Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13122
Peer-review started: October 11, 2022
First decision: November 2, 2022
Revised: November 10, 2022
Accepted: November 24, 2022
Article in press: November 24, 2022
Published online: December 16, 2022
Processing time: 64 Days and 5.1 Hours
Hypoxia-inducible factor prolyl hydroxylase inhibitor is a new class of drugs for treating renal anemia. It is a second-generation hypoxia-inducible factor prolyl hydroxylase-2 (PHD2) inhibitor. Roxadustat can effectively increase hemoglobin in patients with dialysis-dependent chronic kidney disease, with an adverse events profile comparable to that of epoetin alfa. We administered roxadustat to a maintenance hemodialysis patient who was allergic to erythropoiesis-stimulating agents (ESAs) and depended on blood transfusion for five years. After applying Roxadustat, the patient’s anemia improved significantly.
A 77-year-old Chinese man had type 2 diabetes for 16 years, underwent maintenance hemodialysis for five years, and had fatigue for five years. Laboratory tests showed severe anemia (hemoglobin concentration of 42 g/L). The patient was administered a subcutaneous injection of ESAs before dialysis. He suffered an allergic shock immediately and fainted. His blood pressure dropped to undetectable levels. He was not administered ESAs henceforth. The patient was prescribed iron supplements and received blood transfusions occasionally for five years. His hemoglobin concentration ranged from 42-68 g/L. After taking six weeks of oral roxadustat three times weekly (100 mg TIW), the patient’s hemoglobin concentration increased significantly, and his symptoms decreased. We adjusted the doses of roxadustat, and the hemoglobin concentration was maintained between 97 and 126 g/L.
Oral roxadustat is effective in treating anemia in maintenance hemodialysis patients who cannot be administered ESAs.
Core Tip: Anemia of dialysis-dependent chronic kidney disease is commonly treated with erythropoiesis-stimulating agents (ESAs), along with iron supplementation; however, in some cases, patients cannot be administered erythropoiesis-stimulating agents. Roxadustat is a newly developed drug for renal anemia treatment. It is an oral hypoxia-inducible factor-prolyl hydroxylase inhibitor that stimulates erythropoiesis and regulates iron metabolism. In this study, we presented a case in which Roxadustat was administered for the treatment of a patient allergic to ESAs. This was the first case where roxadustat was administered to improve anemia in a patient allergic to ESAs and on maintenance hemodialysis.
- Citation: Xu C, Luo DG, Liu ZY, Yang D, Wang DD, Xu YZ, Yang J, Fu B, Qi AR. Response to roxadustat in a patient undergoing long-term dialysis and allergic to erythropoiesis-stimulating agents: A case report . World J Clin Cases 2022; 10(35): 13122-13128
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13122
Globally, about 2 million patients with chronic kidney disease (CKD) receive renal replacement therapy every year[1]. Anemia is a common complication of chronic kidney disease that increases in prevalence with the progression of the disease[2,3]. It is associated with a poor quality of life and an increase in transfusion, hospitalization, and mortality rates[4,5]. Patients with renal anemia treated with erythropoiesis-stimulating agents (ESAs) may suffer from increased hypertension, stroke, and adverse cardiovascular events, probably associated with the non-physiological increase in erythropoietin[6]. Hypoxia-inducible factor (HIF) prolyl hydroxylase inhibitor (PHI) is a new class of drugs for treating renal anemia. It is a second-generation hypoxia-inducible factor prolyl hydroxylase-2 (PHD2) inhibitor[7]. Roxadustat can effectively increase hemoglobin (Hb) in patients with dialysis-dependent chronic kidney disease, with an adverse events (AE) profile comparable to that of epoetin alfa[8]. Here, we described the case of a dialysis-dependent chronic kidney disease patient with refractory renal anemia who was allergic to ESAs and required blood transfusions for five years. He was then administered Roxadustat therapy. Finally, the patient’s anemia improved significantly and he did not need further blood transfusions.
The patient complained about general fatigue and poor appetite.
The 77-year-old male patient was suffering from type 2 diabetes for 16 years and maintained hemodialysis for five years. He was found to suffer from an allergic shock when he was injected with ESAs subcutaneously. His Hb level fluctuated between 42 and 68 g/L. He complained of general fatigue, which prevented him from even walking. He had a poor appetite. The patient was administered oral iron supplements and underwent blood transfusions occasionally.
The medical history of the patient included type 2 diabetes, which was controlled by insulin, and hypertension, which was controlled by metoprolol and nifedipine.
His personal history and family history were unremarkable.
The vital signs of the patient were as follows: Body temperature, 36.5°C; blood pressure, 126/59 mmHg; pulse rate, 72 beats/min; respiratory rate, 20 breaths/min. His eyelid conjunctiva was pale, his heart rate was 72 beats/min, the rhythm was clear, and there was no obvious murmur.
The blood examination of the patient showed a hemoglobin concentration of 42 g/L, erythrocyte count of 1.29 × 109/L, hematocrit of 12.6%, serum ferritin level of 588.7 ng/mL, transferrin saturation of 20.9%, a serum iron level of 8.3 µmol/L, and an intact parathyroid hormone concentration of 14.6 ng/L.
Doppler: Both kidneys are small and the cortex of both kidneys is thin.
The patient was diagnosed with CKD stage 5, renal anemia, maintenance hemodialysis, type 2 diabetes, and hypertension.
On January 8, 2020, the hemoglobin concentration of the patient was 42 g/L. He received 400 mL of blood in the Emergency Room. His Hb concentration increased to 68 g/L. We then administered Roxadustat three times every week (100 mg TIW). His Hb concentration increased to 105 g/L after six weeks. Regular hemodialysis was performed thrice a week.
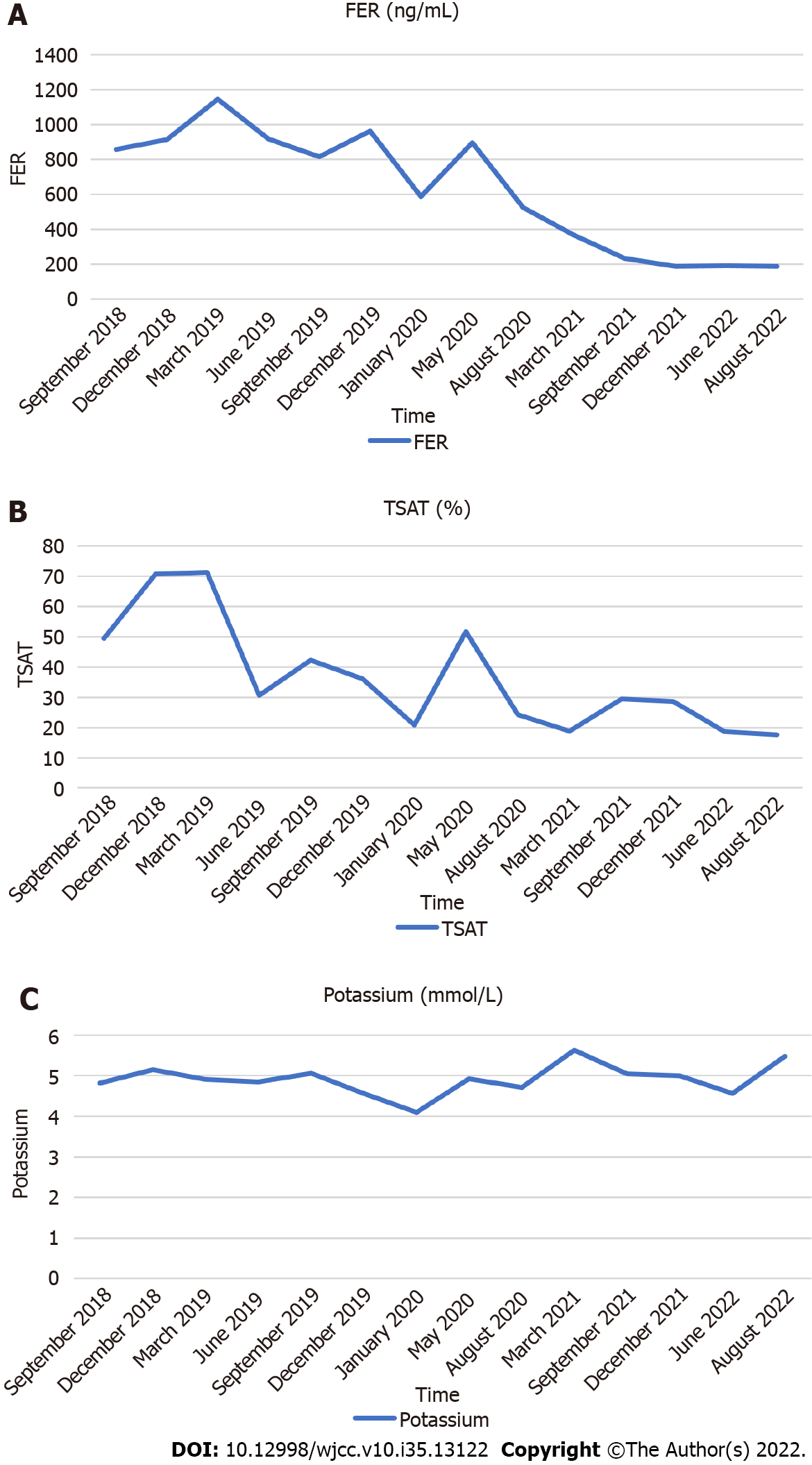
Before administering Roxadustat, the hemoglobin level of the patient was 42 g/L (Table 1). In total, the patient was infused 400 mL of red blood cell suspension, and his Hb level increased to 68 g/L. On the sixth week after starting Roxadustat, the Hb level of the patient rapidly increased to 105 g/L, and his hematocrit (HCT) also increased. The patient felt that his symptoms of fatigue were relieved, and his appetite improved. In the next 29 mo, we adjusted the dose of roxadustat, and the Hb concentration was maintained between 105 and 126 g/L (Figure 1A). His HCT also increased (Figure 1A). No more blood transfusions were performed. The dosage was adjusted between 100 mg (50 mg BIW) to 300 mg/time (100 mg TIW). We reduced the dose of Roxsdustat from 100 mg (50 mg BIW) to 50 mg (50 mg QW) when the hemoglobin (HGB) concentration reached 120 g/L on June 28, 2022. The HGB concentration dropped to 97 g/L after seven weeks. The patient is still under Roxadustat treatment (50 mg BIW). The patient stopped taking iron supplements after six weeks. His iron metabolism (Figures 2A and B) improved significantly. His serum ferritin (FER) and transferrin saturation (TSAT) increased to a peak value, and then, decreased and stabilized (Figures 2A and B). His blood lipid levels improved (Figure 1B), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and LDL-C: high-density lipoprotein cholesterol (HDL-C) were significantly lower than the levels recorded before, in the first four months. His total triglycerides (TG) also decreased initially, rebounded, and stabilized. Although his serum potassium level was slightly higher (Figure 2C), it was within the normal range. The patient needed blood transfusions no more, and the quality of his life improved substantially. He did not feel fatigued, his appetite improved, and he gained weight. His dry weight increased from 48.5 kg to 50.8 kg. We observed no AEs, such as nausea, vomiting, elevated blood pressure, or rash during the follow-up of 31 mo. After the administration of Roxadustat for one year, the patient reported mild hallucinations. On June 2022, his hallucinations aggravated. The reports of the magnetic resonance imaging examination showed encephalatrophy (Figure 3). The patient was administered olanzapine 2.5 mg qn by a neurologist, and the symptom was brought under control.
| Characteristics | ||
| Age | 77 | yr |
| Sex | Male | |
| Dry weight | 48.5 | kg |
| Blood pressure | 126/59 | mmHg |
| Dialysis method | Hemodialysis | |
| HGB | 68 | g/L |
| RBC | 2.13 | 1012/L |
| WBC | 5.56 | 109/L |
| Serum iron | 8.3 | µmol/L |
| FER | 588.7 | ng/mL |
| TSAT | 20.9 | % |
| K | 4.09 | mmol/L |
| PTH | 14.6 | ng/mL |
| Blood lipids | ||
| TG | 1.14 | mmol/L |
| TC | 3.99 | mmol/L |
| HDL-C | 0.84 | mmol/L |
| LDL-C | 2.49 | mmol/L |
HIF and PHI is a new class of drugs for treating renal anemia. The main effects of this drug include the stabilization of the transcription factor HIF, which regulates the expression of those genes that increase the endogenous levels of erythropoietin and hemoglobin while decreasing hepcidin and ferritin levels[9,10].
In a randomized, open-label, phase 3 SIERRAS trial, roxadustat was non-inferior to epoetin alfa for the treatment of anemia in patients with CKD who depended on dialysis and were receiving stable doses of ESAs[11].
Our patient was on hemodialysis for five years and was allergic to ESAs. No such case was reported before this study. The patient only took iron supplements and required occasional blood transfusion. His Hb level ranged from 42 to 68 g/L for five years. He always felt so tired that he could not even walk. However, his anemia improved significantly after the administration of Roxadustat for six weeks and his Hb level reached the target value. The patient needed blood transfusions no more, and the quality of his life improved substantially. He did not feel fatigued, his appetite improved, and he gained weight. Additionally, he was sensitive to the dose of Roxadustat. When we reduced the dose to 50 mg QW, his Hb level dropped to 97 g/L.
Chen et al[12] found that changes in iron biomarker levels were higher with roxadustat than with epoetin alfa. Our patient’s FER and TSAT increased initially, and then, decreased and stabilized. We considered it to be related to an increase in inflammatory status and iron utilization.
In the HIMALAYAS study, the levels of all components of fractionated lipid measures decreased with roxadustat vs epoetin alfa, including LDL-C and HDL-C, which resulted in an overall improvement in the LDL-C: HDL-C ratio[13]. The blood lipid levels, including TG, TC, LDL-C, and the LDL-C: HDL-C ratio were also lower than before. Our findings were similar to those of previous studies. One mechanism hypothesized that lower cholesterol is the hypoxia-induced induction of an insulin-induced gene. It can stimulate the degradation of hydroxymethylglutaryl coenzyme A reductase[14].
In a double-blind trial that compared the effects of roxadustat to those of placebo in patients with chronic kidney disease not undergoing dialysis, hyperkalemia and metabolic acidosis were reported more frequently in the roxadustat group[15]. In our case, serum potassium increased slightly, but it remained within the normal range.
Roxadustat was well-tolerated, and our data showed that the safety profile of this treatment method extended beyond the 26-week treatment period conducted in phase 3 studies in China[12,15]. In two phase 3 studies conducted with Japanese patients suffering from anemia and dialysis-dependent CKD, the most common treatment-emergent AEs with roxadustat included back pain, nasopharyngitis, vomiting, and diarrhea[16,17]. However, no adverse effects were observed in this case. The patient reported having hallucinations after taking Roxadustat for one year, but we did not consider it to be related to the drug.
Roxadustat is a newly developed drug that can improve renal anemia effectively. Oral roxadustat is a good treatment option for patients undergoing hemodialysis, especially for those who are allergic to ESAs. Although the drug is effective and tolerable, further studies are required to monitor possible adverse events.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shirini K, Iran; Yorioka N, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 981] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 2. | Becker K, Saad M. A New Approach to the Management of Anemia in CKD Patients: A Review on Roxadustat. Adv Ther. 2017;34:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting Hypoxia-Inducible Factors for the Treatment of Anemia in Chronic Kidney Disease Patients. Am J Nephrol. 2017;45:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 4. | Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH. Effect of anaemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic kidney disease. Nephrology (Carlton). 2009;14:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Lawler EV, Bradbury BD, Fonda JR, Gaziano JM, Gagnon DR. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol. 2010;5:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | McCullough PA, Barnhart HX, Inrig JK, Reddan D, Sapp S, Patel UD, Singh AK, Szczech LA, Califf RM. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol. 2013;37:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Del Vecchio L, Locatelli F. Roxadustat in the treatment of anaemia in chronic kidney disease. Expert Opin Investig Drugs. 2018;27:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Fishbane S, El-Shahawy MA, Pecoits-Filho R, Van BP, Houser MT, Frison L, Little DJ, Guzman NJ, Pergola PE. Roxadustat for Treating Anemia in Patients with CKD Not on Dialysis: Results from a Randomized Phase 3 Study. J Am Soc Nephrol. 2021;32:737-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 9. | Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31:2448-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Sanghani NS, Haase VH. Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience. Adv Chronic Kidney Dis. 2019;26:253-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 11. | FibroGen. FibroGen announces positive topline results from three global phase 3 trials of roxadustat for treatment of anemia in patients with chronic kidney disease: primary efcacy end points met in all three studies, non-dialysis, incident dialysis, and stable dialysis studies [media release]. 2018. Available from: http://investor.fbrogen.com/phoenix.zhtml?c=253783&p=irol-newsArticle_print&ID=2381297. |
| 12. | Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Cai GY, Hao L, Leong R, Liu C, Neff T, Szczech L, Yu KP. Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis. N Engl J Med. 2019;381:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 13. | Provenzano R, Shutov E, Eremeeva L, Korneyeva S, Poole L, Saha G, Bradley C, Eyassu M, Besarab A, Leong R, Liu CS, Neff TB, Szczech L, Yu KP. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant. 2021;36:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, DeBose-Boyd RA. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292:9382-9393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KP. Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med. 2019;381:1001-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 408] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 16. | Akizawa T, Iwasaki M, Yamaguchi Y, Majikawa Y, Reusch M. Phase 3, Randomized, Double-Blind, Active-Comparator (Darbepoetin Alfa) Study of Oral Roxadustat in CKD Patients with Anemia on Hemodialysis in Japan. J Am Soc Nephrol. 2020;31:1628-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 17. | Akizawa T, Otsuka T, Reusch M, Ueno M. Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study. Ther Apher Dial. 2020;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |