Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13129
Peer-review started: October 20, 2022
First decision: October 28, 2022
Revised: November 8, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 16, 2022
Processing time: 55 Days and 0.3 Hours
Hepatocellular carcinoma (HCC) can occasionally develop with other non-HCC cell types, either in a combined type or collision type. A collision tumor is defined as two histopathologically distinct tumors of the same organ lacking a clear transition zone. Hepatic collision tumors are rare. Among them, “hepatocellular carcinoma-hepatic neuroendocrine carcinoma” (HCC-NEC) collision tumors are especially rare and information about them is rarely published.
A 48-year-old man with typical findings of HCC underwent consecutive therapies, including radiofrequency ablation and embolization prior to resection. Diagnosis of the HCC-NEC collision tumor in the right liver and another HCC in the left liver was established following surgical resection. The patient displayed NEC metastasis following resection and succumbed to septicemia after 2 more rounds of chemotherapy. To our knowledge, this is the 25th reported case of mixed HCC-NEC tumor. The rarity of HCC-NEC collision tumors and the absence of diagnostic criteria make it difficult to differentiate this condition from simple liver tumors, especially in patients with chronic liver disease.
Our case highlights the difficulty in accurately diagnosing HCC-NEC in the absence of histological evidence. The prognosis is poor for this condition, although ultrasound-guided liver biopsy can be helpful to establish a prompt diagnosis. Further accumulation of such cases could help establish an accurate diagnosis earlier. Early discovery of NEC may allow for better treatment strategies and better prognoses.
Core Tip: Collision tumors of the liver are not common. Coexisting hepatocellular carcinomas (HCCs) and neuroendocrine carcinomas (NECs) with collision tumor patterns are extremely uncommon. Herein, we report a case of an HCC-NEC collision tumor of the liver. Definite diagnosis is usually difficult until pathological confirmation. The prognosis is poor. Early discovery of NEC may allow better treatment strategies.
- Citation: Jeng KS, Huang CC, Chung CS, Chang CF. Liver collision tumor of primary hepatocellular carcinoma and neuroendocrine carcinoma: A rare case report. World J Clin Cases 2022; 10(35): 13129-13137
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13129.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13129
Hepatocellular carcinoma (HCC) can occasionally develop with other non-HCC cell types in a mosaic arrangement, either in the combined type or in the collision type. A collision tumor is defined as two histopathologically distinct tumors of the same organ that lack a clear transition zone[1-3]. However, in a combined tumor, both types of tumors intermingle with each other without a clear separation. Hepatic collision tumors are extremely rare[1-4]. Most hepatic collision tumors are composed of HCC and cholangiocarcinoma. HCC and neuroendocrine carcinoma (NEC) present in the liver without a clear transition zone (the so-called HCC-NEC collision tumor) are extremely rare[1-5]. The following case report details our experience treating a patient with an HCC-NEC collision tumor, along with a review of the available literature.
Liver tumors were detected incidentally during a regular liver ultrasound examination in a 48-year-old male patient without symptoms or complaints.
Cirrhosis of the liver as well as two hepatic tumors (3 cm and 2.5 cm, both located in Couinaud’s hepatic segment 8) were discovered incidentally in March 2016 during a regular ultrasound examination.
A 48-year-old male patient was on entecavir (baraclude) antiviral therapy for a number of years for hepatitis B viral infection.
There was no family history of liver disease.
The patient had no symptoms of jaundice, abdominal discomfort, or weight loss and had not received any treatment for the issue prior to admission. The abdomen was soft without any palpable masses or ascites. Sclera was not icteric.
Laboratory results revealed serum alpha fetoprotein (AFP) elevated to 29.91 ng/mL (normal < 7.0 ng/mL). HCC was suspected without tissue evidence.
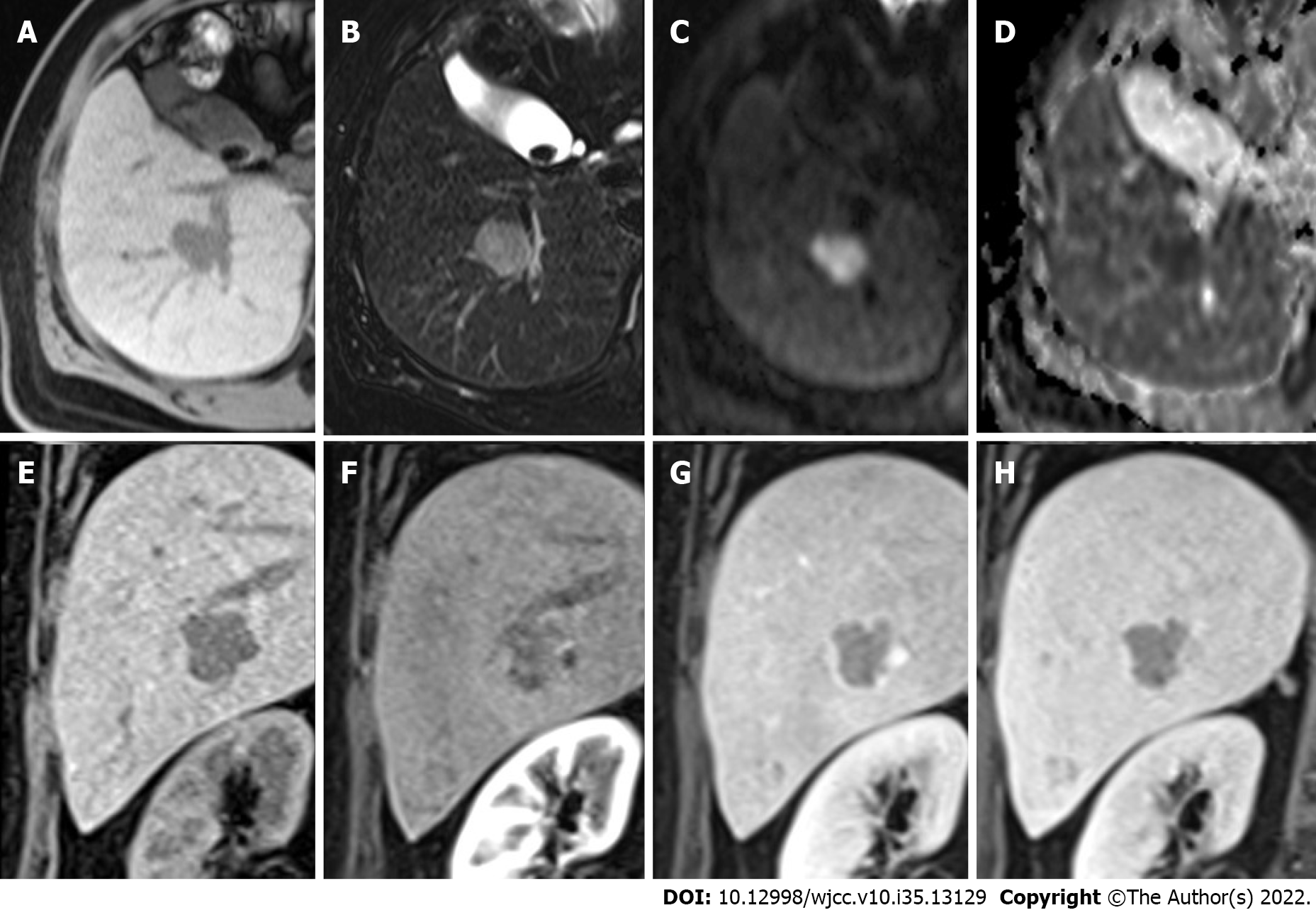
Multiple hepatic nodules at the right hepatic lobe and left hepatic tip were recognized. The representative larger well-defined lesion, 2.5 cm in size, has obvious hypo-intensity on the fat-suppressed T1WI (Figure 1A), hyperintensity on the fat-suppressed T2WI (Figure 1B), significant diffusion restriction with hyperintensity on the diffusion-weighted imaging (Figure 1C) and dark signal on the ADC (apparent diffusion coefficient) map at the corresponding site (Figure 1D), and early hyperenhancement & rapid washout (Figure 1E-H).
Liver collision tumor of primary HCC and NEC.
Ultrasound-guided percutaneous radiofrequency ablation (RFA) was performed on the two lesions in June 2016. One month later, a follow-up liver triphase computed tomography (CT) scan revealed a recurrent hepatic tumor in the left liver (segment 2). Serum AFP was 22.53 ng/mL, and left HCC was suspected.
The patient was prepared for liver transplantation, but no donor was available, and consecutive treatments were therefore given. Transcatheter hepatic arterial chemoembolization with 25 mg doxorubicin loaded in a Hepasphere microsphere was performed once in September 2016, but a follow-up triphase CT scan showed recurrence of liver tumors in segments 2 and 7 in December 2018.
Ultrasound-guided RFA followed by percutaneous ethanol tumor injection (8 mL) at the segment 7 lesion was performed without complications in January 2019. Two weeks later, diagnostic celiac arteriography revealed a large tumor (> 5 cm) located in segment 7 and a smaller tumor located in segment 2. Surgical resection was recommended after consultation with surgeons. The patient then underwent surgical intervention, including cholecystectomy, right hepatectomy (for a large tumor of segment 7), and wedge resection of the segment 2 lesion via intraoperative ultrasound guidance. The perioperative course was uneventful, and the patient was discharged on postoperative Day 14.
Pathological findings revealed a right lobe tumor 5.7 cm × 5 cm × 4.5 cm in size with free resection margins (Figure 2A). The right hepatic lesion was determined to be an HCC-NEC collision tumor composed of a poorly differentiated large NEC with a small amount of HCC tissue after pathological examination (Figure 2B).
Microscopy of the collision tumor revealed organoid nesting NEC tissue with trabecular, frequent, rosette-like structures suggesting atypical nuclear molding, enlarged nuclei, and “salt and pepper” chromatin (Figure 2B). The overall mitotic count was high (> 20/10 HPF), and tumor necrosis and lymphovascular/perineural invasion were observed.
Immunohistochemical analysis revealed the presence of synaptophysin (Figure 2C) and chromogranin A (Figure 2D) in the primary NEC tumor region. CK7, Hepa-1, arginase-1, and CD34 were negative. The smaller HCC region of the collision tumor demonstrated sinusoidal capillarization highlighted by CD34 and focal immunoreactivity for CK7 negative for Hepa-1, arginase-1, synaptophysin (Figure 2D), and chromogranin A (Figure 2E). pTNM staging of NEC tissue was categorized as pT3 (according to the American Joint Committee on Cancer, AJCC 8th Edition) given the presence of vascular and perineural infiltration.
Regarding the segment 2 (of the left lobe) lesion 1.6 cm × 1 cm × 0.7 cm in size (Figure 2F), pathological findings revealed poorly differentiated HCC. No coexisting NEC tissue was found. The resection margin was free.
Modified hepatic activity index (HAI) grading with necroinflammatory scoring of liver pathologies revealed hepatitis of the periportal and periseptal interface (piecemeal necrosis) with mild to moderate portal inflammation. The total modified HAI score was 4/18, and modified Ishak fibrosis staging noted marked bridging with occasional nodules, suggesting incomplete cirrhosis.
During postoperative follow-up, CT and positron emission tomography-CT (PET-CT) scans performed in March 2019 showed metastatic lymphadenopathy of the peripancreatic and para-aortic regions. Endoscopic ultrasound -guided fine needle aspiration of the lymph nodes was performed, and pathological findings revealed metastatic NEC. Two successive rounds of chemotherapy for NEC were given on April 8, 2019 and May 9, 2019. However, a CT scan performed in July 2019 showed the progression of abdominal and thoracic lymphadenopathy.
The patient survived until November 2019, ultimately succumbing to septicaemia and multiple organ failure.
Both collision and combined tumors are uncommon in the liver[1-5]. A collision tumor differs from a combined tumor in that collision tumors involve two tumor types that exist contiguously and without clear delineation[1-3]. Among all primary hepatic malignancies, the incidence of combined tumors is estimated to range from 2.0% to 3.6%[4], while combined tumors are postulated to arise from the same stem cells differentiating into distinct cancers, with the most common combined tumor being hepatocholangiocarcinoma (HCC and cholangiocarcinoma)[1,4]. The incidence of collision-type tumors is even rarer, ranging from 0.1% to 1% among primary liver malignancies[1]. Among primary liver collision tumors, HCC-cholangiocarcinoma, HCC–sarcoma, and HCC–NEC have been reported[1].
In our patient, HCC and NEC coexisted without clear delineation grossly (Figure 2) but were distinguishable by both microscopic features while lacking a focal transition (Figure 2). Moreover, there was no immunoreactivity for the neuroendocrine marker CD56 in the HCC zone. We therefore diagnosed the tumor as HCC-NEC.
Primary NEC in the liver is rare. Usually, NEC presents as metastasis to the liver from other organs. NECs are well known to arise in the pancreas and other extrahepatic organs. However, in this case, neither pre- nor postoperative imaging, including PET-CT, could detect tumors in the pancreas or elsewhere. We therefore regard liver NEC as the primary tumor.
Hepatic HCC-NEC is extremely rare. Only 24 of these tumors (2 female/22 male, ages 43 to 84 (median age 68)) have been reported, including 9 collision tumors, 14 combined tumors, and 1 combined plus collision tumor (Table 1)[1-20].
| Age | Symptoms | Virus | AFP | Diagnosis2 | Type | Therapy | Time1 (Mon) | Status | Ref. | |
| 1 | 43 | Abdominal swelling | HBV | NA | Autopsy2 | Combined | Adriamycin, 5-FU | 26 | Death | Barsky et al[8] |
| 2 | 69 | Abdominal pain | HBV | NA | FNA2 | Combined | NA | NA | NA | Artopoulos et al[10] |
| 3 | 63 | Abdominal pain, jaundice | NA | NA | Resection | Combined | NA | 1 | Death(bleeding spesis) | Vora et al[11] |
| 4 | 72 | NA | HCV | 13.6 | Resection, LND | Collision | NA | NA | NA | Ishida et al[2] |
| 5 | 71 | NA | HCV | 20.7, 479.1 | Resection | Combined (intermingled) | NA | 5 | NA | Yamaguchi et al[6] |
| 6 | 50 | NA | HCV | 1191 | Core biopsy, then Resection | Collision | TACE, cisplatin, doxorubicin, thalidomide and Avastin | 16 | alive | Garcia et al[1] |
| 7 | 65 | Epigastric pain | HBV | Normal | Resection, LND | Combined | NA | 12 | Death | Yang et al[7] |
| 8 | 68 | NA | HBV | 1191 | Resection | Collision | Cisplatin, etoposide | 28 | Alive | Tazi et al[4] |
| 9 | 76 | Echo-detected | HCV | 281.4, 14.9, 2632 (after TACE) | TACE, resection | Combined NE with sarcomatous change | TACE, epirubicin lipiodol | 17 | Death | Nakanishi et al[5] |
| 10 | 51 | Abdominal pain | HCV | NA | Biopsy | Combined | NA | 1 | Death | Hammedi et al[12] |
| 11 | 56 | Abdominal distension | NA | 70 | Resection | Combined | NA | 6 | Alive | Aboelenen et al[13] |
| 12 | 72 | No symptoms | HCV | 24.8 | Resection | Collision | Cisplatin, etoposide | 2 | Death | Nishino et al[14] |
| 13 | 72 | No symptoms | HCV | 3.8 | Resection | Collision | Cisplatin, etoposide | 10 | Alive | Choi et al[15] |
| 14 | 76 | NA | NA | 49.2 | Resection | Collision | Cisplatin | NA | Alive | Baker et al[9] |
| 15 | 71 | NA | HCV | 4791 | Resection | Combined | NA | 8.6 | Death | Nomura et al[16] |
| 16 | 71 | NA | HCV | 10.3 | Resection | Collision | NA | 2.6 | Death | Nomura et al[16] |
| 17 | 58 | NA | HBV | 176.4 | RFA, Resection | Combined | NA | 19.7 | Alive | Nomura et al[16] |
| 18 | 50 | NA | HBV | 473.7 | Resection | Combined | NA | 19.5 | Alive | Nomura et al[16] |
| 19 | 63 | NA | HCV | 2276 | Resection | Combined | NA | 24 | Alive | Nomura et al[16] |
| 20 | 65 | Abdominal discomfort | HCV | 400.06 | Resection | Collision | NA | 1.3 | Death | Liu et al[17] |
| 21 | 70 | Solid mass | HCV | 3.7 | TAE and TPE, Resection | Collision and combined | NA | 3 | Death | Okumura et al[18] |
| 22 | 56 | Incidental | NA | 2.8 | Liver transplantation (Resection specimen) | Collision | NA | 10 | Alive | Yılmaz et al[3] |
| 23 | 79 | Abnormal liver function | NA | 3231.8 | Resection | Combined | NA | 4 | Death | Ikeda et al[19] |
| 24 | 84 | Higher AFP values | NA | 399 | Laparoscopic resection | Combined | NA | 9 | Alive | Nakano et al[20] |
| 25 | 48 | Incidental | HBV | 29.91 | RFA, TACE, RFA, Resection | Collision | Chemotherapy | 40 | Death (sepsis) | Our study |
The coexistence of an HCC-NEC collision tumor (right liver) and a pure HCC tumor (left liver) in one patient is especially rare. The contributing factor of HCC could be attributed to hepatitis B and cirrhosis. However, these two coexisting tumors with different characteristics are difficult to ascribe.
Making an accurate preoperative diagnosis for such an uncommon disease without histological evidence is difficult, and this patient was diagnosed using surgically obtained tissue samples. Although the serum marker AFP is valuable in ruling out HCC, it cannot rule out HCC-NEC comorbidity. Of the remaining 24 case reports described in the literature, none were able to establish an accurate diagnosis using only preoperative imaging (Table 1).
The prognosis for HCC-NEC is poor, although this is based on a small number of reported cases[4]. The majority of HCC-NEC cases described in the literature resulted in mortality (Table 1). Among these 24 reported cases, 20 underwent resection[1-20], and 1 received a hepatectomy with a liver transplant[3]. Most cases are either fatal or result in recurrence[8,10,12]. Long-term survival only occurred in the patient undergoing liver transplantation and in two receiving partial hepatectomy (Table 1), suggesting that liver transplantation is effective for HCC-NEC.
HCCs with NEC components usually present aggressive behavior and dismal prognosis[15,17]. Although both primary NEC and HCC are malignant, aggressive activity is driven by primary hepatic NEC[6,10,17]. The poor prognosis could be attributed to the coexistence of primary NEC of the liver rather than HCC. Yamaguchi et al[6] emphasized elevated expression of p53 and a higher Ki-67 proliferation index in the NEC zone than in the HCC zone, suggesting that NEC exhibits strong malignant behavior. Metastatic tumors usually only arise from the NEC portion of tumor. In our patient, NEC metastasis and growth proceeded soon after hepatic resection despite chemotherapy.
The cellular origins of NEC in NEC-HCC remain elusive[7]. Some researchers have proposed that NEC originates either from the ectopic pancreas[5] or from neuroendocrine cells in the intrahepatic bile duct epithelium[21]. In the patient under discussion, preoperative imaging found no ectopic pancreatic tissue proximal to the tumor. Furthermore, the tumor was negative for CK7, usually expressed by the bile duct epithelium. However, based on the literature, two further hypotheses can be put forward to explain the origin of the tumor in this patient. First, hepatic stem cells may have undergone a malignant transformation, as in other case studies[8,17,21]. Hepatic progenitor cells present in the epithelial lining of the intrahepatic bile ducts can be the origin of NECs. NECs can manifest in the liver as isolated carcinoids or high-grade small-cell carcinomas in noncirrhotic livers[4]. Nevertheless, the majority of primary hepatic NEC patients do not survive past 1 year, regardless of tumor resection[2,7]. With HCC, superior 1-year and 5-year survival outcomes are better following hepatectomy. Furthermore, Baker et al[9] recently published a case study where both components of a mixed HCC/NEC tumor shared a mutation in the CTNNB1 gene (S33F located at exon 3), suggesting that they might have derived from the same cellular origin. The second hypothesis states that pluripotent stem cells become precursors to HCC, neuroendocrine malignancy, and other tumors with polyphenotypic expression[2,6]. This hypothesis is supported by the existence of HCCs possessing neuroendocrine features[21]. Zhao et al[21] found neuroendocrine differentiation in 60% of HCC patients. Moreover, there is a discrepancy between this high rate and the rarity of primary NECs in the liver. If an underdeveloped HCC clone experiences neuroendocrine differentiation, the result could be an NEC that completely replaces the HCC[2,7].
This case report allowed us to review an HCC-NEC collision tumor. To our knowledge, this is the 25th reported case of HCC-NEC. One HCC-NEC collision tumor and another HCC coexisting in one patient (such as our patient) are especially rare. We have detailed a case report involving an HCC-NEC type collision tumor in a male patient who developed NEC metastasis despite repeated interventions. The rarity of HCC-NEC collision tumors and the absence of diagnostic criteria make it difficult to differentiate this condition from simple liver tumors, especially in patients with chronic liver disease. Our patient highlights the difficulty in accurately diagnosing HCC-NEC in the absence of histological evidence. The prognosis is poor for this condition, although ultrasound-guided liver biopsy could be helpful in establishing a prompt diagnosis. The collection of data from more combined or collision HCC-NEC patients in the future may improve the diagnostic details and early treatment. Early discovery of NEC may allow for better treatment strategies and better prognoses.
We would like to thank for the support of the Core Laboratory and the human Biobank of Far Eastern Memorial Hospital.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Limaiem F, Tunisia; Yan YL, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Garcia MT, Bejarano PA, Yssa M, Buitrago E, Livingstone A. Tumor of the liver (hepatocellular and high grade neuroendocrine carcinoma): a case report and review of the literature. Virchows Arch. 2006;449:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Ishida M, Seki K, Tatsuzawa A, Katayama K, Hirose K, Azuma T, Imamura Y, Abraham A, Yamaguchi A. Primary hepatic neuroendocrine carcinoma coexisting with hepatocellular carcinoma in hepatitis C liver cirrhosis: report of a case. Surg Today. 2003;33:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Yılmaz DB, Bayramoğlu Z, Ünay G, Ayık E, Başsorgun Cİ, Elpek GÖ. Incidental Collision Tumor of Hepatocellular Carcinoma and Neuroendocrine Carcinoma. J Clin Transl Hepatol. 2018;6:339-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Tazi EM, Essadi I, M'rabti H, Errihani H. Hepatocellular Carcinoma and High Grade Neuroendocrine Carcinoma: A Case Report and Review of the Literature. World J Oncol. 2011;2:37-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Nakanishi C, Sato K, Ito Y, Abe T, Akada T, Muto R, Sakashita K, Konno T, Kato H, Satomi S. Combined hepatocellular carcinoma and neuroendocrine carcinoma with sarcomatous change of the liver after transarterial chemoembolization. Hepatol Res. 2012;42:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Yamaguchi R, Nakashima O, Ogata T, Hanada K, Kumabe T, Kojiro M. Hepatocellular carcinoma with an unusual neuroendocrine component. Pathol Int. 2004;54:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yang CS, Wen MC, Jan YJ, Wang J, Wu CC. Combined primary neuroendocrine carcinoma and hepatocellular carcinoma of the liver. J Chin Med Assoc. 2009;72:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Barsky SH, Linnoila I, Triche TJ, Costa J. Hepatocellular carcinoma with carcinoid features. Hum Pathol. 1984;15:892-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Baker E, Jacobs C, Martinie J, Iannitti DA, Vrochides D, Swan RZ. Mixed Hepatocellular Carcinoma, Neuroendocrine Carcinoma of the Liver. Am Surg. 2016;82:1121-1125. [PubMed] |
| 10. | Artopoulos JG, Destuni C. Primary mixed hepatocellular carcinoma with carcinoid characteristics. A case report. Hepatogastroenterology. 1994;41:442-444. [PubMed] |
| 11. | Vora IM, Amarapurkar AD, Rege JD, Mathur SK. Neuroendocrine differentiation in hepatocellular carcinoma. Indian J Gastroenterol. 2000;19:37-38. [PubMed] |
| 12. | Hammedi F, Rammah S, Trabelsi A, Bdioui A, Jomaa W, Anjorin A, Bakir D, Mokni M. Carcinome hépatocellulaire avec composante neuroendocrine: à propos d’un cas. Journal Africain du Cancer / African Journal of Cancer. 2012;4:120-123. [DOI] [Full Text] |
| 13. | Aboelenen A, El-Hawary AK, Megahed N, Zalata KR, El-Salk EM, Fattah MA, Sorogy ME, Shehta A. Right hepatectomy for combined primary neuroendocrine and hepatocellular carcinoma. A case report. Int J Surg Case Rep. 2014;5:26-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Nishino H, Hatano E, Seo S, Shibuya S, Anazawa T, Iida T, Masui T, Taura K, Haga H, Uemoto S. Histological features of mixed neuroendocrine carcinoma and hepatocellular carcinoma in the liver: a case report and literature review. Clin J Gastroenterol. 2016;9:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Choi GH, Ann SY, Lee SI, Kim SB, Song IH. Collision tumor of hepatocellular carcinoma and neuroendocrine carcinoma involving the liver: Case report and review of the literature. World J Gastroenterol. 2016;22:9229-9234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Nomura Y, Nakashima O, Akiba J, Ogasawara S, Fukutomi S, Yamaguchi R, Kusano H, Kage M, Okuda K, Yano H. Clinicopathological features of neoplasms with neuroendocrine differentiation occurring in the liver. J Clin Pathol. 2017;70:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Liu YJ, Ng KF, Huang SC, Wu RC, Chen TC. Composite hepatocellular carcinoma and small cell carcinoma with early nodal metastasis: A case report. Medicine (Baltimore). 2017;96:e7868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Okumura Y, Kohashi K, Wang H, Kato M, Maehara Y, Ogawa Y, Oda Y. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma with aggressive biological behavior (adverse clinical course): A case report. Pathol Res Pract. 2017;213:1322-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ikeda A, Aoki K, Terashima T, Itokawa Y, Kokuryu H. A fat containing combined neuroendocrine carcinoma and hepatocellular carcinoma in the liver: A case report. Ann Hepatol. 2021;22:100183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Nakano A, Hirabayashi K, Yamamuro H, Mashiko T, Masuoka Y, Yamamoto S, Ozawa S, Nakagohri T. Combined primary hepatic neuroendocrine carcinoma and hepatocellular carcinoma: case report and literature review. World J Surg Oncol. 2021;19:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Zhao M, Laissue JA, Zimmermann A. "Neuroendocrine" differentiation in hepatocellular carcinomas (HCCs): immunohistochemical reactivity is related to distinct tumor cell types, but not to tumor grade. Histol Histopathol. 1993;8:617-626. [PubMed] |