Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13313
Peer-review started: October 7, 2022
First decision: November 11, 2022
Revised: November 13, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: December 26, 2022
Processing time: 80 Days and 4.6 Hours
Thoracic para-aortic lymph node (TPLN) recurrence in esophageal squamous cell carcinoma (ESCC) is rare and its impact on survival is unknown. We studied survival in patients with ESCC who developed TPLN recurrence.
To study the survival in patients with ESCC who developed TPLNs recurrence.
Data were collected retrospectively for 219 patients who had undergone curative surgery for ESCC during January 2012 to November 2017 and who developed recurrences (36.29% of 604 patients who had undergone curative surgeries for ESCC). The patients were classified into positive (+) and negative (-) TPLN metastasis subgroups. We also investigated TPLN recurrence in 223 patients with ESCC following definitive chemoradiotherapy during 2012-2013. Following propensity score matching (PSM) and survival estimation, factors predictive of overall survival (OS) were explored using a Cox proportional hazards model.
Among the patients with confirmed recurrence, 18 were TPLN (+) and 13 developed synchronous distant metastases. Before PSM, TPLN (+) was associated with worse recurrence-free (P = 0.00049) and OS [vs TPLN (-); P = 0.0027], whereas only the intergroup difference in recurrence-free survival remained significant after PSM (P = 0.013). The Cox analysis yielded similar results. Among the patients who had received definitive chemoradiotherapy, 3 (1.35%) had preoperative TPLN enlargement and none had developed recurrences.
TPLN metastasis is rare but may be associated with poor survival.
Core Tip: Lymph node recurrence is common in resected esophageal squamous cell carcinoma (ESCC). However, thoracic para-aortic lymph nodes (TPLNs) recurrence in ESCC is rare and its impact on survival is unknown. Our study identified the incidence of TPLNs recurrence in ESCC after curative surgery and revealed TPLNs recurrence negatively associated with the overall survival.
- Citation: Li XY, Huang LS, Yu SH, Xie D. Thoracic para-aortic lymph node recurrence in patients with esophageal squamous cell carcinoma: A propensity score-matching analysis. World J Clin Cases 2022; 10(36): 13313-13320
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13313.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13313
Esophageal cancer is the fourth most common cause of cancer-related deaths in China[1]. Although the incidence of esophageal squamous cell carcinoma (ESCC) has decreased in Western countries[2], this subtype accounts for more than 90% of the esophageal cancer diagnoses in China. Currently, surgical resection is the mainstay of curative treatment, and preoperative chemoradiotherapy followed by esophagectomy is considered the standard treatment for locally advanced esophageal cancer based on the accumulated evidence over the past 15 years[3-5].
Lymph node metastasis is among the most crucial negative prognostic factors affecting cancer patients[6]. Thoracic para-aortic lymph node (TPLN) metastasis, a rare complication of esophageal cancer, has only been described in a few case reports[7,8], although in one case report, it was observed that there was a higher incidence of metastasis with recurrent disease[9]. Generally, two-field lymphadenectomy is commonly performed in Western countries and China[3-5], whereas extended three-field lymphadenectomy is performed in Japan[10]. A Chinese consensus recommends the dissection of nine stations of mediastinal lymph nodes to achieve better local control and survival outcomes[11]. However, extensive lymph node dissection increases the number of postoperative complications[12]. Nevertheless, the established guidelines in China and Western countries do not specify dissection of the TPLNs, likely because of the low incidence of TPLN metastasis.
In this study, we reviewed the outcomes of patients who had undergone curative surgery for ESCC at our center to determine the incidence of TPLN metastasis and its impact on survival outcomes. We also reviewed patients who had received definitive chemoradiotherapy for ESCC and compared the effects of different treatment modalities on TPLN metastasis.
This was a retrospective cohort study of anonymized data extracted from medical records. Approval for the use of medical records was obtained from the Ethics Committees of Shantou Central Hospital, China, prior to the study. All study protocols were approved by this committee.
We reviewed the medical records of consecutive patients who had undergone curative esophagectomy for pathologically proven ESCC between January 2012 and November 2017 at our Department of Surgery. The following information was extracted: Patient age, sex, work-up, treatments, and follow-up. Patients who met the following inclusion criteria were considered eligible: (1) Pathological diagnosis of squamous cell carcinoma; (2) Disease stage I-III; and (3) Surgical treatment with curative intent. The main exclusion criteria were a diagnosis of adenocarcinoma or other pathological type and stage IV disease. We further reviewed the records of consecutive patients who had received definitive chemoradiotherapy between January 2012 and December 2013 at the Department of Radiation Oncology. The inclusion criteria were as follows: (1) Pathological diagnosis of squamous cell carcinoma; (2) Disease stage I-III; and (3) Radiochemotherapy with curative intent.
At our center, the operative procedure for ESCC comprised en bloc esophagectomy with two-field (mediastinal and upper abdominal) lymphadenectomy, gastric tube reconstruction, and cervical anastomosis. Mediastinal lymphadenectomy was performed via a right thoracic approach and included the left and right recurrent, paraesophageal, paratracheal, and subcarinal regions and the inferior pulmonary ligament, as per the Chinese expert consensus on mediastinal lymph node dissection[11]. The pathological tumor stage was determined according to the seventh edition of the American Joint Committee on Cancer TNM classification (2009).
The location of the TPLN was defined as previously described[13], namely, the posterior mediasti
Recurrence was defined as the first documented radiographic evidence of disease relapse. The recurrence-free survival (RFS) and overall survival (OS) rates were estimated using the Kaplan-Meier method and compared using the log-rank test. Comparisons of categorical data were performed using the chi-squared test. A P value < 0.05 indicated statistical significance.
A Cox regression model was used to identify prognostic factors, and logistic regression was used to explore the relationships between clinicopathological factors and TPLN recurrence. We performed a propensity score-matching analysis (with variables including age, sex, tumor location, tumor grade, T stage, nodal status, and type of adjuvant therapy) based on the one-to-many nearest neighbor method (caliper width: 0.1)[14]. All P values were two-sided, and all statistical analyses were performed using R software (version i386 3.3.2; R Project for Statistical Computing, Vienna, Austria).
Between January 2012 and November 2017, 604 patients who had been diagnosed with pathologically proven ESCC underwent curative esophagectomy and lymphadenectomy at our center. The R0 reaction rate was 98.34% (594/604). Regarding perioperative mortality, no deaths occurred within 30 d after surgery. A median of 20 lymph nodes were harvested, and positive lymph nodes were observed in 294 (48.68%) of the 604 patients. The median follow-up was 38.63 mo. Among the patients (Supple
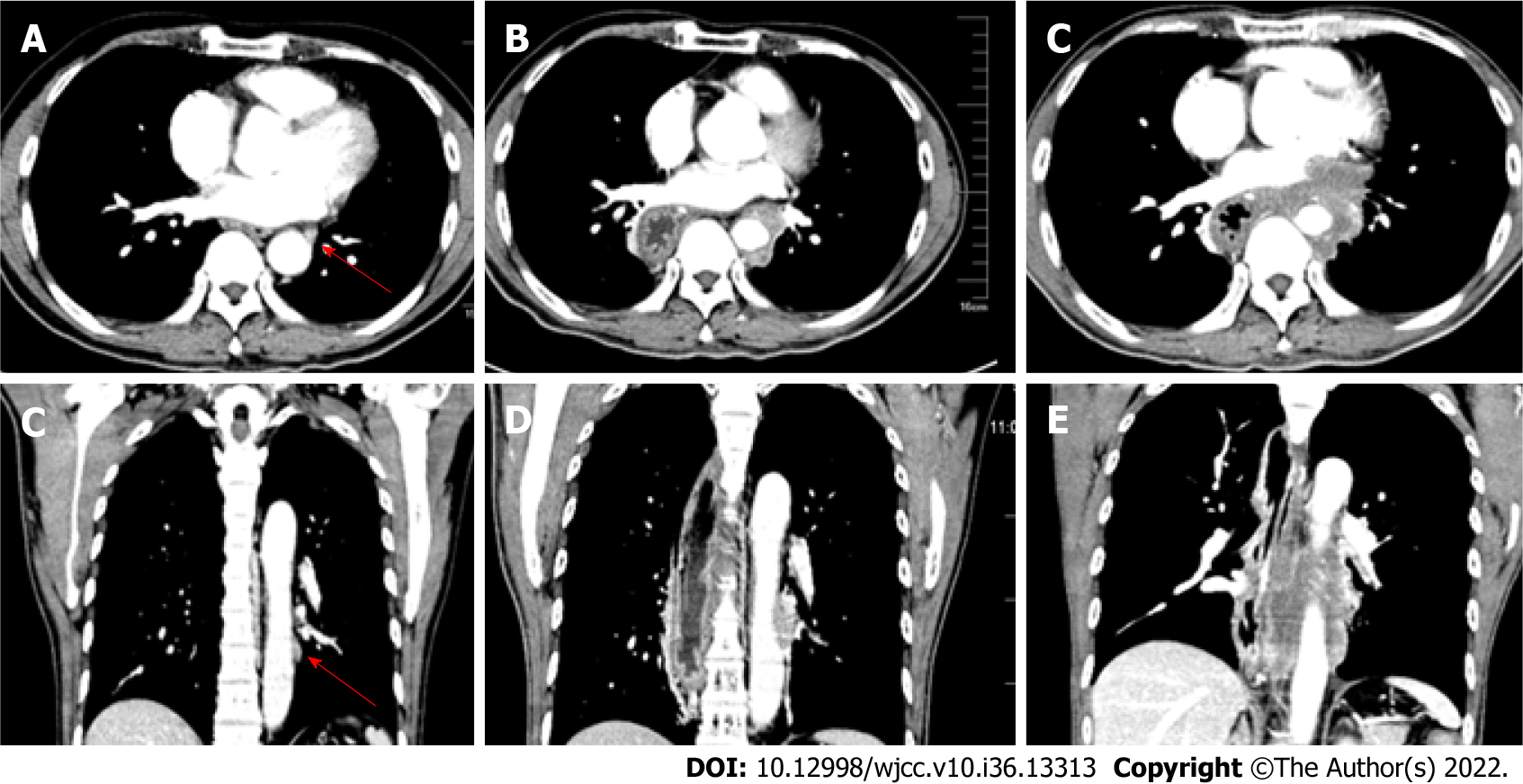
Figure 1 shows an example of TPLN recurrence after surgery in a representative patient. In the TPLN (+) group, 4 (4/604, 0.66%) patients presented with small TPLNs before surgery. The diameters of these small TPLNs ranged from 5.5 to 8.2 mm (mean, 6.73 mm). The TPLNs in these 4 patients were not dissected during surgery. Two patients received adjuvant chemoradiotherapy postoperatively, and two received no adjuvant treatment.
Of the 223 patients diagnosed with thoracic ESCC who had undergone definitive chemoradiotherapy between January 2012 and December 2013, 3 (1.35%) presented with enlarged TPLNs before treatment (diameters: 5.4, 6.2, and 11.0 mm). After definitive therapy, all the TPLNs shrank and did not relapse during follow-up. No other TPLN recurrences were observed in this group. The characteristics of the two groups are shown in Table 1. The groups were well balanced before and after propensity score matching, and the logistic regression analysis did not indicate that any of these characteristics contributed to TPLN metastasis.
| TPLN (+) | Before matching | After matching | |||
| TPLN (-) | P value (df) | TPLN (-) | P value (df) | ||
| Age, median, yr | 56.5 | 59 | 0.19 | 57.5 | 0.76 |
| Sex | 1.00 (1) | 1.00 (1) | |||
| Male | 14 | 156 | 78 | ||
| Female | 4 | 45 | 20 | ||
| Location | 0.73 (2) | 0.87 (2) | |||
| Upper | 0 | 6 | 1 | ||
| Middle | 10 | 115 | 50 | ||
| Lower | 8 | 80 | 47 | ||
| Grade | 0.31 (3) | 0.50 (3) | |||
| I | 0 | 21 | 5 | ||
| II | 11 | 133 | 68 | ||
| III | 6 | 39 | 23 | ||
| Unknown | 1 | 8 | 2 | ||
| T stage | 0.85 (3) | 0.82 (3) | |||
| T1 | 0 | 5 | 1 | ||
| T2 | 3 | 33 | 11 | ||
| T3 | 14 | 157 | 83 | ||
| T4 | 1 | 6 | 1 | ||
| N stage | 0.43 (3) | 0.76 (3) | |||
| N0 | 4 | 75 | 25 | ||
| N1 | 6 | 61 | 28 | ||
| N2 | 7 | 48 | 32 | ||
| N3 | 1 | 17 | 13 | ||
| Adjuvant therapy | 0.70 (2) | 0.91 (2) | |||
| Chemoradiotherapy | 3 | 24 | 14 | ||
| Chemotherapy | 8 | 109 | 49 | ||
| None | 7 | 68 | 35 | ||
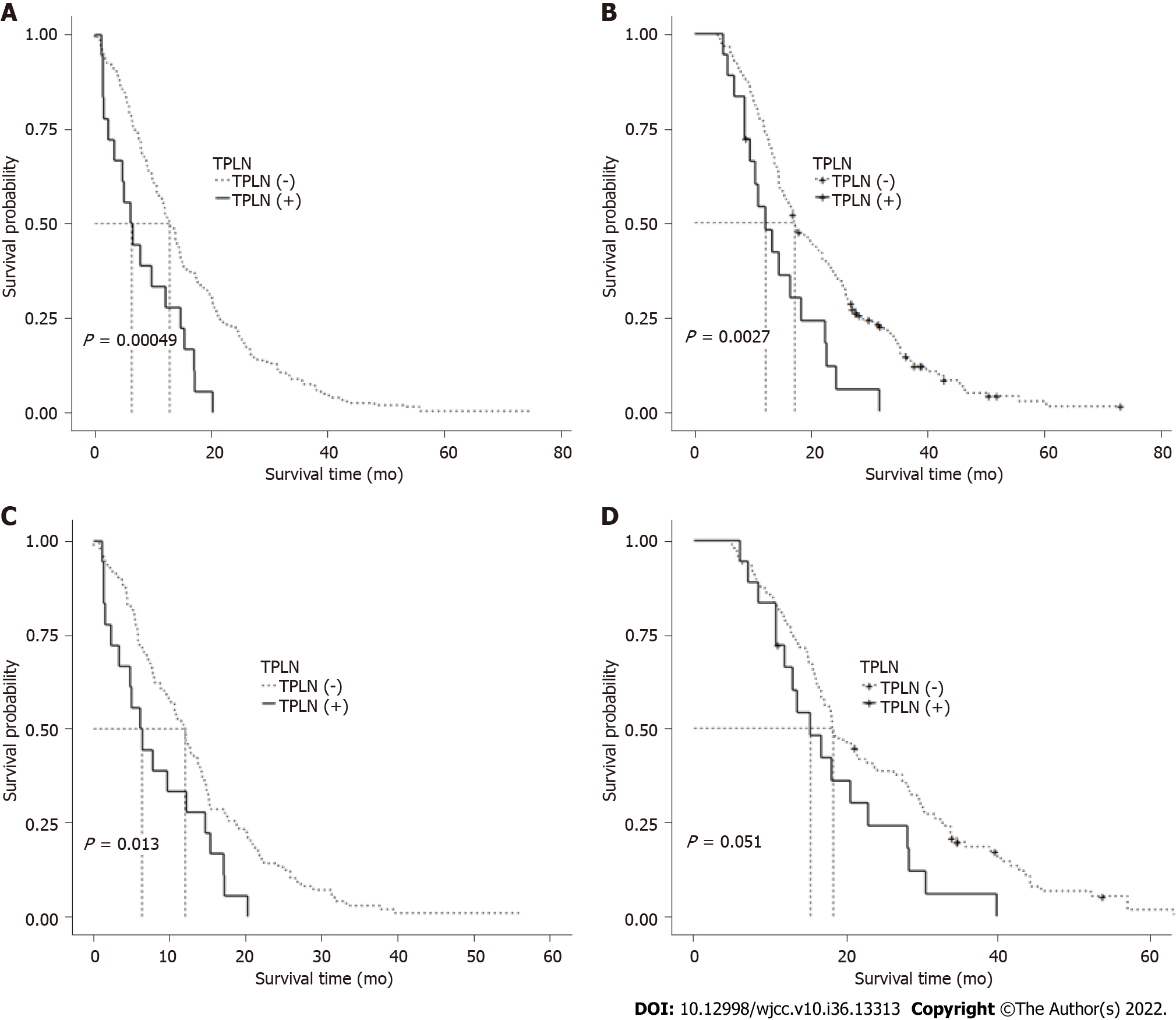
Before matching, the median RFS duration was significantly longer in the TPLN (-) group than in the TPLN (+) group [12.83 mo, 95% confidence interval (CI): 11.5-14.6 vs 6.35 mo, 95%CI: 3.4-15.4, P = 0.00049; Figure 2A). Similarly, the median OS duration was significantly longer in the TPLN (-) group than in the TPLN (+) group (21.47 mo, 95%CI: 19.37-26.60, vs 15.30 mo, 95%CI: 11.90-28.30, P = 0.0027; Figure 2B).
After matching, the median RFS duration remained significantly longer in the TPLN (-) group than in the TPLN (+) group (12.00 mo, 95%CI: 9.8-14.0 vs 6.35 mo, 95%CI: 3.4-15.4, P = 0.013; Figure 2C). However, the difference in OS duration between the TPLN (-) and TPLN (+) groups was only borderline significant, despite the longer duration in the former group (18.25 mo, 95%CI: 16.7-24.1 vs 15.30 mo, 95%CI: 11.90-28.30, P = 0.051; Figure 2D). A Cox model analysis of potential prognostic factors similarly indicated the presence of TPLN metastasis as a risk factor for OS before matching but not after matching.
Globally, the treatment of esophageal cancer remains challenging. For patients with locally advanced esophageal cancer, neoadjuvant chemoradiotherapy or chemoradiotherapy followed by surgery has led to significant improvements in survival relative to surgery alone[15]. However, in some scenarios, surgery represents mainly a curative method, along with definitive chemoradiotherapy.
As noted previously, lymph node metastasis is a strong prognostic factor in cancer cases. A higher number of dissected lymph nodes and increased station clearance are associated with more precise staging, better local control, and perhaps better OS, although these benefits may come at the cost of an increased risk of complications[16]. Consequently, efforts to enhance the radical dissection of lymph nodes include the progression from two-field to three-field lymphadenectomy[17] and a shift from a limited left thoracic approach to an extended right thoracic approach[18]. Despite these advances, surgeons might neglect stations associated with a low incidence of lymph node metastasis, such as TPLN. Few reports have described TPLN recurrence in cases of resectable esophageal cancer.
To the best of our knowledge, our study is the largest cohort study of TPLN recurrence, and our findings demonstrate that TPLN recurrence is associated with poor RFS and OS. We observed a TPLN recurrence rate of approximately 3%. In our cohort, TPLN recurrence did not occur in tumors located in the upper thoracic esophagus, in low-grade tumors (grade 1), or in tumors that had invaded the lamina propria, muscularis mucosae, or submucosa (T1 stage). However, we failed to identify any parameters significantly predictive of TPLN recurrence.
Despite our inability to identify predictive factors, our results have several implications for clinical practice. Previously, Shishido et al[8] reported on 2 patients with enlarged TPLNs (10 mm) that were confirmed pathologically after dissection. In contrast, we identified small TPLNs (longest transverse diameter < 10 mm) in 4 patients via CT scans. Although such small TPLNs had not been previously considered indicative of a metastasis-positive status[19], all 4 of the TPLNs in our patients were swollen after surgery, indicating that the lymph nodes in the thoracic para-aortic area should be treated cautiously, irrespective of the transverse diameter. Moreover, in cases of esophageal cancer, we always intraoperatively harvest small lymph nodes that are pathologically proven to be positive after surgery. However, the value of positron emission tomography/CT scans has not been validated, and therefore, this imaging technique is not routinely applied at our center.
As mentioned above, TPLN is not included in the description of standard lymph node dissection in the guidelines used in China or Western countries, and the extent of LN station nine (i.e., the pulmonary ligament area) does not necessarily include TPLNs[20]. Although we have performed esophageal surgery in more than 1700 cases at our center, we do not routinely clear this area. Moreover, some small lymph nodes in this area are often overlooked, which might explain the poor survival outcomes among our patients with TPLN recurrence.
In Japan, TPLNs are further classified as anterior or posterior. Posterior TPLNs are associated with worse RFS and OS outcomes than anterior TPLNs[13]. Currently, a left thoracic approach is recom
Our study had several limitations. First, this was a retrospective study of a small sample of patients at a single treatment center, which had an inherent risk of bias by the nature of the study design. To overcome this limitation, we are currently building a database dedicated to capturing data on TPLNs. Second, before 2017, nearly all the patients with resectable esophageal cancer at our center were offered surgery as an initial treatment. This practice contrasts with the preferred initial treatment options in Western countries. However, most patients in Western countries present with esophageal adenocarcinoma, but the majority of patients in China have ESCC. Additionally, the benefit of neoadjuvant chemoradiotherapy in Chinese patients with ESCC has been confirmed only recently[5]. A further evaluation of TPLN is needed once this treatment paradigm has been fully introduced into clinical practice.
Our study results confirm the low incidence of TPLN metastasis and reveal a potential relationship between this complication and survival outcomes.
Thoracic para-aortic lymph nodes (TPLNs) recurrence in esophageal squamous cell carcinoma (ESCC) is rare but with poor survival outcomes in clinical observation.
TPLNs recurrence had negative impact on survival outcomes in patients with ESCC.
TPLN (+) was associated with worse recurrence-free and overall survival (OS) in patients with ESCC.
The propensity score-matching and survival estimation were applied, and factors predictive of OS were explored using a Cox proportional hazards model.
To study survival in patients with ESCC who developed TPLNs recurrence.
To determine TPLNs recurrence rate and its impact on survival.
TPLNs recurrence in ESCC is rare but with poor survival outcomes in clinical observation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Shantou Central Hospital.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohamed SY, Egypt; Srpcic M, Slovenia S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer. 2016;35:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Ilson DH. Adenocarcinoma of the esophagus: controversies and consensus. Chin Clin Oncol. 2017;6:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1808] [Article Influence: 180.8] [Reference Citation Analysis (0)] |
| 4. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4051] [Article Influence: 311.6] [Reference Citation Analysis (0)] |
| 5. | Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, Mao W, Xiang J, Han Y, Chen Z, Yang H, Wang J, Pang Q, Zheng X, Li T, Lordick F, D'Journo XB, Cerfolio RJ, Korst RJ, Novoa NM, Swanson SJ, Brunelli A, Ismail M, Fernando HC, Zhang X, Li Q, Wang G, Chen B, Mao T, Kong M, Guo X, Lin T, Liu M, Fu J; AME Thoracic Surgery Collaborative Group. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol. 2018;36:2796-2803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 680] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 6. | Sugimura K, Miyata H, Shinno N, Ushigome H, Asukai K, Yanagimoto Y, Hasegawa S, Takahashi Y, Yamada D, Yamamoto K, Nishimura J, Motoori M, Wada H, Takahashi H, Yasui M, Omori T, Ohue M, Yano M. Prognostic Factors for Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Docetaxel/Cisplatin/5-Fluorouracil Followed by Surgery. Oncology. 2019;97:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Németh G, Schlegel W. Radiation therapy of intrathoracic paraaortic lymph node metastases. Three-dimensional treatment planning. Acta Oncol. 1987;26:203-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Shishido Y, Miyata H, Sugimura K, Motoori M, Miyoshi N, Yasui M, Omori T, Ohue M, Fujiwara Y, Yano M. Successful resection after neoadjuvant chemotherapy for esophageal cancer with posterior thoracic paraaortic lymph node metastasis: a case report and literature review. Gen Thorac Cardiovasc Surg. 2017;65:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 9. | Ninomiya I, Okamoto K, Tsukada T, Saito H, Fushida S, Ikeda H, Ohta T. Thoracoscopic radical esophagectomy and laparoscopic transhiatal lymph node dissection for superficial esophageal cancer associated with lymph node metastases in the dorsal area of the thoracic aorta. Surg Case Rep. 2015;1:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Matsuda S, Takeuchi H, Kawakubo H, Kitagawa Y. Three-field lymph node dissection in esophageal cancer surgery. J Thorac Dis. 2017;9:S731-S740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Li H, Fang W, Yu Z, Mao Y, Chen L, He J, Rong T, Chen C, Chen H, Chen K, Du M, Han Y, Hu J, Fu J, Hou X, Gong T, Li Y, Liu J, Liu S, Tan L, Tian H, Wang Q, Xiang J, Xu M, Ye X, You B, Zhang R, Zhao Y; Society of Esophageal Tumor, Chinese Anti-Cancer Association. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis. 2018;10:2481-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Ma GW, Situ DR, Ma QL, Long H, Zhang LJ, Lin P, Rong TH. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol. 2014;20:18022-18030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 13. | Yamamoto M, Yamasaki M, Tanaka K, Miyazaki Y, Makino T, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y. New classification for the thoracic paraaortic lymph nodes of patients with esophageal squamous cell carcinoma. Surg Today. 2018;48:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 2:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 370] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 15. | Kelly RJ. Emerging Multimodality Approaches to Treat Localized Esophageal Cancer. J Natl Compr Canc Netw. 2019;17:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 16. | Hagens ERC, van Berge Henegouwen MI, Cuesta MA, Gisbertz SS. The extent of lymphadenectomy in esophageal resection for cancer should be standardized. J Thorac Dis. 2017;9:S713-S723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Shao L, Ye T, Ma L, Lin D, Hu H, Sun Y, Zhang Y, Xiang J, Chen H. Three-field versus two-field lymph node dissection for thoracic esophageal squamous cell carcinoma: a propensity score-matched comparison. J Thorac Dis. 2018;10:2924-2932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Li B, Hu H, Zhang Y, Zhang J, Miao L, Ma L, Luo X, Ye T, Li H, Zhou J, Li Y, Shen L, Zhao K, Fan M, Zhu Z, Wang J, Xu J, Deng Y, Lu Q, Jia H, Cheng X, Li C, Pan Y, Liu S, Shao L, Sun Y, Xiang J, Chen H. Extended Right Thoracic Approach Compared With Limited Left Thoracic Approach for Patients With Middle and Lower Esophageal Squamous Cell Carcinoma: Three-year Survival of a Prospective, Randomized, Open-label Trial. Ann Surg. 2018;267:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Yamasaki M, Miyata H, Miyazaki Y, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y. Evaluation of the nodal status in the 7th edition of the UICC-TNM classification for esophageal squamous cell carcinoma: proposed modifications for improved survival stratification: impact of lymph node metastases on overall survival after esophagectomy. Ann Surg Oncol. 2014;21:2850-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | van der Horst S, de Maat MFG, van der Sluis PC, Ruurda JP, van Hillegersberg R. Extended thoracic lymph node dissection in robotic-assisted minimal invasive esophagectomy (RAMIE) for patients with superior mediastinal lymph node metastasis. Ann Cardiothorac Surg. 2019;8:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |