Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13058
Peer-review started: September 5, 2022
First decision: October 27, 2022
Revised: November 4, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 16, 2022
Processing time: 99 Days and 15.2 Hours
Acute myeloid leukemia is often associated with gene mutation or chromosome abnormality, which is an important factor affecting prognosis. The 5-year survival rate of patients with acute myeloid leukemia without hematopoietic stem cell transplantation is low. For patients who only received chemotherapy and whose first remission lasted > 5 years, there are few reports of gene spectrum changes between relapse and initial diagnosis.
We report a 41-year-old woman who presented to our hospital with complaints of dizziness, poor appetite and wasting. She was diagnosed with acute myelomo
Mutations in WT1 (R394fs/A387fs)/PTPN11 T73I/ETV6 S350P and JAK2 W659R may be related to relapse and chemotherapy resistance in acute myeloid leukemia.
Core Tip: We report a patient with relapsed refractory acute myelomonocytic leukemia. Compared with the gene spectrum at initial diagnosis, new genetic variants were detected. We speculate that mutations in WT1 (R394fs/A387fs)/PTPN11 T73I/ETV6 S350P and JAK2 W659R may be related to relapse and chemotherapy resistance.
- Citation: Wang SL. Genetic changes in refractory relapsed acute myeloid leukemia with NPM1 mutation: A case report. World J Clin Cases 2022; 10(35): 13058-13063
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13058.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13058
Chemotherapy is the main treatment for acute myeloid leukemia (AML) patients who cannot undergo hematopoietic stem cell transplantation, but 5-year overall survival is < 40%[1,2]. It is known that some genetic variation or cytogenetic abnormalities are important factors affecting the prognosis of AML[3,4]. Here, we report a case of acute monocytic leukemia with normal karyotype and NPM1 mutation. The patient had complete remission with chemotherapy but relapsed 5 years later. New genetic variants and chromosomal abnormalities were detected when she relapsed. We found new gene mutations that may be related to chemoresistance of AML.
A 41-year-old woman presented to our hospital in July 2016 to address a 2-mo history of dizziness, poor appetite and wasting.
At admission, the patient was actively suffering from dizziness, headache, poor appetite, gum pain and unintentional weight loss (approximately 10 kg in the 2 mo prior).
In 2011, the patient had undergone hysterectomy for cervical cancer. Since it was early-stage cancer, no chemotherapy or radiotherapy was performed after the operation.
The patient’s personal and family histories were unremarkable.
The patient had moderate anemia and slightly swollen gums. There was no sternal tenderness or superficial lymphadenopathy. Her liver and spleen were not palpable under the ribs.
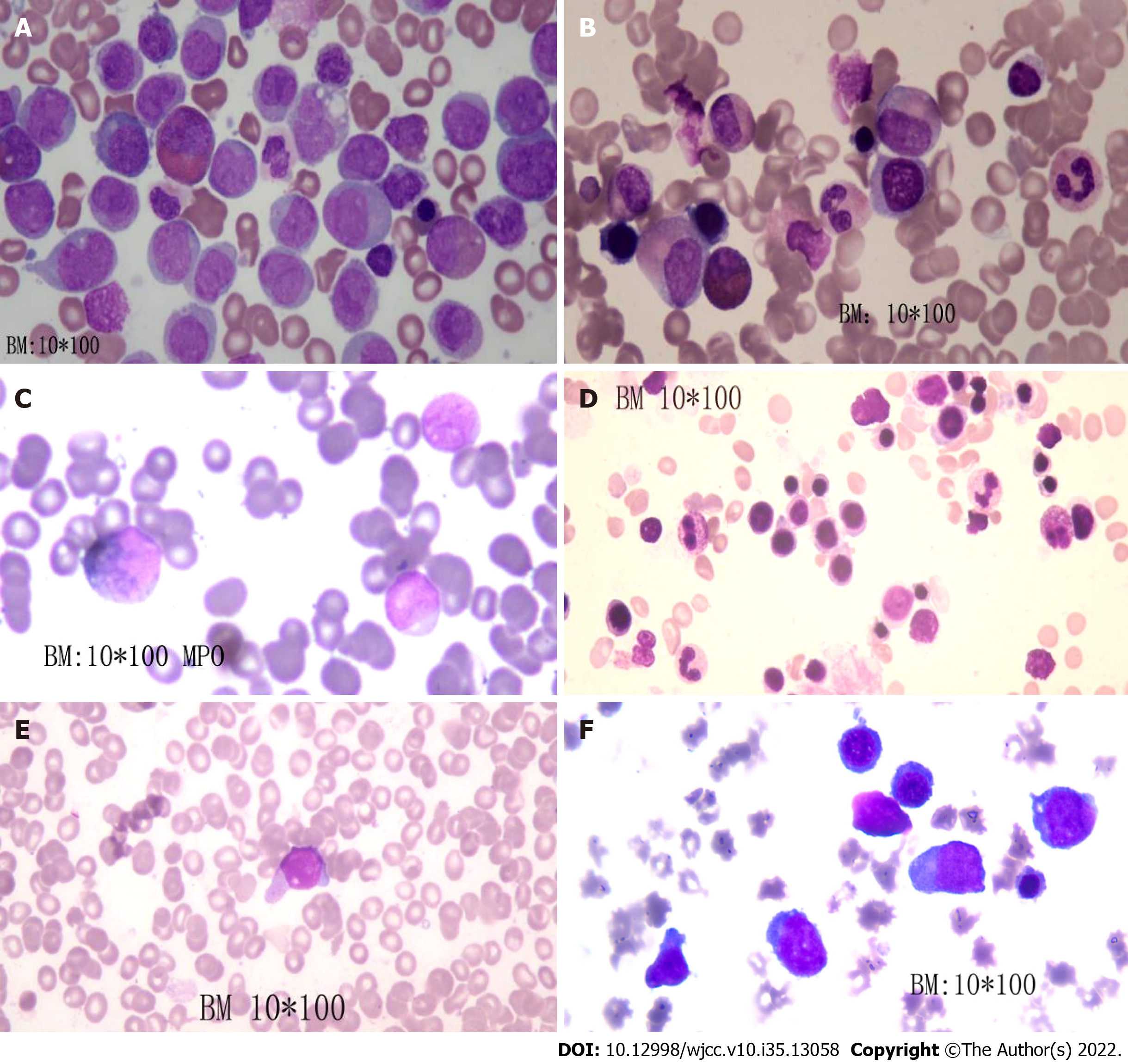
On July 16, 2019, bone marrow showed that the proportion of naive monocytes was 53%, and the proportion of promyelocytes was 18% (Figure 1A). A routine blood test indicated a white blood cell count of 19.4 × 109/L (normal range: 4-10 × 109/L), hemoglobin level of 87 g/L (normal range: 110-150 g/L) and platelet count of 113 × 109/L (normal range: 100-300 × 109/L).
Imaging examination showed no obvious abnormality.
Karyotype analysis showed 46, XX [14]. The following 11 common genes showed mutations: NPM1 exon 12; DNMT3A exon 2 c.27C>T, p.P9P; TET2 exon 3 c.652G>A, p.V218M, c.3117G>A, p.S1039S; TET2 exon 9 c.4140T>C, p.H1380H; and ASXL1 exon 12 c.3759T>C, p.S1253S (Table 1).
| Gene | Variation content |
| NPM1 | Exon 12 |
| ASXL1 | Exon 12 c.3759T>C; p.S1253S |
| DNMT3A | Exon 2 c.27C>T; p.P9P |
| TET2 | Exon 9 c.4140T>C; p.H1380H |
| Exon 3 c.652G>A; p.V218M c.3117G>A; p.S1039S | |
| FLT3-ITD | No gene mutation was detected |
Acute myelomonocytic leukemia (M4b) with NPM1 mutation.
On July 19, 2016, the “idarubicin - cytarabine (IA)” regimen was administered, consisting of idarubicin at 10 mg for days 1-3 plus cytarabine at 0.1 g q12h on days 1-7. On August 3, 2016, bone examination showed complete remission (Figure 1B). On August 25, 2016, the IA regimen was given again. On September 18, 2016, the patient was treated with lumbar puncture and intrathecal injection of cytarabine plus dexamethasone. No abnormality was found in the cerebrospinal fluid examination. However, the patient refused hematopoietic stem cell transplantation for economic reasons. We chose high-dose cytarabine (3.0 g q12h on days 1, 3 and 5 q4w) as consolidation therapy, and four cycles of cytarabine were given starting on September 28, 2016. The minimal residual leukemia cell count was 5.23 × 10-2 after consolidation treatment. Due to minimal residual leukemia, two courses of the “MA” regimen (mitoxantrone 10 mg days 1-3 + cytarabine 75 mg q12h days 1-7 q12w) and the “DA” regimen (daunorubicin 40 mg days 1-3 + cytarabine 75 mg q12h days 1-7 q12w) were given, starting on September 8, 2017. After the last chemotherapy treatment, bone marrow examination showed complete remission (Figure 1C). Routine blood tests were conducted every 3-6 mo.
In August 2021, the patient was re-hospitalized in our department due to recurrent leukemia (Figure 1D). The karyotype analysis of bone marrow cells showed: 46, X, t (X; 3) (p11.2; P13) [6]/46, XX [14]. Molecular pathological gene mutation reports showed mutations of the following genes: NPM1 exon 11 NM_002520:c.863_864insCCAG (p.W288fs); TET2 exon 3 NM_001127208: c.1588C>T(p.Q530*); WT1 exon 7 NM_024426:c.1180_1186delins8 (p.R394fs), NM_024426:c.1157dupC (p.A387fs); PTPN11 exon 3 NM_002834:c.218C>T (p.T73I); ETV6 exon 6 NM_001987:c.1048T>C (p.S350P); and JAK2 exon 15 NM_004972:c.1975T>C (p.W659R) (Table 2).
| Gene | Variation content | Variation ratio |
| NPM1 | Exon 11 NM_002520:c.863_864insCCAG (p.W288fs) | 11.8% (1276X) |
| TET2 | Exon 3 NM_001127208:c.1588C>T (p.Q530*) | 6.6% (2572X) |
| PTPN11 | Exon 3 NM_002834:c.218C>T (p.T73I) | 16.2% (2056X) |
| ETV6 | Exon 6 NM_001987:c.1048T>C (p.S350P) | 6.9% (1822X) |
| JAK2 | Exon 15 NM_004972:c.1975T>C (p.W659R) | 40.2% (1044X) |
| WT1 | Exon 7 NM_024426:c.1157dupC (p.A387fs) | 14.0% (1826X) |
| Exon 7 NM_024426:c.1180_1186delins8 p.R394fs) | 10.1% (1926X) |
IA chemotherapy was given on September 27, 2021. On November 1, 2021, we observed 8% naive cells in the peripheral blood. A regimen of azacytidine at 100 mg on days 1-7 and venetoclax at 400 mg on days 1-28 was given on November 5, 2021. The minimal residual leukemia cell count was 5.9 × 10-3 after the first cycle of treatment and 8.6 × 10-3 after four cycles of treatment (Figure 1E). After seven cycles of treatment, naive cells reappeared in the peripheral blood.
The patient was subsequently treated with the “HA” regimen (cytarabine 0.15 g q12h days 1-7 + homoharringtonine 3.5 mg days 1-7) and the “CV” regimen (cytarabine 15 mg q12h days 1-10 + venetoclax 400 mg days 1-28) on June 10, 2022. Unfortunately, remission was not achieved (Figure 1F). At the last follow-up, the patient had pancytopenia, and the proportion of naive cells was 70%. Blood transfusion was administered.
AML is a highly heterogeneous hematological tumor, often accompanied by gene mutations and chromosomal abnormalities. According to the European LeukemiaNet, AML can be divided into low, medium and high risk[5,6]. It is known that AML with normal karyotype and NPM1 mutation without FLT3-ITD mutation has a better prognosis, but our patient also had mutations in DNMT3A, TET2 and ASXL1. According to the European LeukemiaNet risk classification (2017), this patient was classified as high risk due to the ASXL1 mutation.
After induction of remission, bone marrow transplantation was the best treatment, but the patient refused the recommendation. She relapsed 5 years later, and the gene spectrum and chromosome examination were performed again. Compared with the gene spectrum at diagnosis, WT1 (R394fs/A387fs), PTPN11 T73I, ETV6 S350P and JAK2 W659R mutations replaced the ASXL1 and DNMT3A mutations. This is the first report of mutations in WT1 (R394fs/A387fs), PTPN11 T73I, ETV6 S350P and JAK2 W659R in a patient with relapsed refractory AML. Eisfeld et al[7] reported that the combination of NPM1 and WT1 mutations is an adverse prognostic combination. Krauth et al[8] confirmed that WT1 mutations are independent adverse factors for event-free survival in AML patients.
TET2 mutation is an early event in the stepwise progression from hematopoietic stem cells to myeloid malignancy, but there is still controversy over the prognostic impact of TET2 mutation in AML. TET2 mutation might be correlated with relapse of leukemia[9-11]. JAK2 mutation has not been observed in the absence of TET2 mutations[12]. JAK2 V617F is the most common type of JAK2 mutation but occurs rarely in ML[13]. Currently, there are no reports of the JAK2 W659R mutation in AML. Alfayez et al[14] concluded that mutations in PTPN11 had deleterious effects on survival. ETV6 rearrangements often accompany other molecular mutations[15].
Routine chemotherapy was ineffective for this patient. However, azacytidine and venetoclax treatment achieved morphological remission and progression-free survival for 6 mo. We speculate that mutations in WT1 (R394fs/A387fs), PTPN11 T73I, ETV6 S350P and JAK2 W659R may be related to relapse and chemotherapy resistance.
We report a patient with relapsed refractory AML. Compared with the gene spectrum at diagnosis, new mutations were detected. We speculate that mutations in WT1 (R394fs/A387fs), PTPN11 T73I, ETV6 S350P and JAK2 W659R may be related to relapse and chemotherapy resistance. Larger studies are warranted to confirm this.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diamantidis MD, Greece; Sultana N, Bangladesh S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Alsobhi E, Farahat F, Daghistani M, Awad K, Al-Zahran O, Al-Saiari A, Koshak F. Overall survival of adult acute myeloid leukemia based on cytogenetic and molecular abnormalities during 5 years in a single center study. Saudi Med J. 2019;40:1171-1176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453-474. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2353] [Cited by in RCA: 2567] [Article Influence: 171.1] [Reference Citation Analysis (0)] |
| 3. | Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N, Gundem G, Van Loo P, Martincorena I, Ganly P, Mudie L, McLaren S, O'Meara S, Raine K, Jones DR, Teague JW, Butler AP, Greaves MF, Ganser A, Döhner K, Schlenk RF, Döhner H, Campbell PJ. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374:2209-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2905] [Cited by in RCA: 3144] [Article Influence: 349.3] [Reference Citation Analysis (0)] |
| 4. | Nakajima H. Genetic abnormalities in AML. Rinsho Ketsueki. 2019;60:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3010] [Cited by in RCA: 4320] [Article Influence: 480.0] [Reference Citation Analysis (0)] |
| 6. | Pastore F, Dufour A, Benthaus T, Metzeler KH, Maharry KS, Schneider S, Ksienzyk B, Mellert G, Zellmeier E, Kakadia PM, Unterhalt M, Feuring-Buske M, Buske C, Braess J, Sauerland MC, Heinecke A, Krug U, Berdel WE, Buechner T, Woermann B, Hiddemann W, Bohlander SK, Marcucci G, Spiekermann K, Bloomfield CD, Hoster E. Combined molecular and clinical prognostic index for relapse and survival in cytogenetically normal acute myeloid leukemia. J Clin Oncol. 2014;32:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Eisfeld AK, Kohlschmidt J, Mims A, Nicolet D, Walker CJ, Blachly JS, Carroll AJ, Papaioannou D, Kolitz JE, Powell BE, Stone RM, de la Chapelle A, Byrd JC, Mrózek K, Bloomfield CD. Additional gene mutations may refine the 2017 European LeukemiaNet classification in adult patients with de novo acute myeloid leukemia aged <60 years. Leukemia. 2020;34:3215-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Krauth MT, Alpermann T, Bacher U, Eder C, Dicker F, Ulke M, Kuznia S, Nadarajah N, Kern W, Haferlach C, Haferlach T, Schnittger S. WT1 mutations are secondary events in AML, show varying frequencies and impact on prognosis between genetic subgroups. Leukemia. 2015;29:660-667. [RCA] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, Perna F, Pandey S, Madzo J, Song C, Dai Q, He C, Ibrahim S, Beran M, Zavadil J, Nimer SD, Melnick A, Godley LA, Aifantis I, Levine RL. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1109] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 10. | Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre‐leukemic stem cells in acute myeloid leukemia. Int J Hematol. 2013;98:648-657. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Wang RQ, Chen CJ, Jing Y, Qin JY, Li Y, Chen GF, Zhou W, Li YH, Wang J, Li DW, Zhao HM, Wang BH, Wang LL, Wang H, Wang MZ, Gao XN, Yu L. Characteristics and prognostic significance of genetic mutations in acute myeloid leukemia based on a targeted next-generation sequencing technique. Cancer Med. 2020;9:8457-8467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Sasaki K, Kanagal-Shamanna R, Montalban-Bravo G, Assi R, Jabbour E, Ravandi F, Kadia T, Pierce S, Takahashi K, Nogueras Gonzalez G, Patel K, Soltysiak KA, Cortes J, Kantarjian HM, Garcia-Manero G. Impact of the variant allele frequency of ASXL1, DNMT3A, JAK2, TET2, TP53, and NPM1 on the outcomes of patients with newly diagnosed acute myeloid leukemia. Cancer. 2020;126:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Aynardi J, Manur R, Hess PR, Chekol S, Morrissette JJD, Babushok D, Hexner E, Rogers HJ, Hsi ED, Margolskee E, Orazi A, Hasserjian R, Bagg A. JAK2 V617F-positive acute myeloid leukaemia (AML): a comparison between de novo AML and secondary AML transformed from an underlying myeloproliferative neoplasm. A study from the Bone Marrow Pathology Group. Br J Haematol. 2018;182:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Alfayez M, Issa GC, Patel KP, Wang F, Wang X, Short NJ, Cortes JE, Kadia T, Ravandi F, Pierce S, Assi R, Garcia-Manero G, DiNardo CD, Daver N, Pemmaraju N, Kantarjian H, Borthakur G. The Clinical impact of PTPN11 mutations in adults with acute myeloid leukemia. Leukemia. 2021;35:691-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Zhou F, Chen B. Acute myeloid leukemia carrying ETV6 mutations: biologic and clinical features. Hematology. 2018;23:608-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |