Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13108
Peer-review started: October 2, 2022
First decision: October 21, 2022
Revised: November 2, 2022
Accepted: November 23, 2022
Article in press: November 23, 2022
Published online: December 16, 2022
Processing time: 73 Days and 0.9 Hours
Immune checkpoint inhibitors (ICIs) are a new class of antitumor drugs that have been approved to treat a variety of malignant tumors. However, the occurrence of immune related adverse events (irAEs) has become an important reason for terminating treatment. ICIs sometimes lead to diarrhea and colitis, with severe enterocolitis potentially causing the hemorrhage of the lower gastrointestinal tract and colonic perforation. ICI-associated colitis is primarily treated with glucorticosteroids and/or agents targeting tumor necrosis factor-α. Here, we describe a case of severe ICI-associated colitis due to anti-programmed cell death ligand 1 (PD-L1) (durvalumab) treatment for small cell lung cancer with liver metastasis. The patient exhibited a poor response to rescuable therapy, and eventually received a laparoscopic subtotal colectomy and ileostomy. The data presented here will contribute to optimizing current treatment strategies for patients with severe ICI-associated colitis.
A 71-year-old man was admitted for a second course of anti-PD-L1 + IP (durvalumab + irinotecan + cisplatin) treatment to manage lung cancer with liver metastasis, diagnosed 1 mo previously. Four days after the second dose, the patient developed abdominal pain and bloody diarrhea. Due to the anti-PD-L1 medication history and colonoscopy findings of the patient, he was diagnosed with a colitis associated with ICI treatment. After treatment with sufficient glucocorticoids and two courses of infliximab, the patient developed severe lower gastrointestinal bleeding. After adequate assessment, the patient was treated by laparoscopic surgery, and was discharged in stable condition.
The early screening and hierarchical management of irAEs need the joint participation of a multidisciplinary team. For ICI-related colitis with ineffective medical treatment, timely surgical intervention could prevent the death of patients.
Core Tip: Immune checkpoint inhibitors (ICIs) sometimes lead to diarrhea and colitis, which are routinely treated with immunosuppressive therapy. For ICI-related colitis with ineffective medical treatment, timely surgical intervention could prevent the death of patients.
- Citation: Lu L, Sha L, Feng Y, Yan L. Multidisciplinary treatment of a patient with severe immune checkpoint inhibitor-induced colitis: A case report. World J Clin Cases 2022; 10(35): 13108-13114
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13108.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13108
Immune checkpoint inhibitors (ICIs) are a new class of antitumor drugs that mainly target cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) as well as programmed death receptor-1 (PD-1) and its ligand (programmed cell death ligand 1, PD-L1). These drugs exert antitumor effects by blocking the binding of CTLA-4 and PD-1/PD-L1 to corresponding receptors. This action enhances T-lymphocyte activity, activates the body’s immune recognition, and prevents the immune escape of tumor cells[1,2].
Several studies have reported a noticeable accumulation of CD8+ T cells with high cytotoxicity and proliferative states in ICI-induced colitis. Analysis of the T cell receptor sequence showed that a large part of colitis-related CD8+ T cells originates from tissue resident populations, explaining the frequent and early onset of colitis symptoms after the onset of treatment[3]. ICIs sometimes lead to diarrhea and colitis. Although most patients have only self-limiting diarrhea, without the presence of blood in feces and other associated enterocolitis symptoms, severe colitis directly causes death in < 1% of patients, due to poor responses to medical treatment[4].
However, data remain limited on adverse gastrointestinal effects associated with anti-PD-L1 agents. Here, we describe a patient with severe ICI-associated colitis who failed to respond to corticosteroids and infliximab (IFX). The patient eventually had to receive a laparoscopic colectomy and ileostomy, which prevented the death due to hemorrhagic shock or colon perforation.
The patient, a 71-year-old man, was hospitalized for a second course of anti-PD-L1 + IP (durvalumab + irinotecan + cisplatin) treatment at the Department of Oncology of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, (Shanghai, China) to manage lung cancer with liver metastasis, which was diagnosed 1 mo previously.
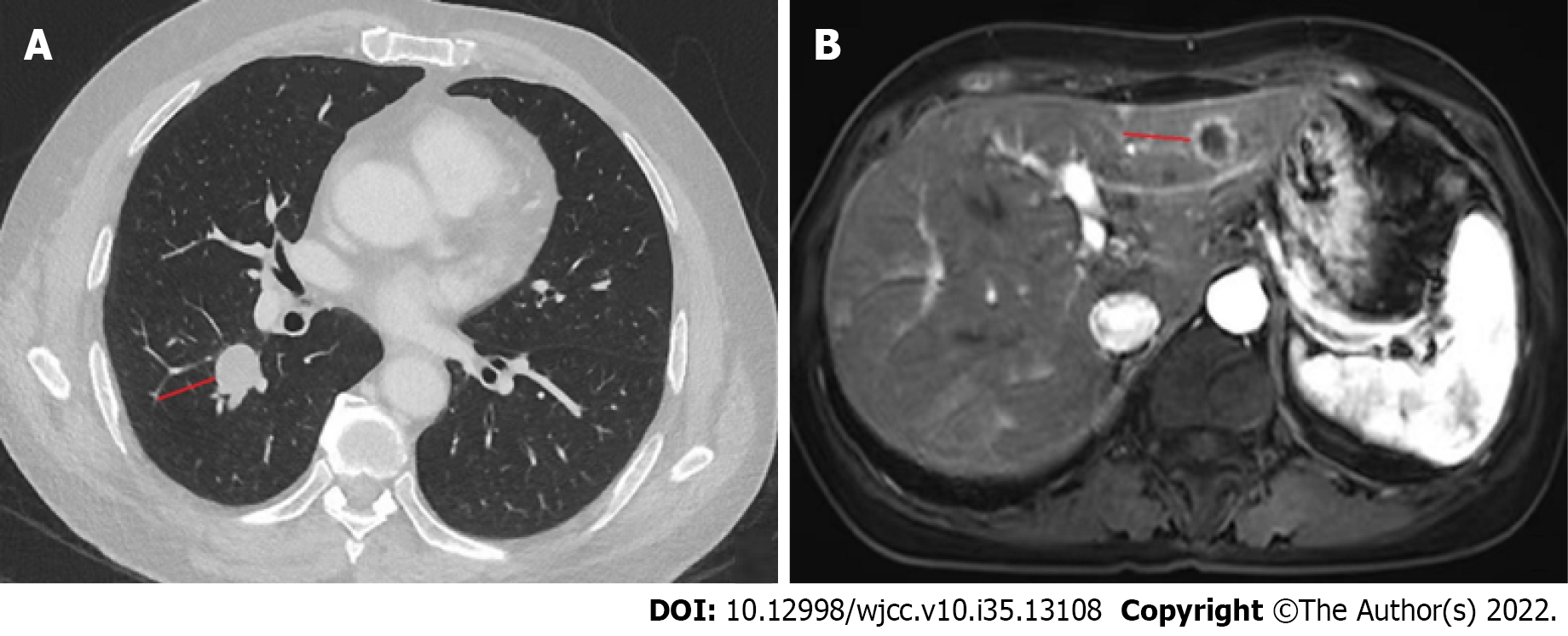
The patient originally presented with a cough that had lasted 2 mo and middle abdominal pain. A chest computed tomography (CT) scan was performed, which showed a high-density mass in the middle lobe of the right lung, and chronic inflammation in the lower lobe of both lungs. An upper abdominal magnetic resonance imaging (MRI) scan was then performed, showing the presence of abnormal signal foci in the liver, with a high probability of metastases (Figure 1). A pathological examination of right lung lobe puncture specimen was consistent with the presence of a poorly differentiated carcinoma (small cell carcinoma).
From January 10, 2022, the patient was treated with anti-PD-L1 + IP: Durvalumab (1500 mg, AstraZeneca) on day 1, together with irinotecan (115 mg, Pfizer) and cisplatin (50 mg, Teva Pharma) on days 1 and 8. No obvious adverse effects were recorded over the next 21 d. Therefore, the patient received the second dose on February 1, 2022. After 4 d of treatment, the patient presented mild diarrhea (3-4 times/d); however, there was no significant improvement in symptoms after taking antidiarrheal medication. The patient’s symptoms worsened, with dark-red bloody diarrhea (6-10 times/d) at a volume of about 50-100 mL each time, and moderate abdominal pain. The patient was transferred to the Department of Gastroenterology on February 6, 2022 for further therapy.
The patient had no notable medical history.
The patient had no specific personal or family history.
The patient weighed 61 kg and had a body mass index of 18.81. The patient’s temperature was normal. He had rough tracheal sounds, and there were no obvious lung rale (bubbling, clicking, or rattling). His abdomen was soft and flat, with tenderness in the left lower part.
The complete blood cell count revealed severe anemia with a hemoglobin concentration of 69 g/L, and a normal platelet count. The patient had a high C-reactive protein level of 45 mg/L, and a high white blood cell count (10.4 × 109/L). The transaminases were slightly elevated (alanine aminotransferase, 62 U/L; aspartate aminotransferase, 59 U/L). Urea nitrogen and creatinine levels were normal. Blood electrolytes, prothrombin, and partial thromboplastin levels were normal. His electrocardiogram was also normal. Further investigation included evaluation for intestinal infectious diseases, such as Epstein-Barr virus and cytomegalovirus (CMV) infection. All results were negative based on blood serology. Clostridium difficile (C. diff) infection was excluded by histocytotoxin testing.
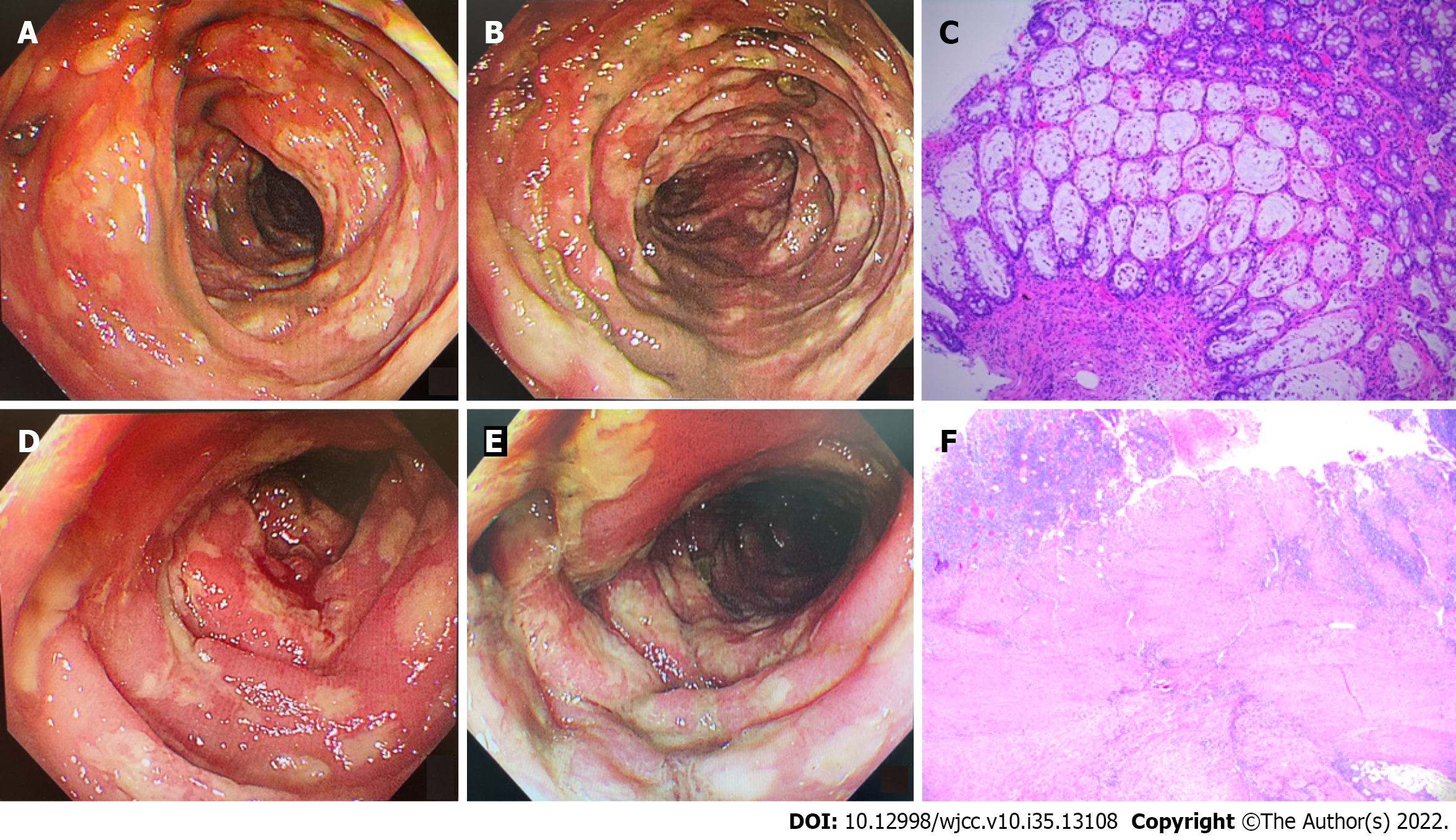
Abdominal CT and mesenteric artery CT angiography (CTA) were performed to exclude colonic malignant tumor, colonic perforation, and ischemic enteritis. Gastroscopy showed the presence of chronic atrophic gastritis, while colonoscopy showed the presence of mucosal congestion, edema, and roughness, indistinct vascular texture, multiple diffuse superficial ulcers with a purulent discharge, and a white moss adhering to the surface below the transverse colon, 40 cm from the anus. Endoscopic examination diagnosed the presence of multiple ulcers in the colon (from the descending colon to the rectum) (Figure 2A and B). Colonoscopic pathology showed the presence of multiple sites containing an intestinal mucosa with localized purulent exudate and necrosis. These features indicated multifocal ulcer formation, surrounding mucosal erosion, marked interstitial hemorrhagic edema, crypt atrophy, and focal glandular epithelium with mild heterogeneous hyperplasia. Immunohistochemistry showed that tissue samples were CMV (-) and EBER (-) (Figure 2C).
Due to the anti-PD-L1 medication history and colonoscopy findings of the patient, he was diagnosed with severe colitis associated with ICI treatment.
We immediately discontinued immunotherapy and chemotherapy. The patient was treated with 120 mg methylprednisolone (once a day) for 3 d. Enteral nutrition, empiric antibiotic therapy, and oral probiotics were administered to the patient; however, his abdominal pain and hematochezia were not significantly relieved.
On February 9, 2022, the treatment was transitioned to intravenous IFX (5 mg/kg), and a reduced dose (60 mg) of intravenous methylprednisolone once a day for 4 d, before switching to oral delivery. During this period, the patient’s abdominal pain resolved, and there was significant improvement to the bloody diarrhea, which only occurred 2-3 times a day and at a reduced amount. Given the significant improvement in symptoms, the patient was discharged.
Approximately 1 wk later, on February 15, 2022, the patient presented again with abdominal pain and bloody diarrhea (5-10 times/d). At this time, the laboratory findings reflected the patient’s clinical degradation, including a hemoglobin concentration of 45 g/L and high C-reactive protein level of 191 mg/L. A whole abdomen CT scan showed colorectal edema of the intestinal wall. An emergency colonoscopy showed severe mucosal inflammation from the sigmoid colon to the rectum with active bleeding on multiple ulcers (Figure 2D and E). After treatment with blood transfusions and fluid resuscitation, the patient continued to have rectal bleeding. A second IFX (5 mg/kg) was administered to the patient. Unfortunately, the bloody diarrhea became more severe (> 20 times/d), resulting in hemorrhagic shock on February 16, 2022.
Considering that the patient had severe lower gastrointestinal bleeding, and had not responded to the immunosuppressive therapy, we decided to perform a laparoscopic colectomy and ileostomy to save his life, after adequate assessment and communication with the patient’s family. Postoperative pathologic evaluation revealed chronic ulcer and intestinal abscess formation in the colon (Figure 2F).
The patient was placed on a fluid diet on the second postoperative day. He was discharged 10 d postoperatively, and continued treatment in the Department of General Surgery.
The colon is one of the most common target organs for irAEs, and patients may present with clinical manifestations associated with diarrhea and colitis. irAEs range from mild, self-limiting symptoms to severe, life-threatening diseases that limit the use of medication[4]. Overall, the proportion of gastrointestinal adverse events is higher with anti-CTLA-4 treatment (8%-54%) compared to anti-PD-1/PD-L1 treatment (< 2%)[5-7]. A retrospective study in the Netherlands showed that patients had mainly moderate (39%) and severe (44%) ICI-related colitis, and that the pathological type was mainly extensive colon (68%). In comparison, severe enterocolitis may result in hemorrhage of the lower gastrointestinal tract, colonic perforation, and death[8]. Evaluation of the global adverse drug reaction database Vigilyze-Vigibase (http://www.vigiaccess.org/) for 31059 immune-related cases by Wang et al[9] showed that ICI irAE was fatal in 613 (1.97%) cases. Overall, fatality was recorded in 5% of patients with colitis. Therefore, timely diagnosis and intervention of ICI irAE could improve the treatment and prevent related mortality. Patients who are, or have been, treated with an ICI, especially within 5-10 wk, should be placed on high alert for irAEs if they develop diarrhea or colitis[10].
Because the clinical symptoms of ICI colitis are usually poorly correlated with endoscopic severity, radiological findings, and response to treatment, the Common Terminology Criteria for Adverse Events are often used to classify ICI colitis as grades 1 (mild) to 5 (death)[11]. For patients with moderate to severe colitis or diarrhea, it is recommended that ICI be discontinued, and that 1-2 mg/kg/d intravenous glucocorticoids be applied for 3-5 d. Glucocorticoids are effective for approximately 60% of patients[12]. For patients who do not respond well to intravenous glucocorticoid therapy, 5 mg/kg IFX should be given as soon as possible. Furthermore, the patient should be carefully monitored for complications, such as lower gastrointestinal hemorrhage and intestinal perforation[13]. Vedolizumab or fecal microbiota transplantation should be considered as a potential substitute for IFX, due to encouraging clinical outcomes and safety profile. Thus, these are recommended as third-line options if IFX treatment fails[14,15]. Ustekinumab and tofacitinib also represent potentially effective treatments for refractory ICI colitis for individual cases, and could be used as alternatives to long-term glucocorticoid dependency when managing ICI-associated colitis[16,17].
The patient had an unresectable lung cancer with liver metastasis. Thus, we formulated a treatment plan of anti-PD-L1 + chemotherapy. In addition to durvalumab, our treatment included irinotecan and cisplatin. The side effects of cisplatin in the digestive tract are mainly nausea, vomiting, and loss of appetite, with relatively few symptoms of bloody diarrhea. The side effects of irinotecan include gastrointestinal mucosal inflammation, which is called chemotherapy-induced colitis. However, this situation mostly occurs under the chemotherapy regimen for colorectal cancer patients. In the FOLFIRI chemotherapy regimen, the combination of fluorouracil (5-FU) and irinotecan increases the severity and incidence of gastrointestinal mucosal reaction[18]. This phenomenon might be associated with the destruction of the mucosal barrier by colorectal cancer. However, there are few reports of chemotherapy-induced colitis when irinotecan is used alone, or in combination with cisplatin to treat non-colorectal tumors[19]. In addition, most pathogenic bacteria that cause chemotherapy-induced colitis are associated with a C. diff infection[20]. In this patient, C. diff infection was excluded by histocytotoxin testing. Therefore, the case in the current study was considered to have ICI-induced colitis, with chemotherapy possibly causing the outcome to occur earlier.
The patient in our study failed to respond to immunosuppressive therapy and developed sever lower gastrointestinal bleeding. Consequently, laparoscopic colectomy and ileostomy were required. We chose these procedures based on the following two conditions. First, the patient was in hemorrhagic shock, and laparoscopic surgery provided the lowest surgical trauma. Resection of the diseased colon could terminate the lower gastrointestinal bleeding symptoms. Second, during the operation, the patient had distinct inflammation and edema in the sigmoid colon. After resection of the diseased intestinal segment, there were no obvious lesions in the mucosa of the proximal intestinal canal, including the descending colon and splenic flexure of the colon. Therefore, only partial resection of the sigmoid colon was performed to remove the lesion. Based on the reasons for the patient’s underlying disease, the rate of anastomotic fistula tends to be much higher in patients with inflammatory bowel disease compared to the general population. Once anastomotic fistula occurs, acute peritonitis and, even, further toxic shock occur. The surgical goals in the acute setting are designed to remove the bulk of the diseased bowel, restore the patient’s health in the most reliable and least risky way, and preserve the patient’s potential of reestablishing intestinal continuity. Subtotal colectomy and terminal ileostomy are safe and effective surgical approaches[21]. Therefore, we performed a prophylactic stoma in the terminal ileum, followed by ileostomy retraction 3-6 mo later, when the patient’s condition permitted.
The early screening and hierarchical management of irAEs need the joint participation of a multidisciplinary team. For patients who present ICI-related colitis when medical treatment is not effective, timely surgical intervention might prevent their death.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atanasova EG, Bulgaria; Du XR, China; Tieranu CG, Romania; Zaltman C, Brazil S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1583] [Article Influence: 197.9] [Reference Citation Analysis (0)] |
| 2. | Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 793] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 3. | Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, Bowling P, Hodi FS, Rahma O, Sullivan RJ, Boland GM, Nowak JA, Dougan SK, Dougan M, Yuan GC, Wucherpfennig KW. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell. 2020;182:655-671.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 4. | Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, McQuade JL, Shoushtari AN, Tsai KK, Eroglu Z, Klein O, Hassel JC, Sosman JA, Guminski A, Sullivan RJ, Ribas A, Carlino MS, Davies MA, Sandhu SK, Long GV. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 601] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 5. | Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 647] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 6. | Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J Immunother. 2018;41:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Abu-Sbeih H, Wang Y. Gastrointestinal Tract Adverse Events. Adv Exp Med Biol. 2020;1244:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1850] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 10. | Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1108] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 11. | Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA Clinical Practice Update on Diagnosis and Management of Immune Checkpoint Inhibitor Colitis and Hepatitis: Expert Review. Gastroenterology. 2021;160:1384-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 12. | Dougan M. Gastrointestinal and Hepatic Complications of Immunotherapy: Current Management and Future Perspectives. Curr Gastroenterol Rep. 2020;22:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Mitchell KA, Kluger H, Sznol M, Hartman DJ. Ipilimumab-induced perforating colitis. J Clin Gastroenterol. 2013;47:781-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, Richards DM, Charabaty A, Wang Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J Immunother Cancer. 2018;6:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, Jiang ZD, Abu-Sbeih H, Sanchez CA, Chang CC, Parra ER, Francisco-Cruz A, Raju GS, Stroehlein JR, Campbell MT, Gao J, Subudhi SK, Maru DM, Blando JM, Lazar AJ, Allison JP, Sharma P, Tetzlaff MT, Wargo JA, Jenq RR. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 16. | Thomas AS, Ma W, Wang Y. Ustekinumab for Refractory Colitis Associated with Immune Checkpoint Inhibitors. N Engl J Med. 2021;384:581-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 17. | Bishu S, Melia J, Sharfman W, Lao CD, Fecher LA, Higgins PDR. Efficacy and Outcome of Tofacitinib in Immune checkpoint Inhibitor Colitis. Gastroenterology. 2021;160:932-934.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 18. | Wang L, Wang R, Wei GY, Zhang RP, Zhu Y, Wang Z, Wang SM, Du GH. Cryptotanshinone alleviates chemotherapy-induced colitis in mice with colon cancer via regulating fecal-bacteria-related lipid metabolism. Pharmacol Res. 2021;163:105232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Abu-Sbeih H, Mallepally N, Goldstein R, Chen E, Tang T, Dike UK, Al-Asadi M, Westin S, Halperin D, Wang Y. Gastrointestinal toxic effects in patients with cancer receiving platinum-based therapy. J Cancer. 2020;11:3144-3150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Ree AH, Nygaard V, Boye K, Heinrich D, Dueland S, Bergheim IR, Johansen C, Beiske K, Negård A, Lund-Iversen M, Hovig E, Nakken S, Nasser S, Julsrud L, Reisse CH, Ruud EA, Kristensen VN, Flørenes VA, Geitvik GA, Lingjærde OC, Børresen-Dale AL, Russnes HG, Mælandsmo GM, Flatmark K. Molecularly matched therapy in the context of sensitivity, resistance, and safety; patient outcomes in end-stage cancer - the MetAction study. Acta Oncol. 2020;59:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ross H, Steele SR, Varma M, Dykes S, Cima R, Buie WD, Rafferty J; Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2014;57:5-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |