Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13088
Peer-review started: September 30, 2022
First decision: October 17, 2022
Revised: October 26, 2022
Accepted: November 25, 2022
Article in press: November 25, 2022
Published online: December 16, 2022
Processing time: 74 Days and 23.8 Hours
Anesthesia for tracheal tumor resection is challenging, particularly in patients with a difficult upper airway. We report a case of a difficult upper airway with a metastatic tracheal tumor causing near-total left bronchial obstruction and requiring emergency tracheostomy and venovenous extracorporeal membrane oxygenation (VV-ECMO) support for rigid bronchoscopy-assisted tumor resection.
A 41-year-old man with a history of right retromolar melanoma treated by tumor excision and myocutaneous flap reconstruction developed progressive dyspnea on exertion and syncope episodes. Chest computed tomography revealed a 3.0-cm tracheal mass at the carinal level, causing 90% tracheal lumen obstruction. Flexible bronchoscopy revealed a pigmented tracheal mass at the carinal level causing critical carinal obstruction. Because of aggravated symptoms, emergency rigid bronchoscopy for tumor resection and tracheal stenting were planned with standby VV-ECMO. Due to limited mouth opening, tracheostomy was necessary for rigid bronchoscopy access. While transferring the patient to the operating table, sudden desaturation occurred and awake fiberoptic nasotracheal intubation was performed for ventilation support. Femoral and internal jugular vein were catheterized to facilitate possible VV-ECMO deployment. During tracheostomy, progressive desaturation developed and VV-ECMO was instituted immediately. After tumor resection and tracheal stenting, VV-ECMO was weaned smoothly, and the patient was sent for intensive postoperative care. Two days later, he was transferred to the ward for palliative immunotherapy and subsequently discharged uneventfully.
In a difficult airway patient with severe airway obstruction, emergency tracheostomy for rigid bronchoscopy access and standby VV-ECMO can be life-saving, and ECMO can be weaned smoothly after tumor excision. During anesthesia for patients with tracheal tumors causing critical airway obstruction, spontaneous ventilation should be maintained at least initially, and ECMO deployment should be prepared for high-risk patients, such as those with obstructive symptoms, obstructed tracheal lumen > 50%, or distal trachea location.
Core Tip: Perioperative management of obstructive tracheal masses is challenging for anesthesiologists. The patient’s history should be combined with thoroughly preoperative examinations to establish meticulous anesthesia plans and prepare for alternatives in case of emergency. Here, we report a rare case of tracheal melanoma concomitant with a difficult upper airway. We also review the case management of tracheal melanomas in the literature. No global consensus exists on the indications and timing of extracorporeal membrane oxygenation (ECMO) use in patients with tracheal masses. Spontaneous ventilation should be maintained until the airway is definitely secured, and preparing for the availability of ECMO is also suggested.
- Citation: Liu IL, Chou AH, Chiu CH, Cheng YT, Lin HT. Tracheostomy and venovenous extracorporeal membrane oxygenation for difficult airway patient with carinal melanoma: A case report and literature review. World J Clin Cases 2022; 10(35): 13088-13098
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13088.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13088
Perioperative management of patients with an obstructive tracheal tumor is challenging for anesthesiologists, but it would be even more troublesome if the patient also has a difficult upper airway. Futility of tracheal intubation and the risks of total airway obstruction should be recognized in such cases, and any degree of sedation or muscle relaxation may compromise the patient’s respiratory drive[1]. Preemptively elective venovenous extracorporeal membrane oxygenation (VV-ECMO) deployment under local anesthesia before any sedation has been advocated in previous reports[1,2]. However, ECMO deployment has some inherent potential complications such as bleeding and thrombosis. Clinicians should have in-depth discussions with the patient and endeavor to minimize these risks once ECMO institution is required.
Herein, we describe the perioperative management of an emergency rigid bronchoscopy-facilitated tumor resection and tracheal stenting for metastatic tracheal melanoma in a 41-year-old man with a difficult upper airway. We also review the literature on the managements of primary and metastatic tracheal melanoma.
A 41-year-old man had progressive shortness of breath for three months.
The patient developed progressive dyspnea on exertion, which was aggravated by sitting or walking, and relieved by lying down. He also complained of cough with blood-tinged sputum and body weight loss of more than 10 kg in the previous three months, and experienced two syncope episodes in a week accompanied by cyanotic lips and a decreased respiratory rate.
The patient had a history of right retromolar melanoma diagnosed at age 37 years (in 2015) with subsequent lung and brain metastases. At that time, he underwent retromolar tumor excision, marginal mandibulectomy, tracheostomy, and myocutaneous flap reconstruction. The patient subsequently underwent wedge resection for right lung metastasis at age 39 (in 2018); right temporal craniotomy for brain metastasis at age 40 (in 2019), and oral commissure reposition surgery at age 41 (in February 2020). He had previously received palliative chemotherapy and adjuvant immunotherapy with nivolumab since 2019 for his stage IV M1d melanoma and was in relatively good health (independent daily activity, Karnofsky Performance Scale score of 80).
The patient denied smoking, alcohol consumption, betel nuts chewing, and a family history of malignancy.
The patient weighed 63 kg, and his height was 168 cm. He exhibited clear consciousness (Glasgow Coma Score, 15). His vital signs in the operating room were as follows: body temperature, 36.8 °C; blood pressure, 115/86 mmHg; heart rate, 92 beats/min; and respiratory rate, 17 breaths/min. His general appearance suggested chronic illness. The patient’s face and neck exhibited an old surgical scar, and he demonstrated trismus with a limited mouth opening (2 cm wide) and limited neck mobility. On auscultation, there was no obvious wheezing, crackling, or stridor, except for a decrease in breathing sounds in his left lung.
Laboratory assessments revealed an elevated white blood cell count (10800/uL, reference range 3250–9160/uL) and a high level of C-reactive protein (2.6 mg/L, reference range < 1.0 mg/L). No other abnormal results were found in routine blood biochemical analyses.
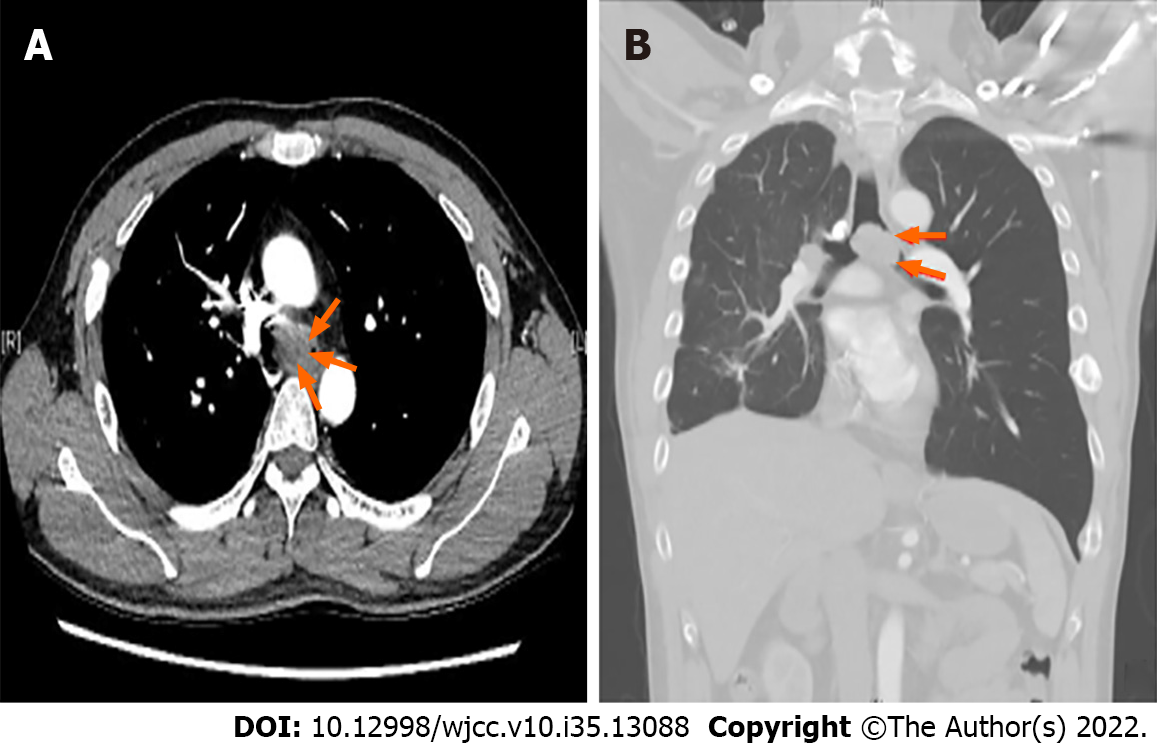
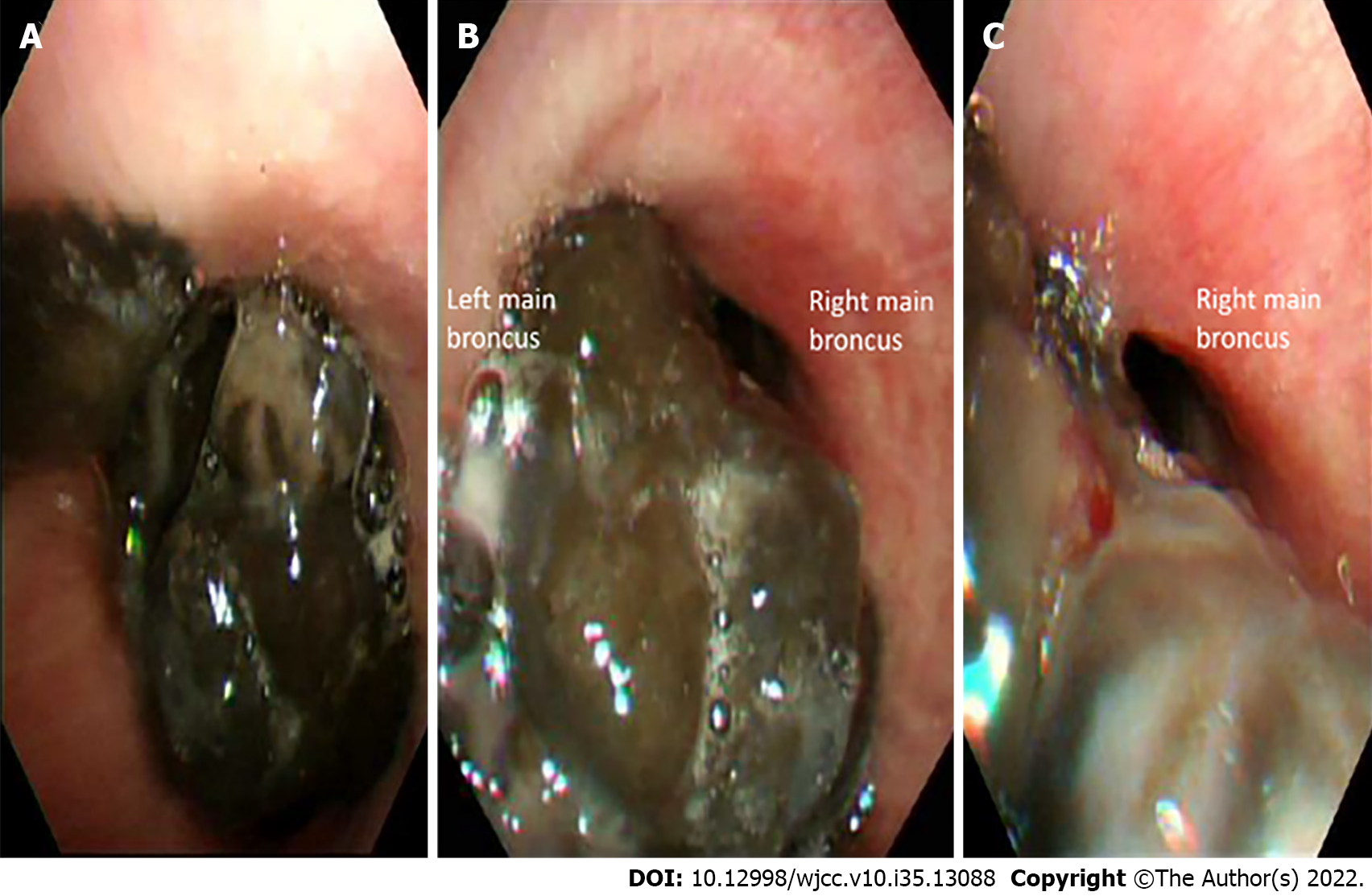
Chest computed tomography (CT) with contrast revealed a large tracheal polypoid mass above the carinal level, approximately 3 cm × 2.5 cm × 2 cm in size (3 mm in the minimum residual tracheal diameter), filling 90% of the tracheal lumen and causing nearly total obstruction of the left main bronchus and left lung hyperinflation (Figure 1). Flexible bronchoscopy revealed a large, black-pigmented, protruding carinal mass, causing nearly total occlusion of the left main bronchus and partial obstruction of the right main bronchus (Figure 2).
Combined with the patient’s medical history and imaging findings, the main diagnosis for this patient was a protruding tracheal mass at the carinal level (most likely a metastatic melanoma) causing nearly total occlusion of the left main bronchus and partial obstruction of the right main bronchus.
After a multidisciplinary discussion between surgeons, oncologists, interventional pulmonologists, and anesthesiologists, rigid bronchoscopy-facilitated carinal tumor resection and tracheal stent placement were suggested for the palliative treatment of this soft, easy-bleeding metastatic melanoma. Additionally, tracheostomy was scheduled for rigid bronchoscopic access because the patient had a limited mouth opening (2 cm in width). Considering the critical airway obstruction, possible intra-operatively VV-ECMO support and potential complications were also explained to the patient and his family. Because of the aggravated symptoms, emergency surgery was planned.
The patient was transferred to the operating room under oxygen support via a simple facemask providing oxygen at 10 L/min (his oxygen saturation (SpO2) was 100%). Standard non-invasive monitoring was initially established, including electrocardiography, pulse oximetry, non-invasive blood pressure, and bispectral index. The thoracic and cardiovascular surgeons and the ECMO team were on standby. A sudden drop in oxygen saturation to 72% occurred while transferring the patient to the operating table. Awake fiberoptic nasotracheal intubation was performed using a 6.5 Fr endotracheal tube fixed at 27 cm (tip above the carina 3–4 cm confirmed by direct visualization using a flexible fiberscope), and SpO2 was stabilized at > 95% after intermittent positive pressure ventilation. Moderate sedation was achieved with target-controlled propofol infusion and adjusted to maintain a bispectral index value of 60–80 while maintaining spontaneous ventilation. Subsequently, invasive hemodynamic monitoring was established, including arterial pressure monitoring via the right radial arterial line and central venous pressure monitoring. Two central venous catheterizations were established via the right internal jugular vein and left femoral vein for possible VV-ECMO. During tracheostomy for rigid bronchoscopy access, progressive desaturation occurred despite intermittent volume ventilation and continuous insufflation (blood gas analysis showed hypoxemia and hypercapnia, Table 1). Consequently, VV-ECMO was instituted immediately via femoro-jugular cannulation using a heparin-coated ECMO circuit with minimal heparinization (activated clotting time < 180 s). With adequate oxygenation ensured by VV-ECMO, muscle relaxation was initiated, and tracheostomy and rigid bronchoscopy were performed, revealing a grade III subglottic stenosis (71%–99% obstruction) and diffuse tumor oozing. Subsequently, the carinal mass resection and Dumon Y-stent insertion were performed uneventfully. Because of difficulty in achieving hemostasis, estimated blood loss of 400 mL, and a decrease in hemoglobin level from 15.7 gm/dL to 10.7 gm/dL, blood transfusion was prescribed with two units of leukocyte-poor red blood cells and six units of fresh frozen plasma. With adequate oxygenation (evidenced by blood gas analysis, Table 1) and stable hemodynamic status postoperatively, 50 mg of protamine was administered intravenously, and VV-ECMO was weaned smoothly. The total VV-ECMO duration was 158 min. Subsequently, the patient was transferred to the intensive care unit for postoperative care.
| Time point | Surgery | Previous symptoms | Airway | Induction anesthetics | Airway management | Muscle relaxation | Oxygen support | PaO2 (mmHg)a | PaCO2 (mmHg)a | Subsequent management | Outcome | |
| October 8, 2019 (age 40 yr) | Right temporal craniotomy | No respiratory distress | 20% obstruction at carinal level | Fentanyl, propofol | Awake nasal fiberoptic intubation | Yes (after intubation) | 1 L/min, FiO2 100% | 461 | 28.8 | Extubation at OR | Uneventful | |
| February 19, 2020 (age 41 yr) | Oral commissure reposition | No respiratory distress | 50% obstruction at carinal level | Fentanyl, propofol | Awake nasal fiberoptic intubation | Yes (after intubation) | 1 L/min, FiO2 70% | 368 | 32 | Extubation at OR | Uneventful | |
| May 23, 2020 (age 41 yr) | At OR, before induction | Smooth respiration in supine position | 90% obstruction at carinal level | Spontaneous respiration under O2 mask | No | O2 mask 10 L/min | 63.7 | 42.3 | Oxygen support, set monitoring | Prepare for tracheal tumor excision | ||
| After intubation | Tracheostomy, rigid bronchoscopy-assisted tracheal tumor excision, and tracheal stent insertion | Sudden desaturation after transferring to OR bed | 90% obstruction at carinal level | Fentanyl, propofol | Awake nasal fiberoptic intubation, spontaneous respiration via ETT | No | 2 L/min, FiO2 100% | 58.2 | 46.6 | Intermittent positive pressure ventilation; prepare VV ECMO access | Progressive desaturation | |
| During tracheostomy, before VV ECMO | Desaturation due to cough, tumor bleeding, or airway compression | 90% obstruction at carinal level | Propofol | Spontaneous respiration via ETT | No | 2 L/min, FiO2 100% | 48.9 | 45.4 | Intermittent positive pressure ventilation, prepare ECMO deployment | Decompensation | ||
| After VV ECMO deployment | Desaturation during tracheostomy | 90% obstruction at carinal level | Propofol, cisatracurium | Tracheostomy, mechanical ventilation | Yes (after ECMO deployment) | 2 L/min, FiO2 100% | 84.2 | 27.1 | Rigid bronchoscopy-assisted tracheal tumor excision, and tracheal stent insertion | Improved oxygenation | ||
| After tracheal tumor excision | Improved oxygenation under ECMO support | Tracheal stent | Propofol, cisatracurium | Tracheostomy, mechanical ventilation | Yes | 1 L/min, FiO2 100% | 220.3 | 41.3 | ECMO weaned | ICU care | ||
| After ECMO removal | Improved oxygenation after tumor removal | Tracheal stent | Propofol, cisatracurium | Tracheostomy, mechanical ventilation | Yes | 1 L/min, FiO2 80% | 260.3 | 39.4 | ICU care | Uneventful | ||
After two days in the intensive care unit, the patient was transferred to the oncology ward for immunotherapy under spontaneous breathing. Histopathological analysis of the resected carinal mass revealed metastatic melanoma. After palliative immunotherapy, the patient was discharged two weeks post-operatively in relatively stable condition. The tracheostomy tube was removed five months later under stable respiratory conditions, while the tracheal stent was adequately left in place to ensure tracheal patency. The patient died one year after the surgery due to multiple melanoma metastases, which had no direct correlation with his tracheal stenosis.
Most tracheal tumors are malignant and secondary to direct mural invasion from carcinomas of the lung, esophagus, and thyroid, or hematogenous spread from distal sites[3]. Our patient had malignant melanoma, which often metastasizes to the lungs, brain, liver, and bones, whereas tracheal metastasis has rarely been reported[4]. Table 2 Lists the publications retrieved from the PubMed and Embase databases from inception through March 8, 2022 (spanning from the oldest available case report in 1965[5] to the latest one in 2020[25]), describing management of primary and metastatic tracheal melanoma. Judging from the listed reports, the mean age of patients who developed tracheal melanoma was 56 years, and the more commonly observed tumor location was at the mid-trachea level. Compared to the very rare primary tracheal melanoma, metastatic tracheal melanoma appeared to be more common, but had a worse prognosis.
| Ref. | Year | Age | Sex | Primary/metastatic | Original site | Symptoms | Obstructiona | location | Size | Management | IHC Stain | Outcome |
| Rosenberg et al[5] | 1965 | 46 | F | Metastatic | cheek | Hemoptysis | Grade III stenosis | Carina | 3.5 cm | Partial excision | - | Died 9.5 yrs later |
| Mori et al[6] | 1977 | 47 | F | Primary | tracheal | Dyspnea | Grade I stenosis | Mid-trachea | 1.5 cm | Resection and anastomosis | -- | Alive 1 yr later |
| Andrews et al[7] | 1981 | 28 | M | Metastatic | shoulder | Dyspnea, hemoptysis | 50% obstruction | distal trachea | --- | laser | - | Died 10 days later |
| Castro et al[8] | 1990 | 68 | M | Metastatic | nasal | Dyspnea | 90% obstruction | Carina | Large | YAG laser | - | No recurrence in 9 months |
| Duarte et al[9] | 1998 | 32 | F | Primary | tracheal | Exertional dyspnea | 85% obstruction | 6cm below vocal cord | 2.3 cm × 1.3 cm | Resection and anastomosis | HMB-45(+), S100(+) | Died 13 months later |
| Koyi et al[10] | 2000 | 54 | M | Metastatic | shoulder | Dyspnea | 50% obstruction | Carina | -- | YAG laser | -- | Died 4 months later |
| Capaccio et al[11] | 2002 | 61 | F | Metastatic | shoulder | Dyspnea | Grade III stenosis | Mid-trachea | 1.5 cm × 1 cm | Tracheostomy, Argon plasma coagulation | - | Died 3 months later |
| Bardia et al[12] | 2006 | 42 | F | Metastatic | foot | Dyspnea | 50% obstruction | Subglottic | --- | Tracheostomy and radiotherapy | -- | Alive 1 yr later |
| Terra et al[13] | 2008 | 29 | F | Primary | tracheal | Dyspnea | 90% obstruction | 3 cm below vocal cord | 2.5 cm × 1.7 cm × 0.5 cm | Tracheostomy, resection and anastomosis | HMB-45(+), S100(+) | Survived 4 yrs after diagnosis |
| Cekin et al[14] | 2010 | 22 | M | Primary | tracheal | Dyspnea and hoarseness | 80% obstruction | Mid-trachea | 1.5 cm × 2 cm | Tracheotomy, resection and anastomosis | HMB-45(+), Melan-A(+), S100(+) | Alive 3 yrs later |
| Nureki et al[15] | 2012 | 73 | M | Primary | Tracheal | No symptoms | Grade I stenosis | Mid-trachea | 9 mm | Resection and anastomosis | Died 2 yrs later | |
| Shelton et al[16] | 2013 | 58 | M | Metastatic | arm | Hemoptysis | Grade I stenosis | Mid-trachea | - | laser | - | Not mentioned |
| Heyman et al[17] | 2013 | 64 | M | Metastatic | anorectal | Cough | 75% obstruction | Distal trachea | - | Argon plasma coagulation | - | Died 9 days later |
| Mark et al[18] | 2013 | 64 | M | Primary | laryngotracheal | Exertional dyspnea | Grade III stenosis | Subglottic | 1.3 cm × 4.9 cm | Awake tracheostomy, tumor resection | Pan melanin (+), S100(-) | Hospice care 10 months later |
| Imai et al[19] | 2015 | 68 | M | Primary | Tracheal | Stridor, hemoptysis | 90% obstruction | Carina | 2.5 cm × 2 cm × 1.5 cm | Chemotherapy, radiotherapy | HMB-45(+), Melan-A(+), S100(+) | Not mentioned |
| Purcell et al[20] | 2015 | 50 | F | Metastatic | Shoulder | Stridor, dyspnea | 90% obstruction | Mid-trachea | - | Bronchoscopic resection | - | Alive 6 months later |
| Costa et al[21] | 2017 | 85 | F | Thyroid metastasis | Nasal | Dyspnea | > 50% obstruction | Mid-trachea | - | Thyroidectomy | HMB-45(+),S100(+) | Died 5 months later |
| Fung et al[22] | 2018 | 73 | F | Tracheal metastasis | Nasal | Dyspnea at rest | Nearly-total obstruction | Distal trachea | - | Preemptive VV-ECMO, tumor resection | -- | Died 4 months later |
| Trugillo et al[23] | 2019 | 47 | M | Metastatic | Nasal | Dyspnea | 90% obstruction | Mid-trachea | - | resection | - | Not mentioned |
| Siraneci et al[24] | 2020 | 80 | F | Thyroid metastasis | Unknown | Dyspnea, stridor | Grade III stenosis | 1cm below vocal cord | --- | Metal stent | HMB-45(-), S100(+) | Died 2 wks later |
| Cruz et al[25] | 2020 | 45 | M | Primary | Tracheal | Cough, hemoptysis | < 20% obstruction | Cricoid level | Small | Argon plasma coagulation | - | Immunotherapy, alive 2 yrs later |
| Present case | 2022 | 41 | M | Metastatic | Retromolar | Exertional dyspnea, hemoptysis, syncope | 90% obstruction | carina | 3 cm x 2.5 cm x 2 cm | Rescue VV-ECMO, tumor resection, tracheal stent | HMB-45(+), Melan-A(+), S100(+) | Died 1 yr later |
Many patients with tracheal tumors might have a diagnosis delayed for more than 17 months because of nonspecific initial symptoms, until the progressively enlarged tumors cause life-threatening airway obstruction[26]. The symptoms of patients with tracheal tumors vary according to the tumor location and severity of the obstruction. Dyspnea, cough, wheezing, hoarseness, hemoptysis, and stridor were the predominant symptoms in our search[3,26]. A previous review found that dyspnea on exertion might occur if the residual tracheal diameter was < 8 mm, and would progress to dyspnea at rest if the residual tracheal diameter was < 5 mm[26].
Our patient had metastatic tracheal melanoma first visualized on chest CT examination in February 2019 (age 40 years) leading to progressive tracheal obstruction; however, he remained asymptomatic and underwent two uneventful surgeries under general anesthesia. One month before presenting to the hospital in May 2020 (age 41 years), he had symptoms of exertional dyspnea, hemoptysis, and syncope episodes, with 90% tracheal lumen obstruction (3 mm minimum residual diameter) revealed on chest CT. These symptoms implied a severe tracheal obstruction requiring immediate surgical intervention, including curative treatment with tracheal resection and anastomosis, or palliative therapies with laser, argon plasma coagulation, or tumor resection and tracheal stent[26]. Since our patient had stage IV metastatic melanoma with a five-year survival rate of < 30%, palliative therapy to relieve obstructive symptoms was chosen as a better option after shared decision-making[27,28]. In addition, rigid bronchoscopy-assisted tumor resection and tracheal stent placement were thought to be more beneficial as they would facilitate hemostasis and ensure subsequent airway patency.
Airway patency is well maintained through negative pressure ventilation in the awake state; however, the respiratory drive and muscle tone are compromised during anesthesia with sedation and muscle relaxation. The loss of spontaneous diaphragm movement after muscle relaxation further decreases the transpleural pressure gradient, leading to a decrease in airway diameter and an increased risk of tracheal tumor bleeding[29]. If central airway obstruction occurs at the distal trachea, airway maintenance and ventilation are more difficult because the obstruction cannot be bypassed with endobronchial intubation or tracheostomy[26]. Therefore, maintaining spontaneous ventilation is recommended for patients with critical tracheal tumor obstruction, especially those with distal tracheal obstruction. In addition, muscle relaxation should be commenced to reduce cough during airway management only after the airway is definitely secured or ECMO is initiated[30].
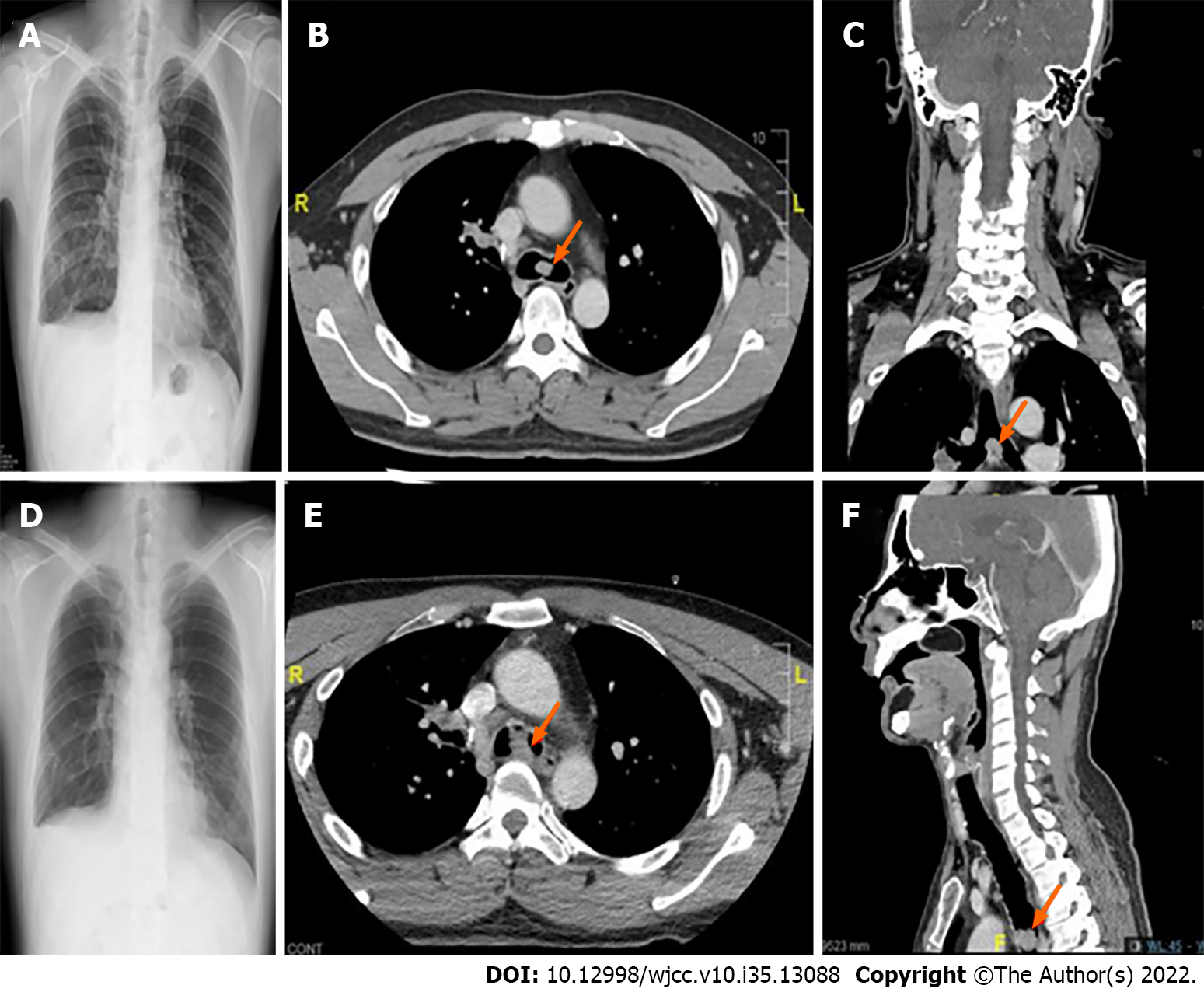
After examining our patient’s previous anesthesia records and related examinations, we found that his carinal mass was first visualized on chest CT in February 2019 (age 40) with a size of only 0.2 cm. Subsequently, the patient underwent two uneventful procedures with general anesthesia and muscle relaxation for a right temporal craniotomy in October 2019 (age 40 years) and oral commissure reposition surgery in February 2020 (age 41 years), with the presence of progressively enlarged carinal masses measuring 1.2 cm and 2.1 cm in size, respectively. During these two procedures, the patient remained asymptomatic, and no perioperative hypoxemia or hemoptysis was observed, even though the carinal mass had already obstructed 50% of the tracheal lumen (or grade II subglottic stenosis by the Cotton-Myer grading system) (Figure 3). The patient developed progressive dyspnea on exertion when 90% of lumen obstruction occurred at the carinal level, and the ECMO team was on standby before the induction of anesthesia in case of “cannot intubate, cannot ventilate” occurred. A comparison of the clinical situation and anesthesia management for different degrees of tracheal obstruction in this patient is shown in Table 1. Serial blood gas analysis revealed that this patient with 90% tracheal obstruction had progressive CO2 retention and hypoxemia in supine position even under an O2 mask 10 L/min support. Due to sudden desaturation, a definite upper airway was initially secured at the OR through immediate awake fiberoptic nasotracheal intubation while maintaining spontaneous ventilation. In addition, we had a rigid bronchoscopy standby that could establish airway patency in cases of central airway stenosis, and ventilation could be maintained by options including spontaneous ventilation, jet ventilation, continuous insufflation, or intermittent volume ventilation[31]. In case the above-mentioned ventilation measures fail to provide adequate oxygenation, VV-ECMO is the final ventilation strategy to provide adequate gas exchange[32].
Although rigid bronchoscopy through the mouth is the standard procedure for facilitating tracheal tumor excision, it was not feasible in our patient because of limited mouth opening[33]. Therefore, we chose to perform tracheostomy for rigid bronchoscopy access, as previously described[34]. However, our patient developed progressive desaturation during the tracheostomy. Possible reasons for this development could have included cough, tumor bleeding, airway compression, or even laryngospasm during tracheostomy. Consequently, we instituted VV-ECMO immediately (within 5 min) via femoro-jugular cannulation to ensure adequate oxygenation and reduce hypercapnia (Table 1).
During anesthetic management of anterior mediastinal masses causing central airway obstruction, Blank et al[35] suggested that spontaneous ventilation should be maintained, and preparations for preemptive extracorporeal circulation should be made in patients stratified as high risk (severe postural symptoms such as stridor and cyanosis, tracheal compression > 50%, pericardial effusion, or superior vena cava syndrome). Regarding the management of patients with intrinsic tracheal tumors, Zhu et al[36] and Gao et al[37] shared their case series in which ECMO support was considered in high-risk patients with tracheal lumen obstruction > 50%. Kim et al[29] suggested that preemptive ECMO support should be available prior to any airway procedure when the minimum residual tracheal lumen is < 5 mm on CT scan. For patients with tracheal tumor obstruction, according to the management strategies listed in Table 2, the higher grade of tracheal obstruction and distal trachea location should be stratified as higher risk requiring possible ECMO support. Several case series reported successful establishment of elective ECMO under local anesthesia for bronchoscopy-assisted endotracheal tumor resection, and those patients were successfully weaned from ECMO after tumor resection[1,2,38]. Meyer et al[39] reported 14 cases of elective ECMO support during rigid bronchoscopy-assisted bronchotracheal stenting for central airway stenosis, and all those patients were weaned from ECMO successfully. VV-ECMO is conducted in most cases because it can secure oxygenation, requires less heparinization (compared to venoarterial-ECMO), and has a lower risk of cannulation-related complications[38]. The simplest cannulation method for VV-ECMO is the femoro-jugular approach[40]. Because emergency initiation of ECMO might increase the risk of iatrogenic trauma and an unpredictable period of hypoxemia, prophylactic central venous catheterization can be established to facilitate rescue VV-ECMO[2]. In our case, preemptive ECMO was not considered because the patient and surgeon were concerned about possible complications of ECMO, such as tumor bleeding and thrombosis. Therefore, in our patient with 90% lumen obstruction at the carinal level, we made the necessary preparations by establishing two central venous catheterizations to ensure that VV-ECMO was immediately available in cases of decompensation. Additionally, we used a heparin-coated VV-ECMO circuit with minimal heparinization to reduce the risk of bleeding.
Perioperative management of a patient with an obstructive tracheal mass concomitant with a difficult upper airway is very rare and challenging, warranting multidisciplinary teamwork. To the best of our knowledge, this is the first report of a patient with a progressively enlarged carinal melanoma who underwent serial anesthesia. In this difficult airway patient with metastatic carinal melanoma, we performed an emergency tracheostomy, rigid bronchoscopy-assisted tumor resection, and tracheal stenting under immediate VV-ECMO support. For patients with tracheal tumor obstruction, the obstructive lesion should initially be identified via in-depth preoperative examinations followed by establishment of feasible anesthesia plans, including preparation for alternative plans in case of deterioration. In high-risk patients, such as those with obstructive symptoms, > 50% obstructed tracheal lumen, or obstruction of the distal trachea, spontaneous ventilation should be maintained until the airway is definitely secured and prepared for ECMO deployment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Taiwan Society of Anesthesiologists, 1214; Taiwan Pain Society, 1084.
Specialty type: Anesthesiology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li G, China; Tajiri K, Japan S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Hang D, Tawil JN, Fierro MA. Venovenous Extracorporeal Membrane Oxygenation for Rigid Bronchoscopy and Carinal Tumor Resection in Decompensating Patients. Anesthesiology. 2020;132:156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Dunkman WJ, Nicoara A, Schroder J, Wahidi MM, El Manafi A, Bonadonna D, Giovacchini CX, Lombard FW. Elective Venovenous Extracorporeal Membrane Oxygenation for Resection of Endotracheal Tumor: A Case Report. A A Case Rep. 2017;9:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Madariaga ML, Gaissert HA. Secondary tracheal tumors: a systematic review. Ann Cardiothorac Surg. 2018;7:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Tas F, Erturk K. Recurrence behavior in early-stage cutaneous melanoma: pattern, timing, survival, and influencing factors. Melanoma Res. 2017;27:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Rosengerg LM, Polanco GB, Blank S. Multiple tracheobronchial melanomas with ten-year survival. JAMA. 1965; 24:717-9.. |
| 6. | Mori K, Cho H, Som M. Primary "flat" melanoma of the trachea. J Pathol. 1977;121:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Andrews AH Jr, Caldarelli DD. Carbon dioxide laser treatment of metastatic melanoma of the trachea and bronchi. Ann Otol Rhinol Laryngol. 1981;90:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Castro DJ, Saxton RE, Ward PH, Oddie JW, Layfield LJ, Lufkin RB, Calcaterra TC. Flexible Nd:YAG laser palliation of obstructive tracheal metastatic malignancies. Laryngoscope. 1990;100:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Duarte IG, Gal AA, Mansour KA. Primary malignant melanoma of the trachea. Ann Thorac Surg. 1998;65:559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Koyi H, Brandén E. Intratracheal metastasis from malignant melanoma. J Eur Acad Dermatol Venereol. 2000;14:407-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Capaccio P, Peri A, Fociani P, Ferri A, Ottaviani F. Flexible argon plasma coagulation treatment of obstructive tracheal metastatic melanoma. Am J Otolaryngol. 2002;23:253-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Bardia A, Rao RD. Images in clinical medicine. Intratracheal melanoma metastases. N Engl J Med. 2006;355:1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Terra RM, Minamoto H, Junqueira JJ, Falzoni R, Pêgo-Fernandes PM, Jatene FB. Tracheal malignant melanoma: successful outcome with tracheal resection. Ann Thorac Surg. 2008;86:308-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Cekin E, Cincik H, Yilmaz I, Gungor A. Primary malignant melanoma of the trachea: case report. Ear Nose Throat J. 2010;89:E18-E20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Nureki S, Miyazaki E, Fujisaki H, Ito T, Kumamoto T, Tokuishi K, Kawahara K. Incidentally discovered primary malignant melanoma of the trachea. Intern Med. 2012;51:1743-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Shelton T, Cambron S, Seltzer M, Siegel A. Tracheal metastasis from melanoma detected with 18F-FDG PET/CT. Clin Nucl Med. 2013;38:815-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Heyman BM, Chung MM, Lark AL, Shofer S. Endobronchial metastasis from primary anorectal melanoma. Am J Case Rep. 2013;14:253-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Mark J, Taliercio S, Karakla D. Primary laryngotracheal melanoma. Otolaryngol Head Neck Surg. 2013;148:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Imai H, Kiyohara Y, Yoshikawa S, Kusutani N, Ono A, Taira T, Kenmotsu H, Harada H, Naito T, Murakami H, Sano T, Fuji H, Endo M, Nakajima T, Takahashi T. Primary malignant melanoma of the trachea: A case report. Oncol Lett. 2015;9:657-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Purcell P, Meyer T, Allen C. Tracheal mass. Malignant melanoma metastatic to the trachea. JAMA Otolaryngol Head Neck Surg. 2015;141:291-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Costa MM, Belo S, Capela-Costa J, Costa J, Carvalho D. Malignant melanoma with synchronous thyroid metastases: case report and literature review. Arch Endocrinol Metab. 2017;61:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Fung R, Stellios J, Bannon PG, Ananda A, Forrest P. Elective use of veno-venous extracorporeal membrane oxygenation and high-flow nasal oxygen for resection of subtotal malignant distal airway obstruction. Anaesth Intensive Care. 2017;45:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Fernandez-Trujillo L, Morales E, Lores J, Aguirre M, Sua L. EP1.05-10 Metastasis from a Primary Melanoma of the Sinonasal Cavity: A Case Report. J Thorac Oncol. 2019;14. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Şiraneci ŞR, Yıldırım MB, Duman B, Elbeği İÇ, Dalar L. Malignant Melanoma Metastasis to the Thyroid Gland Causing Severe Central Airway Obstruction. Turk Thorac J. 2020;21:134-137. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Cruz L, Martinez Zayas G, Kalhor N, Grosu HB. Primary Tracheal Melanoma. J Bronchology Interv Pulmonol. 2020;27:e47-e48. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Diaz-Mendoza J, Debiane L, Peralta AR, Simoff M. Tracheal tumors. Curr Opin Pulm Med. 2019;25:336-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Rebecca VW, Herlyn M. Nongenetic Mechanisms of Drug Resistance in Melanoma. Annu Rev. Cancer Biol. 2020;4:315-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | The American Cancer Society. Cancer Facts and Figures 2022. Atlanta, GA: Am. Cancer Soc. 2022. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on August 8, 2022). |
| 29. | Kim CW, Kim DH, Son BS, Cho JS, Kim YD, I H, Ahn HY. The Feasibility of Extracorporeal Membrane Oxygenation in the Variant Airway Problems. Ann Thorac Cardiovasc Surg. 2015;21:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Johansen M, Lakheeram I, Buu N. Report of two cases of endobronchial tumour mass resection in children. Can J Anaesth. 2021;68:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Nicastri DG, Weiser TS. Rigid Bronchoscopy: Indications and Techniques. Oper Tech Thorac Cardiovasc Surg. 2012;17:44-51. |
| 32. | Chen L, Wang Z, Zhao H, Yao F. Venovenous extracorporeal membrane oxygenation-assisted tracheobronchial surgery: a retrospective analysis and literature review. J Thorac Dis. 2021;13:6390-6398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 243] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Ahmed Z, Alalami A, Haupert M, Rajan S, Durgham N, Zestos MM. Airway management for rigid bronchoscopy via a freshly performed tracheostomy in a child with Goldenhar syndrome. J Clin Anesth. 2012;24:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Blank RS, de Souza DG. Anesthetic management of patients with an anterior mediastinal mass: continuing professional development. Can J Anaesth. 2011;58:853-859, 860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 36. | Zhu JH, Lei M, Chen EG, Qiao Q, Zhong TD. Ventilation strategy and anesthesia management in patients with severe tracheal stenosis undergoing urgent tracheal stenting. Acta Anaesthesiol Scand. 2018;62:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Gao R, Gu X, Zhang S, Ma S, Xu L, Li M, Gu L. Intraoperative airway management for patients with tracheal tumors: A case series of 37 patients. Thorac Cancer. 2021;12:3046-3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Malpas G, Hung O, Gilchrist A, Wong C, Kent B, Hirsch GM, Hart RD. The use of extracorporeal membrane oxygenation in the anticipated difficult airway: a case report and systematic review. Can J Anaesth. 2018;65:685-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Meyer S, Dincq AS, Pirard L, Ocak S, D'Odémont JP, Eucher P, Rondelet B, Gruslin A, Putz L. Bronchotracheal Stenting Management by Rigid Bronchoscopy under Extracorporeal Membrane Oxygenation (ECMO) Support: 10 Years of Experience in a Tertiary Center. Can Respir J. 2021;2021:8822591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Hoetzenecker K, Klepetko W, Keshavjee S, Cypel M. Extracorporeal support in airway surgery. J Thorac Dis. 2017;9:2108-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |