Arnason T, Borger DR, Corless C, Hagen C, Iafrate AJ, Makhlouf H, Misdraji J, Sapp H, Tsui WM, Wanless IR, Zuluaga Toro T, Lauwers GY. Biliary Adenofibroma of Liver: Morphology, Tumor Genetics, and Outcomes in 6 Cases. Am J Surg Pathol. 2017;41:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Parada LA, Bardi G, Hallén M, Hägerstrand I, Tranberg KG, Mitelman F, Johansson B. Monosomy 22 in a case of biliary adenofibroma. Cancer Genet Cytogenet. 1997;93:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Akin O, Coskun M. Biliary adenofibroma with malignant transformation and pulmonary metastases: CT findings. AJR Am J Roentgenol. 2002;179:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Garduño-López AL, Mondragón-Sánchez R, Bernal-Maldonado R, Hinojosa-Becerril CA, Meneses-García A. A case of biliary adenofibroma of the liver causing elevated serum CA 19-9 Levels. Rev Oncol. 2002;4:271-273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Gurrera A, Alaggio R, Leone G, Aprile G, Magro G. Biliary adenofibroma of the liver: report of a case and review of the literature. Patholog Res Int. 2010;2010:504584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Lee S, Kim KW, Jeong WK, Yu E, Jang KT. Magnetic Resonance Imaging Findings of Biliary Adenofibroma. Korean J Gastroenterol. 2019;74:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |