Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.13015
Peer-review started: August 1, 2022
First decision: October 17, 2022
Revised: October 29, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 16, 2022
Processing time: 134 Days and 18.9 Hours
Ductal spasm is a rare but life-threatening complication of cardiac catheterization in neonates with pulmonary atresia and an intact ventricular septum. In patients with ductal-dependent pulmonary blood flow, ductal spasm may lead to refractory hypoxemia and severe hemodynamic instability, which need to be treated in perfect order.
We present a male infant with a gestational age of 39 wk, and his fetal echocardiography showed pulmonary atresia. At 28 d of age, transcatheter pulmonary valvuloplasty with balloon dilatation was performed. Two hours after the operation, the patient's pulse oxygen saturation continued to decrease. The patient was then transferred to receive cardiac catheterization. During catheterization, the invasive blood pressure and pulse oxygen saturation suddenly decreased, and repeated aortography revealed partial occlusion of the ductus arteriosus. It no longer changed when pulse oxygen saturation rose to 51% after approximately 20 min of maintenance therapy. Therefore, a ductal stent was used for implantation. Hemodynamics and hypoxemia were improved.
We should know that ductal spasm may occur during pulmonary atresia and intact ventricular septum cardiac catheterization. Understand the pathophy
Core Tip: In patients with ductal-dependent pulmonary blood flow, ductal spasm may lead to refractory hypoxemia and severe hemodynamic instability. Ductal stenting has been widely developed as an initial palliative strategy to secure pulmonary blood flow in such patients. We report a case of a neonate with pulmonary atresia and an intact ventricular septum who developed ductal spasm during ductal stenting. We discussed the appropriate treatment for ductal spasm in a neonate during cardiac catheterization.
- Citation: Zhang X, Zhang N, Song HC, Ren YY. Management of ductal spasm in a neonate with pulmonary atresia and an intact ventricular septum during cardiac catheterization: A case report. World J Clin Cases 2022; 10(35): 13015-13021
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/13015.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.13015
Percutaneous ductal stenting has been widely developed as an initial palliative strategy to secure patients with pulmonary atresia and intact ventricular septum (PA/IVS)[1]. Ductal stenting can be performed simultaneously or in stages after pulmonary valvuloplasty. The management of interventional surgery in such patients is challenging. Ductal spasm (DS) is a rare but life-threatening complication of cardiac catheterization in neonates with PA/IVS. In patients with ductal-dependent pulmonary blood flow (PBF), DS may lead to refractory hypoxemia and severe hemodynamic instability[2]. We report a case of a neonate with PA/IVS who developed DS during ductal stenting. Changes in hemodynamics and respiratory parameters were recorded, and the treatment process was summarized and analyzed.
The patient was a male infant with a gestational age of 39 wk, and his fetal echocardiography showed pulmonary atresia.
Fetal echocardiography of the neonatal patient was performed before delivery (24 wk pregnant) to show pulmonary atresia. Due to abnormal umbilical blood flow, the patient was delivered by cesarean section, without a history of intrauterine distress and premature rupture of membranes. Then, the patient was transferred to the cardiac intensive care unit (CICU) for further treatment. The patient received prostaglandin E1 (PGE1) at 3 ng/kg/min to maintain patency of the ductus arteriosus (DA) after delivery, and the pulse oxygen saturation (SPO2) was 84%–88%.
The patient had no history of other illnesses.
The patient had no family history of illness.
The physical examination revealed normal development (height, 50 cm; weight, 3.26 kg), a blood pressure of 85/47 mmHg, a pulse rate of 145 beats per min, and a respiratory rate (RR) of 30 breaths/min. His pulse oxygen saturation (SPO2) was 84%–88% in room air.
Blood tests showed an Hb level of 12.5 g/dL, hematocrit (Hct) of 37%, platelet count of 266 × 109 cells/L, leukocyte count of 11.22 × 109/L, C-reactive protein of 4.16 mg/L, and N-terminal pro-brain natriuretic peptide (NT-proBNP) of 982.50 pg/mL. Other blood tests showed no significant abnormalities.
Postnatal echocardiography revealed a foramen ovale of approximately 5.5 mm, a right-to-left shunt, and an intact ventricular septum. The pulmonary valve ring was 6.2 mm, with membranous atresia. The descending aorta was connected to the bifurcation of the main pulmonary artery through the patent ductus arteriosus (PDA) (the inner diameter of the end of the pulmonary artery was 3 mm) and a continuous shunt.
Pulmonary atresia and intact ventricular septum; Patent foramen ovale; Patent ductus arteriosus.
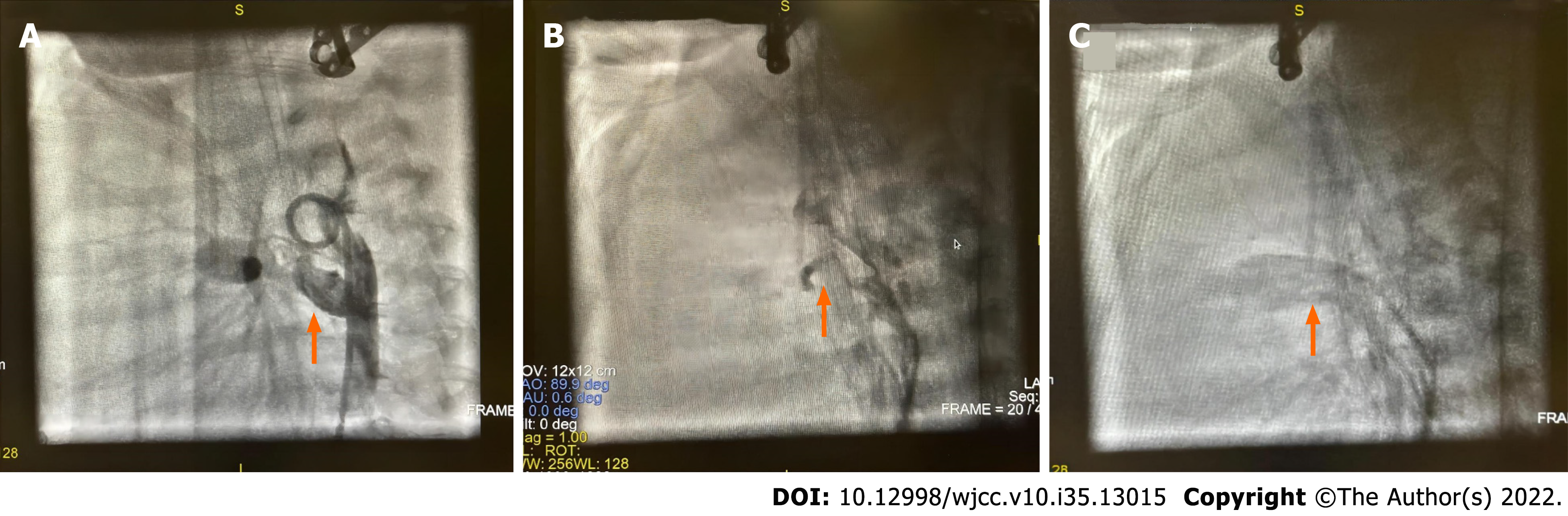
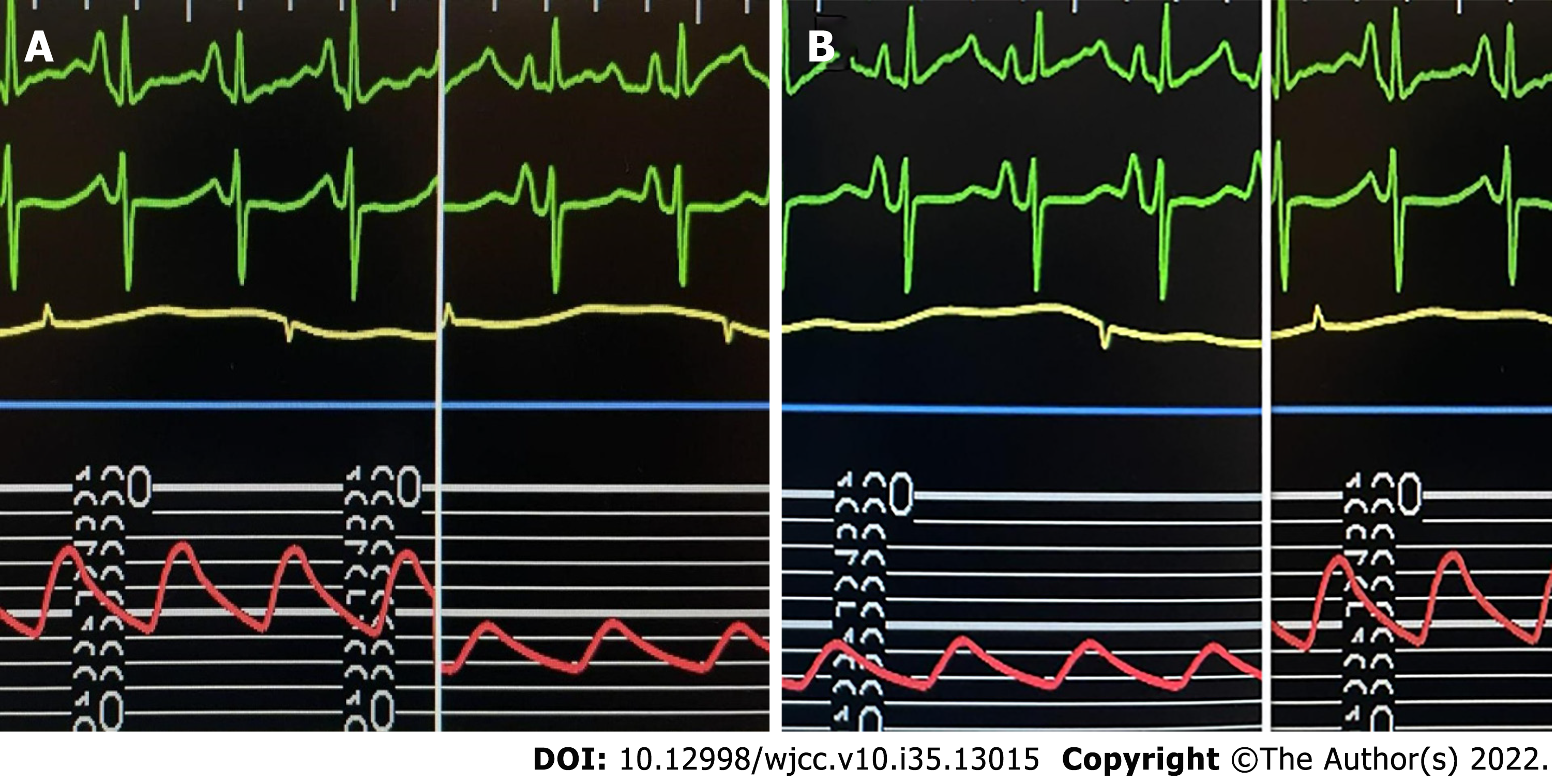
At 28 d of age, the patient planned to undergo pulmonary valve perforation and percutaneous balloon pulmonary valvuloplasty (PBPV). After induction of anesthesia, the patient inhaled 30% oxygen; left internal jugular vein and left radial artery catheterization were performed, and PGE1 was continuously infused at 3 ng/kg/min. Descending aortography showed that the DA was tubular with an internal diameter of 3.0 mm. After pulmonary valve perforation and balloon dilatation, right ventriculography showed that the pulmonary valve was open, and SPO2 was 89% when the patient was returned to the CICU with an endotracheal tube. Two hours later, the patient's SPO2 dropped to 75%. Echocardiography showed a significantly enlarged right atrium, with a right-to-left shunt at the atrial level, patent foramen ovale (diameter, 7 mm), PDA (diameter, 3 mm at the pulmonary end), severe tricuspid regurgitation, and a little forward flow through the pulmonary valve. Intensive care doctors considered the patient to have developed right-sided heart failure. The treatment included infusion of vasoactive drugs and diuretics, inhalation of nitric oxide, intravenous infusion of treprostinil to reduce pulmonary artery pressure, and adjustment of the PGE1 dose to 10 ng/kg/min. However, the SPO2 continued to decrease to as low as 60%. The patient was then transferred from the CICU to the catheterization room. General anesthesia was maintained by inhaling 1.5% sevoflurane in 40% oxygen and cisatracurium 0.5 mg administered intravenously. Descending aortography revealed that the PDA was tortuous and flat (Figure 1A). When the catheter was adjusted, the invasive blood pressure (IBP) suddenly decreased from 68/40 mmHg to 44/28 mmHg (Figure 2A), SPO2 decreased from 68% to 40%, and end-tidal carbon dioxide (PETCO2) decreased from 36 mmHg to 18 mmHg. Repeated aortography revealed partial occlusion of the DA (Figure 1B). The catheter was withdrawn because of a suspected DS. PGE1 was increased to 20 ng/kg/min, and norepinephrine was increased to 0.2 µg/kg/min. Simultaneously, the concentration of inhaled oxygen was reduced to inhaled air. It no longer changed when SPO2 rose to 51% after approximately 20 min of maintenance therapy. Therefore, a ductal stent was used for implantation. After successfully implanting a 4.5 mm × 21 mm stent, the change in invasive arterial pressure was 65/42 mmHg (Figure 2B), SPO2 was 85%, and ETCO2 was 33 mmHg. PGE1 treatment was then discontinued. Aortography showed that the stent was unobstructed without displacement (Figure 1C). The guidewire and balloon were withdrawn, and the patient was returned to the CICU.
The patient was hemodynamically in the immediate postoperative period, and his SPO2 while breathing room air was 85%. Then, the endotracheal tube was removed on day 3 after ductal stenting intervention, and the patient was discharged from the CICU 2 days later. One month later, the patient underwent balloon dilatation of the pulmonary valve again, and catheter angiography showed ductal stent patency without stenosis or occlusion.
A 28-day-old neonate with PA/IVS who underwent PBPV developed persistent hypoxemia and could not be improved after a series of treatments. The patient had to be transferred to receive cardiac catheterization. During catheterization, the invasive blood pressure and pulse oxygen saturation suddenly decreased, and repeated aortography revealed DS. We adopted routine treatment methods, including inhaling air, increasing the infusion dose of PGE1 and vasoactive drugs. It no longer changed when SPO2 rose to 51% after approximately 20 min of maintenance therapy. Therefore, a ductal stent was used for implantation. Hemodynamics and hypoxemia were improved. Pulmonary circulation in patients with PA/IVS depends on DA patency. Postpartum contraction of DA will lead to severe hypoxemia, cyanosis and death[3]. Ductal stenting for DA is usually used for patients who have successfully undergone pulmonary valve perforation but still need pulmonary circulation support because PGE1 infusion cannot be relieved[4]. Compared with a modified Blalock-Taussig (BT) shunt, a meta-analysis demonstrated that ductal stenting had fewer procedural complications and shorter CICU stays[5]. According to the Catheterization for Congenital Heart Disease Adjustment for Risk Method, pulmonary valve perforation and any catheterization within 4 days after surgery are classified as the most high-risk procedures. This classification is because most of these patients are newborns or premature infants with long operation times and high skill requirements. The procedure can easily cause drastic changes in hemodynamics and SPO2. Most ductal stenting procedures are performed later after balloon pulmonary valvuloplasty. As a staged procedure, it was helpful to evaluate the ductal contraction time after PGE1 discontinuation and can avoid stent displacement and dislocation. In addition, some patients had to undergo cardiac catheterization and stent implantation a few days after balloon pulmonary valvuloplasty because they could not tolerate PGE1 infusion or SPO2 continued to decrease[1].
In previous reports, the most common complication of PA/IVS cardiac catheterization was vascular-related injury. Other complications include DS, dissection, acute stent thrombosis, stent migration or dislodgment. However, the incidence of DS has not been reported in detail in previous ductal stenting studies. Chen and colleagues reported a case of ductal dissection and spasm during stenting of a PDA[6]. The catheter was considered sandwiched because the guidewire could not pass through the DA smoothly, and the end was slightly deformed. After pulling out the guidewire, the SPO2 decreased to 60%. Repeated angiography revealed a severe DS. PGE1 was administered again (20 ng/kg/min). The hemodynamics were stable, and SPO2 recovered to 85% after 20 min of PGE1 infusion. Finally, a ductal stent was implanted. According to the literatures[7-9], there are many factors leading to DS. One possible factor is mechanical stimulation. A cardiac interventionalist may unintentionally induce spasm when performing catheter manipulation to enter the aortic arch, main or branch pulmonary artery, or when crossing the PDA[8]. Another possible factor is biochemical stimulation. DS may be stimulated by stress-induced catecholamine (nitric oxide and prostaglandins) release[9] and inhalation of high concentrations of oxygen[10]. In this case, the concentration of inhaled oxygen did not change, and PGE1 was continuously infused before and during the catheterization procedure. Therefore, we considered that the catheter stimulated the DA during aortic angiography, causing contraction and spasm.
Evidence of DS in patients with ductal-dependent PBF includes confirmation of PDA presence by preoperative echocardiography and intraoperative angiography. Second, intraoperative echocardiography and angiography showed that the catheter's ampulla gradually became thinner and completely or nearly occluded[7]. Finally, significant reductions in IBP, SPO2, and ETCO2 (manifestations of decreased PBF) are important clinical indications. Therefore, the prevention of DS is important. In patients with ductal contractions, it is necessary to consider the possibility of further ductal contractions during the procedure. Determining continuous infusion of PGE1 and performing appropriate operations are important. To reduce the DS or expand the stenosis area, Bahaidarah and colleagues recommended that PGE1 should be infused intravenously until the guidewire passes through the DA[11]. After DS, if the catheter or wire has crossed the PDA, the stent can be implanted quickly to re-establish stable PBF[2]. If the stent cannot be implanted as soon as possible, the catheter should be removed immediately to alleviate DS[5]. In this case, the reason for this patient's ductal stenting was that SPO2 continued to decrease, and the pulmonary artery had little forward blood flow, which was not enough to meet the needs of the body. DS during the procedure occurred suddenly, not slowly. The most appropriate response was to withdraw the catheter and provide conservative treatment. It was wise to wait for a few minutes and take an angiogram again to observe the size of the DA to decide the next treatment[12,13]. If the stent is directly implanted, it may be disastrous to further stimulate the ductus arteriosus and cause the ductus arteriosus to close directly. To avoid further aggravation of the DS, we did not immediately cross the wire and catheter to the PDA to implant the stent. Of course, DS is a functional abnormality that can be reversed through treatment, but it takes time to reverse before patient's condition continue to deteriorate. However, there was no significant relief of DS after conservative treatment. The patient could not continue to tolerate long-term hypoxia and hypotension, so we performed ductal stenting.
Specific anesthetic management at this time is not decisive, but measures should be taken to relieve DS and maintain hemodynamic stability as much as possible. Preoperative safe central venous access, inotropes, vasopressors, and sodium bicarbonate are necessary to increase blood pressure, improve acidosis, and help blood flow through the PDA during DS, thereby increasing PBF[14]. IBP monitoring is important to quickly observe the direct hemodynamic changes following DS. Batlivala and colleagues reported 2 cases of DS after angiography. Continuous arterial pressure monitoring helps patients quickly diagnose DS[15]. When DS occurs, the infusion dose of PGE1 should be increased immediately. Some studies suggest that when aggravating cyanosis compatible with impending closure occurs in patients with ductal-dependent PBF, an initial dose of 20 ng/kg/minute PGE1 should be started[16,17]. If there is no improvement in oxygenation, stepwise dose increases of 5 ng/kg/minute are administered until no further increase in oxygenation occurs or SPO2 has reached the preclosure level of the ductus arteriosus, even to 100 ng/kg/min. If the hemodynamics are stable, the catheter is generally reopened within 30 mins to 2 h[18]. Of course, an initial dose of PGE1 of 20 ng/kg/min was given after DS in this case. It was undeniable that our approach was conservative, and we did not continuously increase the dose of PGE1. When hypoxaemia occurs in DS, some researchers believe that increasing the inhaled oxygen concentration cannot improve the condition. Instead, air should be inhaled for ventilation to avoid hyperoxia, stimulating the further development of DA occlusion. Historical animal experiments have shown that when the same isolated DA rings were transferred from low oxygen partial pressure (PO2) to high PO2 and restimulated by administering PGE1, the relaxation effect of PGE1 on DA rings at high PO2 was significantly increased. However, no further study has confirmed whether the DA response to PGE1 is the same from low PO2 to high PO2. Decker and colleagues suggested that inhalation anesthesia should be changed to total intravenous anesthesia with propofol because 8 children with DS were well relieved in their cases[19]. However, there is no clear explanation for this phenomenon. Heparin administration to achieve an activated clotting time of > 250 s should be confirmed. If a stent cannot be implanted and hemodynamics deteriorate rapidly, cardiopulmonary bypass or extracorporeal membrane oxygenation can be performed as soon as possible to save time and life.
Ductal spasm may occur during PA/IVS cardiac catheterization. Understand the pathophysiology of ductal-dependent PBF and make comprehensive perioperative preparations are essential to deal with hemodynamic disorders caused by ductal spasm.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Gendy HA, Egypt; Teragawa H, Japan S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Haddad RN, Hanna N, Charbel R, Daou L, Chehab G, Saliba Z. Ductal stenting to improve pulmonary blood flow in pulmonary atresia with intact ventricular septum and critical pulmonary stenosis after balloon valvuloplasty. Cardiol Young. 2019;29:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Aggarwal V, Petit CJ, Glatz AC, Goldstein BH, Qureshi AM. Stenting of the ductus arteriosus for ductal-dependent pulmonary blood flow-current techniques and procedural considerations. Congenit Heart Dis. 2019;14:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Eilers L, Qureshi AM. Advances in Pediatric Ductal Intervention: an Open or Shut Case? Curr Cardiol Rep. 2020;22:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Ratnayaka K, Nageotte SJ, Moore JW, Guyon PW, Bhandari K, Weber RL, Lee JW, You H, Griffin DA, Rao RP, Nigro JJ, El-Said HG. Patent Ductus Arteriosus Stenting for All Ductal-Dependent Cyanotic Infants: Waning Use of Blalock-Taussig Shunts. Circ Cardiovasc Interv. 2021;14:e009520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Alsagheir A, Koziarz A, Makhdoum A, Contreras J, Alraddadi H, Abdalla T, Benson L, Chaturvedi RR, Honjo O. Duct stenting vs modified Blalock-Taussig shunt in neonates and infants with duct-dependent pulmonary blood flow: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2021;161:379-390.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Chen TY, Chen PW, Wang JN. Patent ductus arteriosus stenting: ductal dissection and spasm in pulmonary atresia with intact ventricular septum. Cardiol Young. 2022;32:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 7. | Batlivala SP, Glatz AC, Gillespie MJ, Dori Y, Rome JJ. Ductal spasm during performance of transcatheter ductal occlusion. Catheter Cardiovasc Interv. 2014;83:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Méot M, Haddad RN, Patkai J, Abu Zahira I, Di Marzio A, Szezepanski I, Bajolle F, Kermorvant E, Lapillonne A, Bonnet D, Malekzadeh-Milani S. Spontaneous Closure of the Arterial Duct after Transcatheter Closure Attempt in Preterm Infants. Children (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Ovalı F. Molecular and Mechanical Mechanisms Regulating Ductus Arteriosus Closure in Preterm Infants. Front Pediatr. 2020;8:516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Coceani F. Oxygen sensing in the ductus arteriosus-A unifying vision for two concepts. Br J Pharmacol. 2022;179:3325-3329. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Bahaidarah S, Al-Ata J, Alkhushi N, Azhar A, Zaher Z, Alnahdi B, Abdelsalam M, Elakaby A, Dohain A, Abdelmohsen G. Outcome of ductus arteriosus stenting including vertical tubular and convoluted tortuous ducts with emphasis on technical considerations. Egypt Heart J. 2021;73:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | MacDonald ST, Bhindi R, Ormerod O, Wilson N. Ductus arteriosus spasm. JACC Cardiovasc Interv. 2009;2:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Alkamali A, Alasrawi S. Dealing with patent ductus arteriosus (PDA) spasm in the cath lab. J Cardiol Curr Res. 2018;11:135-138. [DOI] [Full Text] |
| 14. | Udink Ten Cate FE, Sreeram N, Hamza H, Agha H, Rosenthal E, Qureshi SA. Stenting the arterial duct in neonates and infants with congenital heart disease and duct-dependent pulmonary blood flow: a multicenter experience of an evolving therapy over 18 years. Catheter Cardiovasc Interv. 2013;82:E233-E243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Sarquella-Brugada G, Mivelaz Y, Dahdah N. Hemodynamic changes alert to spontaneous ductus arteriosus spasm. Rev Esp Cardiol (Engl Ed). 2013;66:743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Huang FK, Lin CC, Huang TC, Weng KP, Liu PY, Chen YY, Wang HP, Ger LP, Hsieh KS. Reappraisal of the prostaglandin E1 dose for early newborns with patent ductus arteriosus-dependent pulmonary circulation. Pediatr Neonatol. 2013;54:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Khalil M, Jux C, Rueblinger L, Behrje J, Esmaeili A, Schranz D. Acute therapy of newborns with critical congenital heart disease. Transl Pediatr. 2019;8:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 18. | Cucerea M, Simon M, Moldovan E, Ungureanu M, Marian R, Suciu L. Congenital Heart Disease Requiring Maintenance of Ductus Arteriosus in Critically Ill Newborns Admitted at a Tertiary Neonatal Intensive Care Unit. J Crit Care Med (Targu Mures). 2016;2:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | De Decker R, Comitis G, Thomas J, van der Merwe E, Lawrenson J. A novel approach to ductal spasm during percutaneous device occlusion of patent ductus arteriosus. Cardiol Young. 2016;26:1352-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |