Published online Dec 26, 2022. doi: 10.12998/wjcc.v10.i36.13239
Peer-review started: August 10, 2022
First decision: November 11, 2022
Revised: November 15, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: December 26, 2022
Processing time: 138 Days and 10.7 Hours
Periprosthetic joint infection (PJI) is a catastrophic complication that can occur following total knee arthroplasty (TKA). Currently, the treatment for PJI mainly includes the use of antibiotics alone, prosthetic debridement lavage, primary revision, secondary revision, joint fusion, amputation, etc.
To explore the clinical effect of two-stage revision surgery for the treatment of PJI after TKA.
The clinical data of 27 patients (3 males and 24 females; age range, 47–80 years; mean age, 66.7 ± 8.0 years; 27 knees) with PJI treated with two-stage revision surgery in our hospital between January 1, 2010 and December 31, 2020 were analyzed retrospectively. The following outcomes were compared for changes between preoperative and last follow-up results: Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), visual analogue scale (VAS) scores, Hospital for Special Surgery (HSS) scores, knee range of motion (ROM), and infection cure rates.
All 27 patients were followed up (range, 13–112 mo). The ESR (14.5 ± 6.3 mm/h) and CRP (0.6 ± 0.4 mg/dL) of the patients at the last follow-up were significantly lower than those at admission; the difference was statistically significant (P < 0.001). The postoperative VAS score (1.1 ± 0.7), HSS score (82.3 ± 7.1), and knee ROM (108.0° ± 19.7°) were significantly improved compared with those before the surgery; the difference was statistically significant (P < 0.001). Of the 27 patients, 26 were cured of the infection, whereas 1 case had an infection recurrence; the infection control rate was 96.3%.
Two-stage revision surgery can effectively relieve pain, control infection, and retain good joint function in the treatment of PJI after TKA.
Core Tip: The two-stage revision surgery approach, which includes the first stage of thorough debridement, removal of prosthesis, antibiotic bone cement placeholder exclusion for 3–6 mo, three normal consecutive routine blood examination results for erythrocyte sedimentation rate and C-reactive protein, selection of a suitable prosthesis for the second-stage revision, and the use of a sufficient amount of a full course of sensitive antibiotics, is a reliable method for the treatment of periprosthetic joint infection after total knee arthroplasty A. This approach can effectively relieve pain, control infection, and preserve good joint function.
- Citation: Qiao YJ, Li F, Zhang LD, Yu XY, Zhang HQ, Yang WB, Song XY, Xu RL, Zhou SH. Analysis of the clinical efficacy of two-stage revision surgery in the treatment of periprosthetic joint infection in the knee: A retrospective study. World J Clin Cases 2022; 10(36): 13239-13249
- URL: https://www.wjgnet.com/2307-8960/full/v10/i36/13239.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i36.13239
Periprosthetic joint infection (PJI) is a catastrophic complication after total knee arthroplasty (TKA), with an incidence of approximately 2% after primary TKA[1]. PJI can be classified as early (occurring within 3 mo after surgery), delayed (3–24 mo after surgery), and late (2 years after surgery)[2]. Currently, the treatment for PJI primarily includes the use of antibiotics alone, prosthetic debridement lavage, primary revision, secondary revision, joint fusion, amputation, etc.[3,4]. Tsukayam et al[5] grouped PJIs into four types and proposed corresponding treatment strategies: Type Ⅰ, no symptoms of infection but with positive intraoperative culture, mostly observed in preoperative diagnosis of aseptic loosening of the knee prosthesis and positive culture of the tissue specimen during revision surgery, which can be treated with intravenous antibiotics; type II, an early infection that occurs within 1 mo after surgery and can be treated by debridement to preserve the prosthesis; type III, an acute hematogenous infection that occurs suddenly in a well-functioning joint, which can also be treated, and the prosthesis is preserved; type IV, an advanced chronic infection that requires removal and revision of the prosthesis. PJI staging is the most widely used staging method and provides guidance on when to retain the prosthesis. One-stage revision leads to short hospitalization time, requires minimal cost, and has low impact on joint function[6]. However, its use is controversial because of the associated high recurrence rate of postoperative infection, and its effectiveness needs to be further explored[7]. Second-stage revision requires multiple surgeries and has the disadvantages of long hospital stay, increased costs, and impaired joint function, but it is effective and remains the gold standard of PJI treatment[8-10]. This study aimed to retrospectively analyze 27 patients with PJI who were treated with one-stage debridement, implant removal, antibiotic bone cement spacer exclusion, and two-stage revision and compare their preoperative and follow-up results.
The inclusion criteria were as follows: Patients with PJI treated for the first time after TKA between January 1, 2010, and December 31, 2020; a diagnosis of PJI that conforms to the diagnosis standard set by of the International Consensus Group on Periprosthetic Joint Infection in 2014[11]; patients able to tolerate surgery; provision of informed consent for one-stage debridement, implant removal, antibiotic bone cement spacer exclusion, and two-stage revision; with complete follow-up data.
Patients with previous revision surgery due to infectious or non-infectious causes, those with termination of treatment using antibiotic bone cement spacer, those who were under anti-infection treatment for another infection site, and those lost to follow-up were excluded.
We retrospectively analyzed the clinical data of 27 patients (3 males and 24 females; age range, 47–80 years; mean age, 66.7 ± 8.0 years; 27 knees) with PJI who were treated with phase I absences and phase II revisions from January 1, 2010, to December 31, 2020, at our hospital. There were 15 and 12 PJI cases in the right and left knees, respectively. There were 16 initial TKA cases recorded in our hospital and 11 cases from an outside hospital. The primary disease was osteoarthritis of the knee in 20 cases and rheumatoid arthritis in 7 cases. In all the cases, postoperative pathology was suggestive of acute/chronic septic inflammation. The surgery for all the cases was performed by the same team of surgeons.
Surgery was performed under general anesthesia or combined lumbar epidural anesthesia, and the skin around the knee was cleaned thoroughly with a soft brush dipped in soap and water placed under the table. The surgical incision site was disinfected according to the infected area, the incision was made layer by layer along the original incision, the necrotic tissue and pus were cleared from outside to inside the infection site, any encountered sinus was completely removed, the joint capsule was opened to reveal the prosthesis, and the joint capsule, membranous tissue on the surface of the prosthesis, prosthesis, and bone cement were removed. At least three points of the interface granulation tissue and any other abnormal or septic tissue were sampled for bacterial culture and rapid frozen pathological and postoperative pathological examinations. The joint capsule, synovium, and diseased bone tissue were debrided thoroughly, and the surrounding tissue was completely excised. The bone cement was thoroughly cleaned after the prosthesis and pad were removed, and the remaining diseased tissue of the posterior joint capsule was completely excised again. The joint cavity was rinsed at least three times with hydrogen peroxide, dilute iodophor, and saline, with a liquid volume of more than 5000 mL, and the joint cavity was soaked with concentrated iodophor for at least 15 min and finally rinsed thoroughly with a large amount of saline using a pulse gun. The surgical gown, sterile gloves, and surgical instruments were changed for the next phase of the surgery. Depending on the size of the joint space after the prosthesis had been removed, an antibiotic cemented spacer of appropriate size was handmade (40 g of bone cement + 4 g of vancomycin, vancomycin-resistant, or specific pathogenic bacteria; antibiotics were added according to drug sensitivity results). Two groups of flushing and drainage tubes were left in place, and 4000 mL of saline/24 h of continuous drainage was administered after the surgery. Drainage fluid was collected and sent for culturing three times after 5–7 postoperative days, and the drainage tubes were removed after negative results were obtained.
For the reexamination of the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and routine blood culture to monitor infection control regularly, exclusion for 3–6 mo is generally recommended, and three normal consecutive reexamination results are required for the second revision surgery to be performed. The bone and soft tissue are carefully explored, and three different parts of the soft tissue are sampled for rapid frozen pathological examination to assess the infection control. If the high magnification field was still greater than 10 neutrophils, thorough debridement and reinsertion of a new antibiotic bone cement placeholder is repeated. If the infection was completely controlled, the appropriate knee revision prosthesis was selected according to the degree of bone defect, bone quality, and stability of the collateral ligament, metal pad, and extension rod.
For pain management, advanced, individualized, and multi-mode analgesia was used. For thrombosis prevention, 5000 IU of low molecular weight heparin calcium was injected subcutaneously once a day. For functional exercise, isometric and isotonic exercises of the quadriceps muscle, ankle pump exercises, and other functional exercise were performed as soon as possible after the administration of postoperative analgesia, and the exercise routine was gradually transitioned to active and passive functional exercises of the affected joints. For drainage, continuous knee cavity lavage was performed for 7–10 d after the first-stage spacer exclusion using 4000 mL of normal saline solution every day. Drainage fluid was tested by routine bacterial culture for 3 consecutive days from the 7th postoperative day, and the flushing and drainage tubes were removed after all the results were negative. Based on the drainage condition, the drainage tube should be removed within 48 h after the two-stage revision.
The preoperative puncture fluid, intraoperative pus, necrotic tissue, and postoperative drainage fluid were all subjected to bacterial culture and drug sensitivity tests, and corresponding antibiotics were selected according to the drug sensitivity results. During the results waiting period or when the culture results are negative, empirical antibiotics should be administered for treatment, and broad-spectrum antibiotics should also be selected administered to treat most gram-negative and gram-positive bacterial infections. After first-stage exclusion, an adequate amount of sensitive antibiotics was administered intravenously for 2 wk (if the culture result was negative, intravenous vancomycin combined with third-generation cephalosporins or quinolones and other antibiotics was administered); oral sensitive antibiotics was also administered for 4 wk after the 2 wk (if the culture result was negative, oral rifampicin + ciprofloxacin were administered), and the decision to stop or extend the antibiotics (intravenous + oral sensitive antibiotics) < 6 wk of the drug course was based on a patient’s condition. The initial intravenous antibiotic regimen was continued after second-stage revision until the intraoperative culture results were clear or stopped if the results were negative; treatment was continued according to new PJI if there were 2 or more positive results.
ESR, CRP, visual analogue scale (VAS) score, Hospital for Special Surgery (HSS) score, range of motion (ROM), and infection control rate were recorded before the surgery and at the last follow-up. Infection control was defined as the absence of infection recurrence and prosthesis loosening after revision and follow-up. ESR and CRP were used to evaluate infection control, and pain VAS score, HSS score, and ROM were used to evaluate symptom improvement and recovery of knee function. Two years after the surgery, radiographic images were reviewed to determine whether the prosthesis was loose.
SPSS version 23.0 (IBM, Chicago, IL, United States) was used to analyze the data. ESR, CRP, VAS score, HSS score, and ROM were all expressed as mean ± SD. Paired sample t test was used to compare the results obtained before surgery and at last follow-up, and P < 0.05 was considered to be statistically significant.
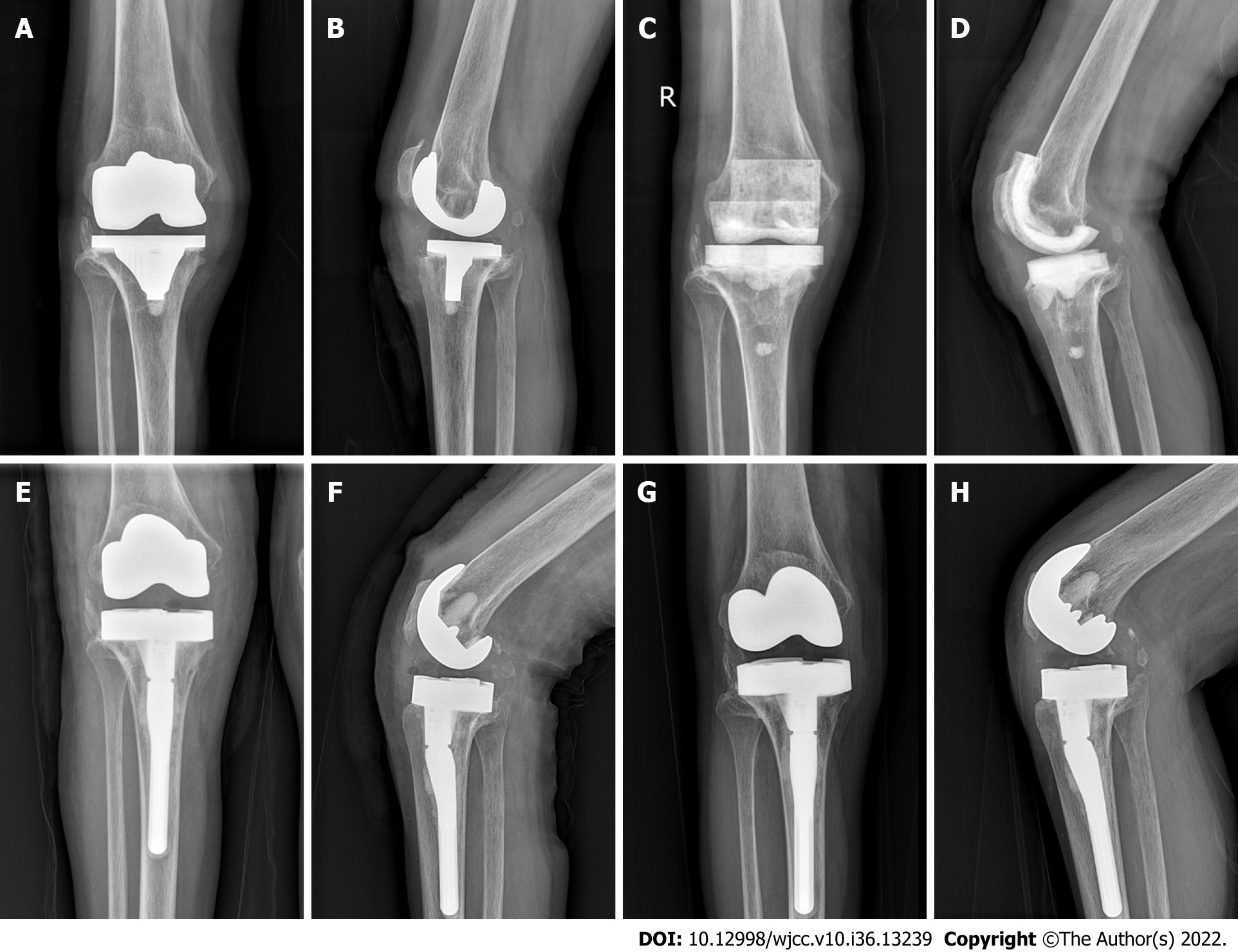
All 27 patients were followed up for 13–112 mo (average, 59 ± 26 mo). Twenty-six patients were cured of the infection, and 1 patient had recurrence after 6 years of follow-up, with an infection control rate of 96.3%. The time between the initial joint replacement and the diagnosis of PJI ranged from 7 to 195 mo (mean, 41 ± 35 mo), with 9 cases (33.3%) of delayed PJI and 18 cases (66.7%) of advanced PJI. The duration of spacer exclusion was 2.8–6.3 mo (mean, 3.8 ± 0.8 mo). Positive bacterial culture results were obtained in 17 of the 27 patients (Table 1), with a positive rate of 63.0%, including 13 g-positive bacterial infection [8 cases of Staphylococcus aureus, 2 cases of methicillin-resistant S. aureus (MRSA), 2 cases of Staphylococcus epidermidis, and 1 case of Streptococcus griseus], 3 g-negative bacterial infection (2 cases of Enterobacter cloacae, 1 case of Pseudomonas aeruginosa), and 1 case of fungal infection (Candida). An articulating spacer was used in all 27 patients. All 27 patients with the following types of prosthesis underwent revision surgery: Surface knee prosthesis (3 cases), Legacy® (Zimmer Biomet, Warsaw, IN, United States) constrained condylar knee (LCCK) prosthesis (20 cases), and rotating hinge (RH) knee prosthesis (4 cases). The ESR (14.5 ± 6.3 mm/h) and CRP (0.6 ± 0.4 mg/dL) levels at the last follow-up were significantly (P < 0.001) lower than those obtained preoperatively (ESR, 57.1 ± 21.8 mm/h; CRP, 2.9 ± 2.1 mg/dL), and this difference was statistically significant (Table 2). The patients’ VAS score (1.1 ± 0.7), HSS score (82.3 ± 7.1), and knee ROM (108.0° ± 19.7°) at the last follow-up improved significantly (P < 0.001) compared with the preoperative scores (VAS, 4.0 ± 0.8; HSS score, 57.9 ± 9.4; knee ROM, 91.9° ± 26.9°) (Table 3).
| Pathogenic bacteria | Number of cases | Proportion (%) | |
| Gram-positive bacteria | S. aureus | 8 | 48.1 |
| Methicillin-resistant S. aureus | 2 | ||
| S. epidermidis | 2 | ||
| Ste. griseus | 1 | ||
| Gram-negative bacteria | E. cloacae | 2 | 11.1 |
| P. aeruginosa | 1 | ||
| Fungi | Candida | 1 | 3.7 |
| Negative bacterial culture | Not assessed | 10 | 37.0 |
| Indicators | Preoperative exclusion | Final follow-up | T value | P values |
| ESR (mm/h) | 57.1 ± 21.8 | 14.5 ± 6.3 | 9.043 | < 0.001 |
| CRP (mg/dL) | 2.9 ± 2.1 | 0.6 ± 0.4 | 5.804 | < 0.001 |
| Indicators | Preoperative exclusion | Final follow-up | T value | P values |
| VAS (score) | 4.0 ± 0.8 | 1.1 ± 0.7 | 18.028 | < 0.001 |
| HSS (score) | 57.9 ± 9.4 | 82.3 ± 7.1 | -21.813 | < 0.001 |
| ROM (°) | 91.9 ± 26.9 | 108.0 ± 19.7 | -7.148 | < 0.001 |
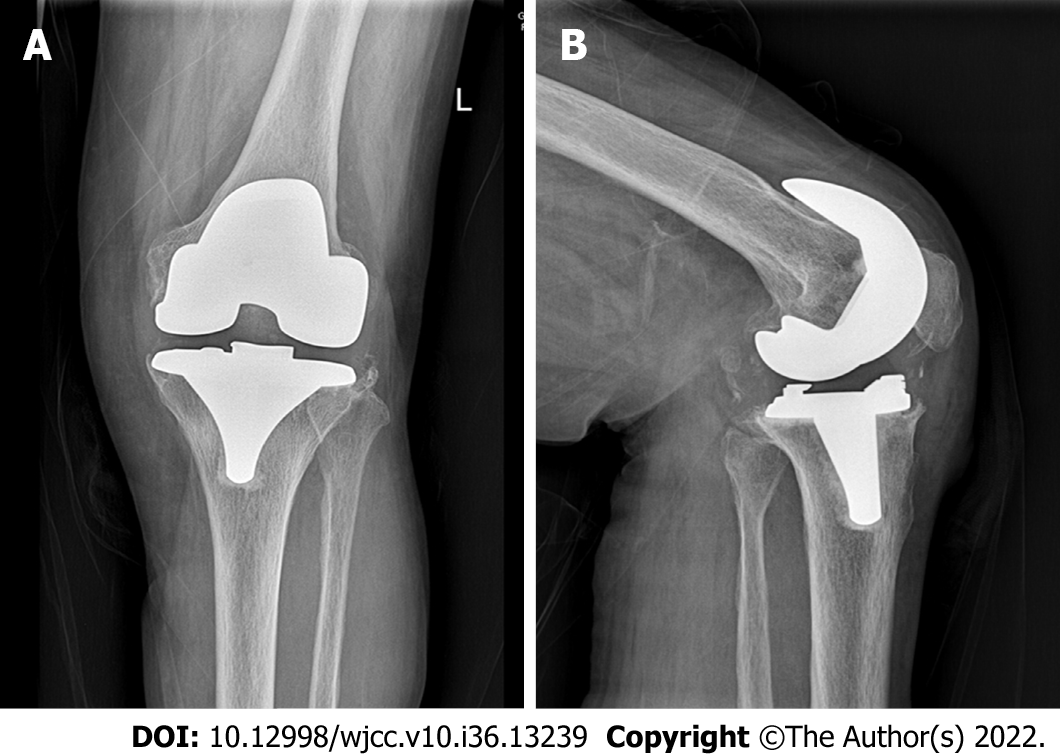
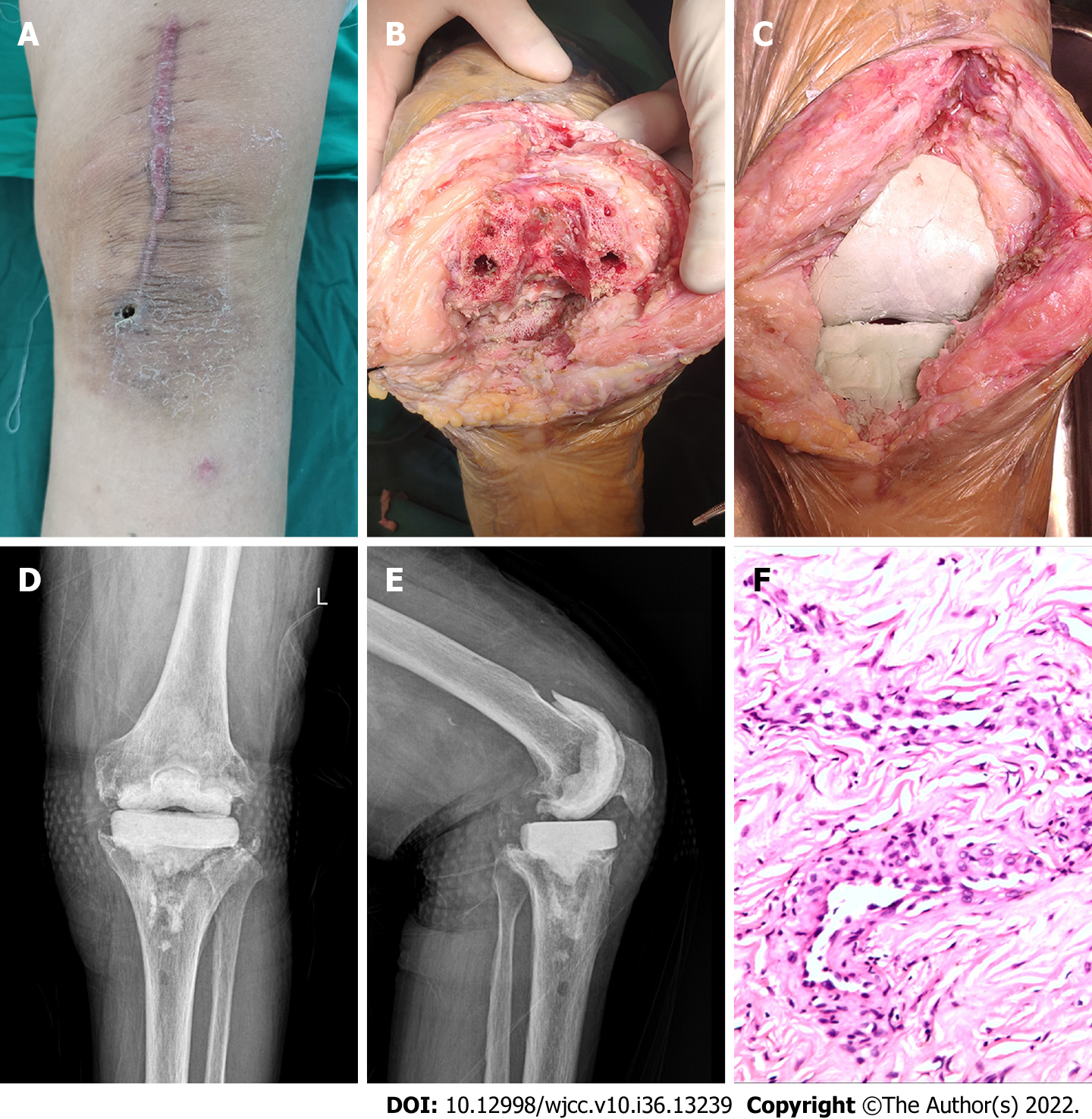
The patient was a 78-year-old woman whose primary disease was left knee osteoarthritis (Kellgren-Lawrence stage VI). More than 4 years after the left TKA was performed in another hospital, her left knee joint became swollen and painful with limited movement for more than 6 mo, and there was sinus tract formation and secretion discharge for 2 mo (Figure 1). After admission, the bacterial culture of the knee joint puncture showed MRSA. Drug sensitivity test showed sensitivity to vancomycin and rifampin. After ensuring no contraindications to surgery, lumbar anesthesia was administered for one-stage debridement, implant removal, and antibiotic bone cement spacer exclusion (Figure 2). Postoperative intravenous vancomycin (1 g, twice a day) was administered for 2 wk. After discharge, rifampicin capsule (0.45 g, once a day) and ciprofloxacin hydrochloride capsule (0.5 g, twice a day) were taken orally for 6 wk; the exclusion time was 4 mo. After three consecutive routine blood examinations, ESR and CRP were normal, and knee arthroplasty was performed in the second stage of the treatment (Figure 3).
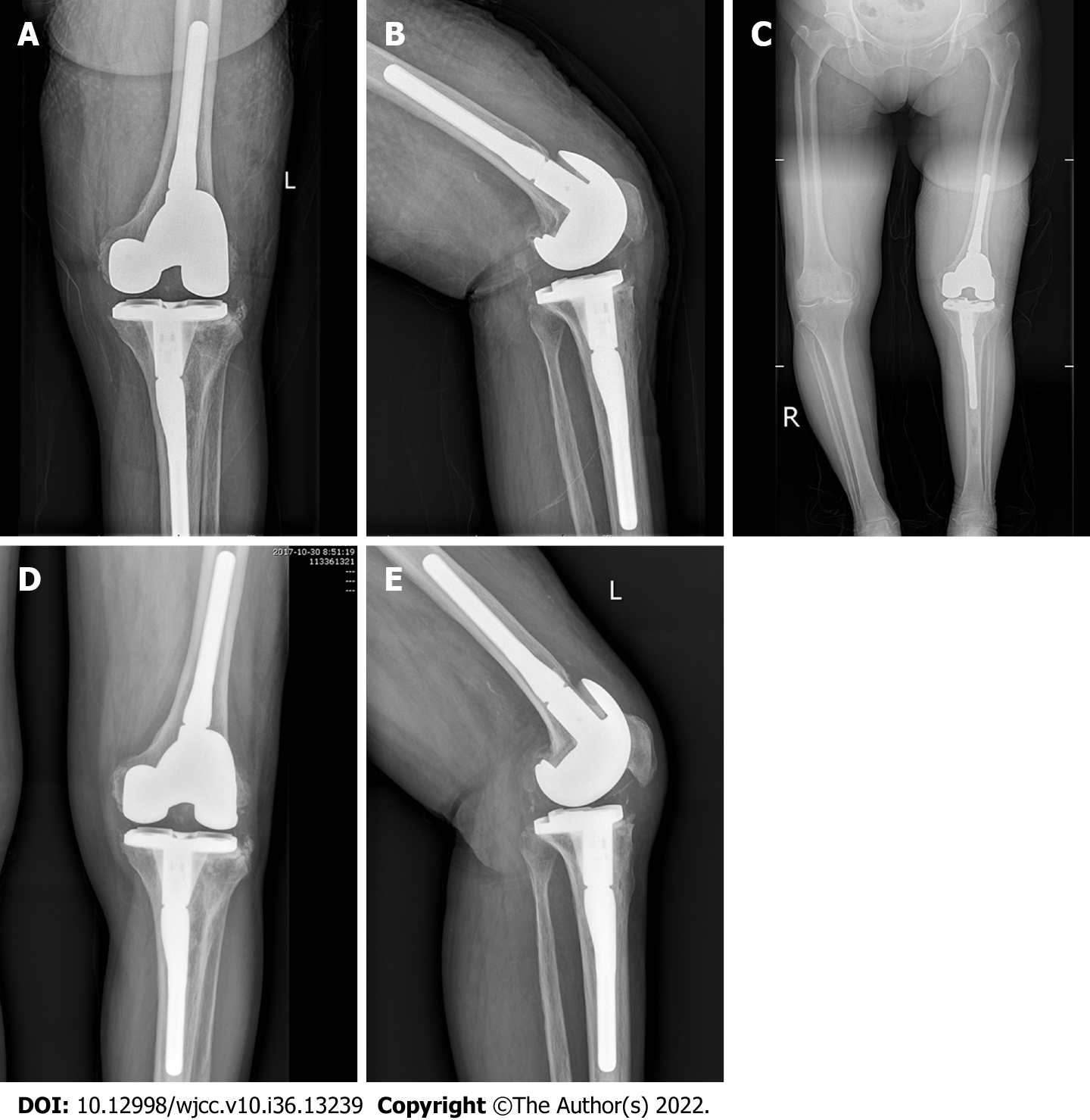
Case 2 was a 51-year-old woman with rheumatoid arthritis of the right knee, which occurred more than 6 years after right TKA (Figure 4). The patient’s chief complaints were right knee swelling and pain with limited movement for 6 mo. Negative bacterial culture results were obtained throughout the treatment, and pathological examination showed chronic purulent inflammation. After ensuring no contraindications to surgery, lumbar anesthesia was administered for stage debridement, implant removal, and antibiotic bone cement spacer exclusion. Postoperative intravenous vancomycin (1 g, twice a day) combined with cefoperazone sulbactam sodium (3 g, twice a day) was administered for 2 wk. After discharge from the hospital, the patient had to take oral rifampin (0.45 g, once a day) and ciprofloxacin (0.5 g, twice a day) for 6 wk, with an exclusion time of 4.8 mo. Three consecutive routine blood examinations revealed that the ESR and CRP were normal on all three instances. Knee arthroplasty was then performed in the second stage of the treatment.
As one of the most serious complications of TKA, PJI is characterized by difficult treatment, long treatment period, high recurrence rate, and high disability rate. Different treatments have been reported in recent years. Prosthetic knee revision, including primary and secondary revision, is one of the basic treatment options for PJI. Currently, second-stage revision is the gold standard for PJI treatment[12], and the cure rate varies greatly among different joint centers. In the present study, we retrospectively analyzed 27 patients with PJI that was diagnosed and treated in our hospital. The infection control rate of the second-stage revision was 96.3%, and the success rate of treatment was significantly higher than that reported in previous studies[13,14].
According to the bacterial biofilm formation theory proposed by Costerton[15], bacteria adhere to the surface of prosthesis and secrete polysaccharide, fibrin, lipoprotein, and other substances to form a multi-membrane structure called biofilm. The bacterial biofilm forms irreversible changes in approximately 3 wk, and it can resist human immunity and the effects of antibiotics. Therefore, the application of antibiotics alone cannot completely eradicate the infection around the joint. The formation of biofilm is the main reason for the difficulty in the late treatment of PJI. Radical treatment of PJI requires removal of the entire prosthesis and intensive debridement to the point of complete exposure. Mechanical operation should be performed to remove the biofilm, which is very important for infection control and is the primary factor for successful surgical outcome[3,16,17]. Debridement should not be forceful, and soft tissue injury and bone defect around the joint should be minimized as much as possible. Soft tissue injury of the skin has a significant influence on wound healing in the first stage, and collateral ligament injury and bone defect increase the difficulty of second-stage revision. Correct application of antibiotics plays a crucial role in the prognosis of PJI. After identifying the pathogenic bacteria and determining drug sensitivity through bacterial culture, selecting a sufficient amount and appropriate course of sensitive antibiotics can improve outcomes. A previous study reported that the main pathogenic bacteria of PJI are gram-positive bacteria such as S. epidermidis and S. aureus, which are basically sensitive to vancomycin[18]. PJI with negative bacterial culture is called refractory PJI and is common in the clinic. In this study, the failure rate of bacterial culture was 37% (10/27), which is approximately equivalent to the rate (20%–50%) reported in the literature[19]. Negative bacterial cultures make the diagnosis of and antibiotic selection for PJI difficult. The main reason for the low pathogen detection rate may be that the patient has been empirically treated with antibiotics before the specimens were obtained[20]. Therefore, it is important to seek effective methods to improve the positive rate of bacterial culture. Emergence of metal-artifact-free magnetic resonance imaging technology, polymerase chain reaction technology, ultrasonic pyrolysis, mass spectrometry analysis, second-generation sequencing, and other technologies[4,21-23] has made identification of pathogens relatively easy. However, with the widespread application and even abuse of antibiotics, the incidence of drug-resistant bacteria, such as MRSA, is increasing, and the treatment is difficult and requires a prolonged cycle. Fungi detected in PJI are extremely rare, accounting for approximately 1%–2%. The diagnosis of fungal PJI is very difficult, and negative bacterial cultures do not rule out fungi as the cause of PJI; therefore, treatment is also extremely challenging[24].
The application of antibiotic bone cement spacer is an important step in second-stage revision. Spacers are classified as non-jointed (stationary) and jointed (mobile). Spacer, made with a ratio of 40 g bone cement to 4 g vancomycin, sustainably releases high concentrations of antibiotics locally to control infection. Individually made articular spacers can appropriately maintain the knee space and lateral collateral ligament tension, effectively prevent soft tissue contracture around the prosthesis, and maximally preserve knee function, which is convenient for the second-stage revision exposure of the surgical field and the recovery of postoperative joint function[25-27]. Studies have shown that 3D-printed articular spacers can provide satisfactory ROM during the spacer exclusion period, prevent bone loss, and reduce the rate of infection and complications[28]. At present, there is no unified standard for the duration of the exclusion period. If the exclusion period is too long, the overall treatment time is prolonged and treatment cost is increased, which is not conducive to knee joint function. Spacer poses the risk of wear, dislocation, and fracture; the infection might not clear within an extremely short exclusion period, and there is a risk of infection recurrence. Our hospital recommends an interval of 3–6 mo between the first and second surgeries, and the knee joint should be normal during the open period. If the patient's general condition is stable, the incision is adequately healed, without symptoms of infection (such as swelling, heat, and pain), and three consecutive routine blood examination results of CRP and ESR are normal, the second revision can be performed. If signs of infection are identified during the surgery, the revision should be abandoned, and debridement and exclusion should be performed again.
Correct selection of revision prosthesis is the key to the success of the second phase of knee revision[29]. Ensuring prosthesis stability and maintaining joint stability are the basic principles for the selection of revision prosthesis. Because of bone defects, injury or loss of collateral ligaments, and other reasons, a special prosthesis system is often required for the second phase of knee revision. The ultimate goal of second-stage revision is to eradicate infection and rebuild a painless, functional, and stable knee. On the premise of achieving soft tissue balance and optimal joint stability, knee prosthesis with less limitation should be selected as much as possible. In the case of good bone structure and mild bone defect, the use of a prosthesis with less limitation can lead to effective load sharing in surrounding soft tissues, reduce prosthesis wear and loosening, and improve the success rate of revision[30]. When the bone structure is poor, the bone defect is obvious, and the stability of the prosthesis is poor. The stress on the contact surface of the prosthesis can be dispersed maximally to the pulp cavity using the extender rod and spacer to increase the stability of the prosthesis. In the case of collateral ligament insufficiency, the LCCK prosthesis not only provides anterior-posterior stability but also controls the balance between varus and varus, partially compensating for the collateral ligament function. RH prosthesis should be used in cases of severe lateral collateral ligament dysfunction combined with severe bone defects or complete loss of stability of the knee joint. RH prosthesis has good internal stability and does not require balancing of soft tissues. A large prosthesis module can replace bone defects, and a long handle can disperse the interface stress of the prosthesis.
This study has some limitations. This is a retrospective study with a relatively small sample size. It is not a multicenter study with a large sample or a randomized controlled trial. Some patients had negative bacterial cultures, and antibiotic use was mainly empirical. The reliability and accuracy of the study should be further confirmed in future studies with large samples.
The two-stage revision surgery approach, which includes the first stage of thorough debridement, removal of prosthesis, antibiotic bone cement placeholder exclusion for 3–6 mo, three normal consecutive routine blood examination results for ESR and CRP, selection of a suitable prosthesis for the second-stage revision, and the use of a sufficient amount of a full course of sensitive antibiotics, is a reliable method for the treatment of PJI after TKA. This approach can effectively relieve pain, control infection, and preserve good joint function.
Periprosthetic joint infection (PJI) is a catastrophic complication after total knee arthroplasty (TKA).
This study aimed to retrospectively analyze 27 patients with PJI who were treated with one-stage debridement, implant removal, antibiotic bone cement spacer exclusion, and two-stage revision and compare their preoperative and follow-up results.
This study aimed to explore the clinical effect of one-stage debridement, implant removal, antibiotic bone cement spacer exclusion, and two-stage revision in the treatment of PJI after TKA.
Of 27 patients with PJI treated with two-stage revision surgery were analyzed retrospectively. The following outcomes were compared for changes between preoperative and last follow-up results: Erythrocyte sedimentation rate, C-reactive protein, visual analogue scale scores, Hospital for Special Surgery scores, knee range of motion, and infection cure rates.
Of the 27 patients, 26 were cured of the infection, whereas 1 case had an infection recurrence; the infection control rate was 96.3%.
Two-stage revision surgery can effectively relieve pain, control infection, and retain good joint function in the treatment of PJI after TKA.
Whether the PJI can be cured using the one-stage debridement, implant removal, antibiotic bone cement spacer exclusion, and two-stage revision.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abulsoud MI, Egypt; Torres RM, Portugal S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Koh CK, Zeng I, Ravi S, Zhu M, Vince KG, Young SW. Periprosthetic Joint Infection Is the Main Cause of Failure for Modern Knee Arthroplasty: An Analysis of 11,134 Knees. Clin Orthop Relat Res. 2017;475:2194-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 2. | Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2193] [Cited by in RCA: 2168] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 3. | Visperas A, Santana D, Klika AK, Higuera-Rueda CA, Piuzzi NS. Current treatments for biofilm-associated periprosthetic joint infection and new potential strategies. J Orthop Res. 2022;40:1477-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Wang K, Li W, Liu H, Yang Y, Lv L. Progress in Prevention, Diagnosis, and Treatment of Periprosthetic Joint Infection. Evid Based Complement Alternat Med. 2021;2021:3023047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A Suppl 1:S75-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Negus JJ, Gifford PB, Haddad FS. Single-Stage Revision Arthroplasty for Infection-An Underutilized Treatment Strategy. J Arthroplasty. 2017;32:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Nguyen M, Sukeik M, Zahar A, Nizam I, Haddad FS. One-stage Exchange Arthroplasty for Periprosthetic Hip and Knee Joint Infections. Open Orthop J. 2016;10:646-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J. 2015;97-B:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 9. | Masters JP, Smith NA, Foguet P, Reed M, Parsons H, Sprowson AP. A systematic review of the evidence for single stage and two stage revision of infected knee replacement. BMC Musculoskelet Disord. 2013;14:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Zahar A, Sarungi M. Diagnosis and management of the infected total knee replacement: a practical surgical guide. J Exp Orthop. 2021;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 684] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 12. | Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. 1983. J Bone Joint Surg Am. 2002;84:490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Luu A, Syed F, Raman G, Bhalla A, Muldoon E, Hadley S, Smith E, Rao M. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control. J Arthroplasty. 2013;28:1490-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Pangaud C, Ollivier M, Argenson JN. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019;4:495-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 15. | Costerton JW. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin Orthop Relat Res. 2005;7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 277] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 16. | Shoji MM, Chen AF. Biofilms in Periprosthetic Joint Infections: A Review of Diagnostic Modalities, Current Treatments, and Future Directions. J Knee Surg. 2020;33:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop. 2015;86:147-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 18. | Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010;34:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Goh GS, Parvizi J. Diagnosis and Treatment of Culture-Negative Periprosthetic Joint Infection. J Arthroplasty. 2022;37:1488-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | McLawhorn AS, Nawabi DH, Ranawat AS. Management of Resistant, Atypical and Culture-negative Periprosthetic Joint Infections after Hip and Knee Arthroplasty. Open Orthop J. 2016;10:615-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Tang Y, Zhao D, Wang S, Yi Q, Xia Y, Geng B. Diagnostic Value of Next-Generation Sequencing in Periprosthetic Joint Infection: A Systematic Review. Orthop Surg. 2022;14:190-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Prinz J, Schmid B, Zbinden R, Zingg PO, Uçkay I, Achermann Y, Bosshard PP. Fast and Sensitive Multiplex Real-Time Quantitative PCR to Detect Cutibacterium Periprosthetic Joint Infections. J Mol Diagn. 2022;24:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Inaoka T, Kitamura N, Sugeta M, Nakatsuka T, Ishikawa R, Kasuya S, Sugiura Y, Nakajima A, Nakagawa K, Terada H. Diagnostic Value of Advanced Metal Artifact Reduction Magnetic Resonance Imaging for Periprosthetic Joint Infection. J Comput Assist Tomogr. 2022;46:455-463. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Chisari E, Lin F, Fei J, Parvizi J. Fungal periprosthetic joint infection: Rare but challenging problem. Chin J Traumatol. 2022;25:63-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Craig A, King SW, van Duren BH, Veysi VT, Jain S, Palan J. Articular spacers in two-stage revision arthroplasty for prosthetic joint infection of the hip and the knee. EFORT Open Rev. 2022;7:137-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Guild GN 3rd, Wu B, Scuderi GR. Articulating vs. Static antibiotic impregnated spacers in revision total knee arthroplasty for sepsis. A systematic review. J Arthroplasty. 2014;29:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Romanò CL, Gala L, Logoluso N, Romanò D, Drago L. Two-stage revision of septic knee prosthesis with articulating knee spacers yields better infection eradication rate than one-stage or two-stage revision with static spacers. Knee Surg Sports Traumatol Arthrosc. 2012;20:2445-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Kong L, Mei J, Ge W, Jin X, Chen X, Zhang X, Zhu C. Application of 3D Printing-Assisted Articulating Spacer in Two-Stage Revision Surgery for Periprosthetic Infection after Total Knee Arthroplasty: A Retrospective Observational Study. Biomed Res Int. 2021;2021:3948638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37:1279-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |