Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12980
Peer-review started: May 17, 2022
First decision: August 21, 2022
Revised: September 11, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 16, 2022
Processing time: 211 Days and 4.1 Hours
Urothelial encrusted pyelo-ureteritis disease is an infrequent condition and there is no unified surgical treatment and reference standard to consult. We have used a minimally invasive endoscopic method to treat three such cases, which yielded excellent results.
The first case was a 45-year-old man who had unilateral ureteropelvic junction (UPJ) atresia and contralateral stenosis and was treated by double endoscopic surgery using an anterograde percutaneous nephroscope coupled with a rigid retrograde ureteroscope. The second case was a 12-year-old boy who received a percutaneous nephroscopy on one side and a percutaneous nephroscopy with a rigid ureteroscope on the other side due to the presence of bilateral UPJ stenosis. The third case was a 32-year-old woman with bilateral lower ureteral stricture treated using a rigid retrograde ureteroscope. Endoscopic surgeries were successfully performed on all the three patients. Varying degrees of encrustation and erosion of the urothelium were observed during the operation. The calcified layer composition analysis showed magnesium ammonium phosphate or carbonate apatite. Two patients achieved a good prognosis.
Minimally invasive endoscopic treatment for urothelial encrusted pyelo-ureteritis disease can yield better results.
Core Tip: We retrospectively summarize our clinical experience on the use of minimally invasive endoscopy in the treatment of urothelial encrusted pyelo-ureteritis disease. Since urothelial encrusted pyelo-ureteritis disease is clinically infrequent, we discuss and analyze the clinical manifestations, laboratory examination, imaging characteristics, morbidity, and treatment process of three patients with this disease. We carefully followed them for a long time and observed good therapeutic effects.
- Citation: Liu YB, Xiao B, Hu WG, Zhang G, Fu M, Li JX. Endoscopic treatment of urothelial encrusted pyelo-ureteritis disease: A case series. World J Clin Cases 2022; 10(35): 12980-12989
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12980.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12980
Urinary epithelial encrusted diseases are a group of conditions including encrusted cystitis, encrusted pyelitis, crusted urethritis, and crusted posterior urethritis[1]. These conditions are rare occurrences in clinical settings and, therefore, remain poorly reported. The most commonly encountered among the group is encrusted cystitis. Encrusted pyelitis and urethritis were first reported by Morales et al[2] in 1992. They described these conditions as an infectious disease characterized by erosion, inflammation, and calcification of the renal pelvis and calyces on mucosal tissues. Reports also showed that the lesions seen on the renal pelvic might extend to ureteral epithelial tissues[3]. Therefore, due to their wide range of lesions, different clinical manifestations, and pathogenic characteristics, there is a degree of complexity and uncertainty requiring targeted treatment according to the involved location and disease characteristics. At present, there is no unified surgical treatment and reference standard to consult. From March 2018 to July 2021, our department admitted and successfully treated three patients with complex urothelial encrusted pyelo-ureteritis disease using a minimally invasive endoscopic method. The overall diagnosis and treatment processes are reported as follows. We also performed a review of the available literature.
The first patient was a 45-year-old man with a 3-mo history of abdominal pain. The second patient was a 12-year-old boy with a 6-wk history of bilateral lumbago pain. The third patient was a 32-year-old female woman who presented with intermittent hematuria for 2 mo.
The first patient developed abdominal pain 3 mo ago, after which he was diagnosed with left ureteral calculi and received left ureteroscopic lithotripsy at a local hospital. Lumen stenosis and mucosal erosion were observed during the operation, and an indwelling ureteral stent was placed. The second patient had a 6-wk history of bilateral lumbago pain, during which he underwent an abdominal non-enhanced computed tomography (NCCT) scan at a local hospital, which showed the presence of bilateral ureteral calculi and hydronephrosis. The initial ureteral stenting procedure failed, and the patient was transferred to our hospital 2 wk after bilateral nephrostomy. The third patient underwent bilateral extracorporeal shock wave lithotripsy 3 mo ago due to bilateral ureteral calculi. Bilateral ureteroscopic lithotripsy was performed 1 mo ago and indicated the presence of ureteral stricture and calcification attached to the urothelial tissue. Therefore, the scheduled operation was abandoned.
The first patient had a history of systemic vasculitis for 8 mo and received intermittent immunosuppression therapy consisting of cyclophosphamide, prednisone, and tacrolimus. The second patient had a history of dermatomyositis for 4 mo and was on regular oral immunosuppressants (methotrexate), intermittent immunoglobulin, and hormonal therapy. The third patient had a history of dermatomyositis for more than 2 years and has been taking a long-term therapy consisting of oral methylprednisolone and methotrexate.
The personal and family history was unremarkable in all the three patients.
The first and second patients had mild percussion pain in the renal region. The third patient was normal.
The first patient had a serum creatinine level of 103.7 μmol/L and urine white blood cell (WBC) count of 100.3/μL, and the results of the urine culture showed the following: Staphylococcus epidermidis (> 100000 CFU/mL) and Xanthomonas (> 100000 CFU/mL). The second patient's urine test showed a urine WBC count of 125/μL, and urine culture suggested the presence of coagulase-negative Staphylococcus (> 100000 CFU/mL). The third patient's urine test and culture showed a WBC of 204 /μL and Candida albicans (16000 CFU/mL).
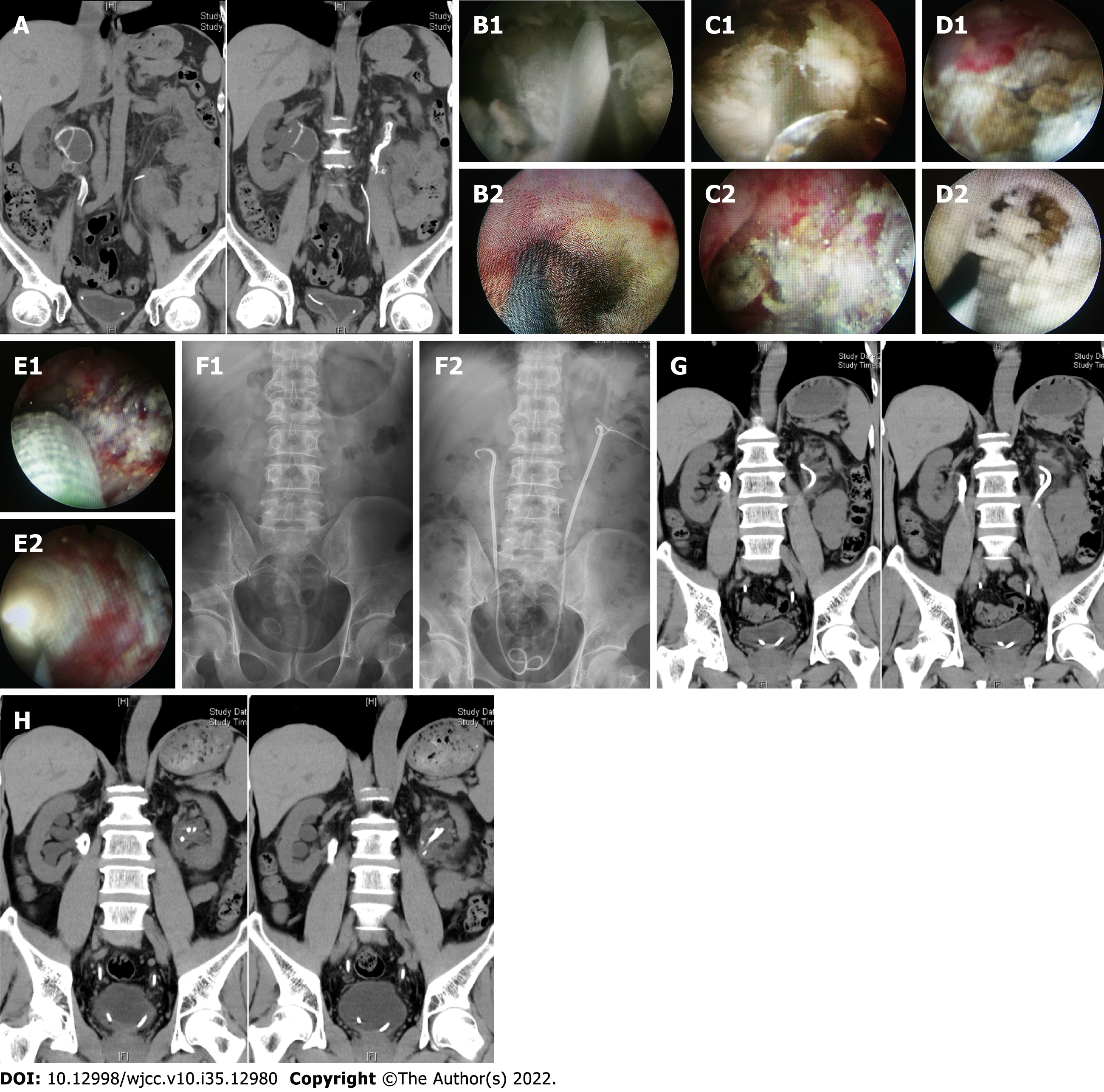
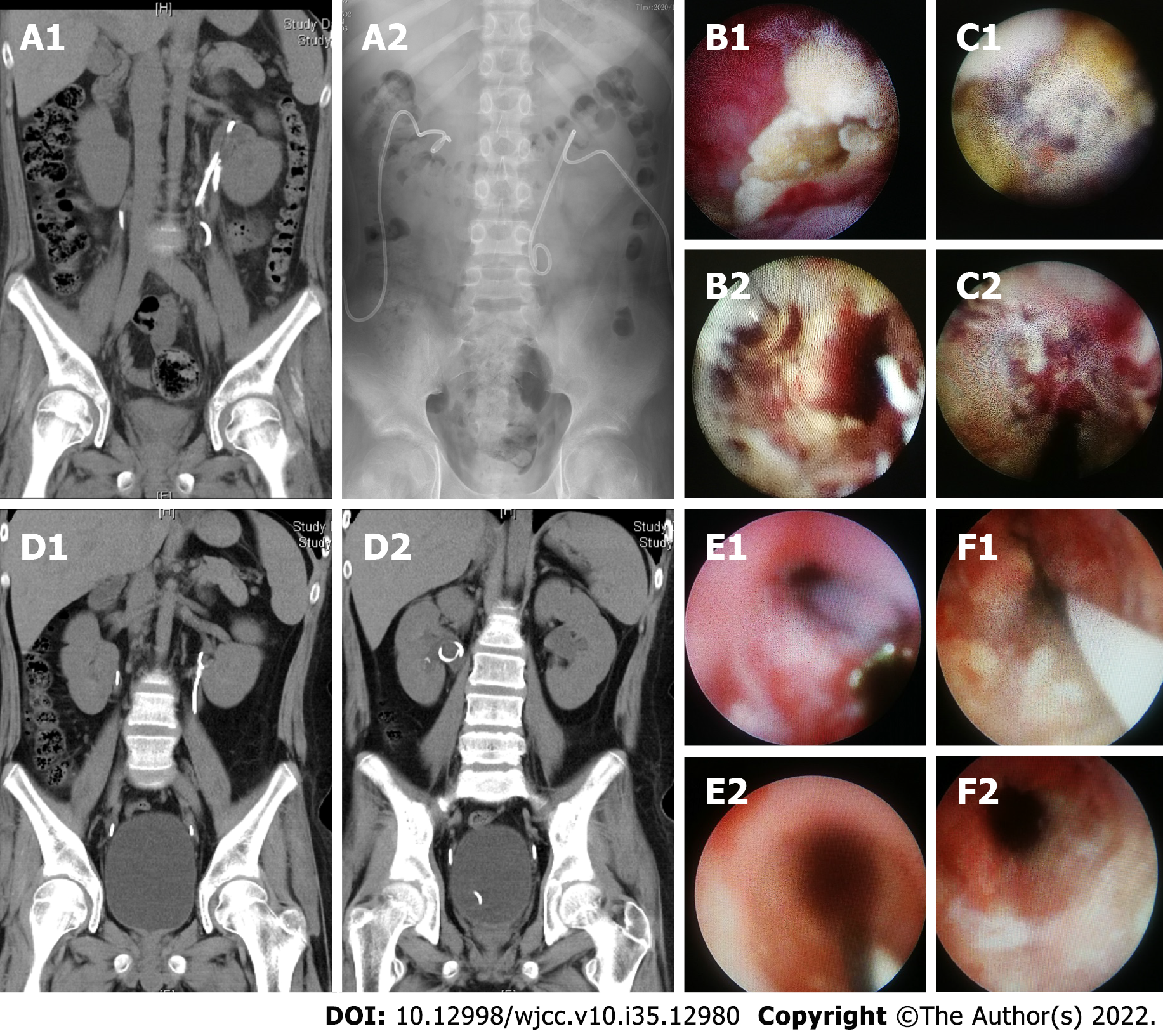
The first patient's abdominal NCCT scan performed at our hospital showed bilateral hydronephrosis and annular calcification of the renal pelvis on both sides (Figure 1A). The kidney, ureter, and bladder (KUB) scan showed bilateral upper urinary tract calcification with a stent in the descending left artery (Figure 1). The second patient's NCCT scan performed at our hospital showed bilateral dilatation of the renal pelvis and the upper ureter, as well as bilateral ureteral calculi (Figure 2). The third patient's NCCT scan performed at our hospital showed signs of bilateral lower ureteral calculi and a left retroperitoneal cystic mass (possibility of urine-derived cyst) (Figure 3).
These three patients were eventually diagnosed with urothelial encrusted pyelo-ureteritis disease.
For the first patient, vancomycin was selected after admission as an anti-infectious agent according to the urine culture and drug sensitivity test results. Additionally, a simultaneous bilateral nephrostomy was performed soon after admission, and the patient underwent bilateral urography 3 d after the first procedure. The test showed right ureteropelvic junction (UPJ) atresia of about 0.5 cm in length. Stenosis of about 1 cm in length was also discovered on the left UPJ. An endoscopic surgery using an anterograde nephroscope combined with a retrograde ureteroscope was performed on the right side after the second urine culture was negative. The surgery was performed in an oblique supine lithotomy position under general anesthesia. An 8 Fr rigid retrograde ureteroscope was placed at about the level of the lower edge of the fourth lumbar spine. A large amount of encrusted tissue can be seen, and the guidewire was blocked (Figure 1B). Meanwhile, an anterograde 24 Fr standard channel was established. The nephroscope showed extensively encrusted and necrotic tissue of the renal pelvis mucosa (Figure 1B). Additionally, a flexible anterograde ureteroscope was used to reveal the presence of lumen atresia at the blockage point indicated by resistance to the FUS and the guidewire, as well as local tissue vibration and weak light. A holmium laser was used to incise the encrusted tissue in order to insert the guidewire into the bladder. Then, a 6-cm 18 Fr balloon dilator was inserted alongside the guidewire and the atresia lumen was unobstructed after 5-min dilation. Next, a combination of a nephroscope and grasping forceps was used to scrape the loose encrusted tissue. Some of the encrusted tissue remained due to dense adhesion between the deep encrusted tissue and the urothelium (Figure 1C). A 7-12 Fr indwelling stent and a 14 Fr indwelling renal catheter were then placed. One week later, the same procedure was repeated on the patient’s left side. The surgical effects and results were significantly better than those of the right side due to the mild hardness and adhesion degree of the encrusted tissue (Figure 1D and E).
For the second patient, following preoperative anti-infection therapy, anterograde urography indicated bilateral UPJ stenosis with a length of about 1.5 cm on the left side and 1 cm on the right. Endoscopic therapy was then administered 5 d later after a negative urine culture. The patient was placed in the prone position, and a 16 Fr micro-channel was established through nephrostomy. Next, a 9.5 Fr rigid ureteroscope was used to visualize the tubular encrusted tissue (Figure 2B). UPJ stenosis extending downward to the upper ureter, with a length of about 1.5 cm. The surface calcification was first crushed using pneumatic lithotripsy and then removed with stone forceps and a basket. Fresh mucosal tissue could be seen after the completion of the procedure (Figure 2B). Meanwhile, additional upper ureteral encrusted tissue was observed on the right side by a rigid anterograde ureteroscope through a 14 Fr micro-channel. Unfortunately, the encrustation was difficult to clear due to its lower position. Therefore, a retrograde procedure was performed using lithotomy. Next, a rigid ureteroscope was inserted to reach the lesion area (Figure 2C). Then, a combination of pneumatic lithotripsy, stone forceps, and a basket was used to remove the encrusted tissue layers. A small amount of deeply encrusted tissue was left (Figure 2C). Finally, a 6 Fr indwelling double J stent and a 14 Fr renal catheter were placed on both sides.
The third patient’s retroperitoneal cystic mass was first punctured, drained, and the fluid tested. The results indicated a creatinine level of 964 μmol/L and confirmed the diagnosis of urinoma. A bilateral ureteroscopy was then performed after antifungal treatments. The left ureteroscopy showed encrusted tissue located about 5 to 8 cm from the urethral opening (Figure 3D). We proceeded to crush the surface layer using pneumatic lithotripsy. Next, the deep adhesion was moderately scraped with grasping forceps (Figure 3D). A suspected fistula was observed after injection of methylene blue through the allantoic drainage tube, and the surrounding encrustations were further removed. The right ureteroscopy showed annular encrusted tissue 1 cm in length at about 4 cm from the urethral opening (Figure 3E) which was later smashed and cleared using pneumatic lithotripsy (Figure 3G). 6 Fr indwelling double J stents were then placed on both sides.
The follow-up reexamination of patient 1 confirmed that the bilateral stents were in good positions (Figure 1F). The encrusted tissue histopathological results indicated chronic inflammation of the urinary mucosa and focal epithelial hyperplasia. Meanwhile, the calcification composition analysis showed the presence of struvite and apatite. An NCCT scan performed 3 mo later showed a small amount of calcification in the left renal pelvis and annular calcification in the right renal pelvis, with both exhibiting significant reductions in size and number compared to the preoperative NCCT (Figure 1G). An additional bilateral ureteroscopy was performed to remove the incompact encrusted tissue, and a 6Fr indwelling double J stent was later placed. NCCT follow-up at 6 mo performed after the procedure did not show any sign of encrustation on the left, and there was no obvious change on the right side either (Figure 1H). The follow-up ureteroscopy revealed no encrustation on the left side. However, an obvious encrustation was observed on the right. Subsequently, bilateral 6 Fr double J stents were replaced. The encrustation and stenosis on the right urinary tract persisted 2 years after the initial follow-up, and stents were replaced regularly because the patient refused to accept an upper urinary tract reconstruction surgery. The left urinary tract did not show abnormalities.
For the second patient, the postoperative KUB scan showed that the double J stents were in good positions. The encrusted tissue histopathological results showed hyperplasia of the urinary mucosa and chronic inflammation. Meanwhile, the calcification composition analysis determined the presence of apatite. NCCT follow-up at 3 mo did not show signs of calcification on either side (Figure 2D). Additionally, ureteroscopy indicated that there was a small amount of encrustation within the primary lesions on both sides. These lesions were immediately removed (Figure 2E and F). Next, the stents were replaced with 5 Fr double J stents on both sides. NCCT and ureteroscopy at 1.5 years showed no calcification and encrustation on either side. The follow-up was continued after removal (both sides) of the double J stents.
The third patient’s KUB scan showed that the bilateral ureteral stents were in good positions (Figure 3F). The encrusted tissue histopathological results indicated the presence of hyperplasia of the urinary mucosa and chronic inflammation. Additionally, the calcification composition analysis indicated that there were struvite and apatite. NCCT and ureteroscopy at 3 and 6 mo showed no encrustation on either side, and the follow-up was continued.
No complications such as postoperative fever, bleeding, or Clavien-Dindo grade ≥ II adverse events occurred in the three patients. Only patient 1’s urine culture indicated twice the presence of Corynebacterium during the follow-up period. The patients' demographic characteristics and clinical diagnosis and treatment are shown in Table 1.
| Gender | Age | Autoimmune diseases | Surgical history | Urine culture | Side | Location | Uronephrosis | Stenosis / atresia | Operation | Composition | Histopathology | Prognosis | Follow-up | |
| Case 1 | Male | 45 | Systemic vasculitis | URL | Staphylococcus epidermidis and Xanthomonas | Bilateral | Renal pelvis and upper ureter | Left: Moderate; Right: Severe | Left stenosis: 1 cm; Right atresia: 0.5 cm | PCNL + URL | Struvite and apatite | Chronic inflammation and focal epithelial hyperplasia | Unilateral recovery | 24 mo |
| Case 2 | Male | 12 | Dermatomyositis | Stent placement and nephrostomy | Coagulase negative Staphylococcus | Bilateral | Upper ureter | Left: Moderate; Right: Moderate | Left stenosis: 1.5 cm; Right stenosis: 1 cm | PCNL + URL | Apatite | Hyperplasia of the urinary mucosa and chronic inflammation | Bilateral recovery | 18 mo |
| Case 3 | Female | 32 | Dermatomyositis | ESWL and URL | Candida albicans | Bilateral | Middle and lower ureter | Left: Mild; Right: Mild | - | URL | Apatite | Hyperplasia of the urinary mucosa and chronic inflammation | Bilateral recovery | 12 mo |
Urothelial encrusted disease is a rare condition with various clinical manifestations and pathogenesis according to lesion location and involved areas. In 1914, François et al[4] were the first to report chronic cystitis with mucous erosion accompanied by calcinosis. During the following decades, only a few pieces of foreign literature reported on the condition, with the majority being sparse cases. The disease is even rarer in China. Currently, there is a lack of a unified standard for naming the condition. In the English literature, most scholars call it encrusted, which means "crust or scab", and some domestic scholars call it leathery lesion. The description of "encrusted" seems to be more suitable for the condition from the morphological perspective and endoscopic treatment. The lesions in the three presented cases all occurred within the upper urinary tract, even though the disease is commonly observed in the middle urinary tract. Therefore, we limit our discussion to only encrusted pyelitis and ureteritis. An encrusted disease of the upper urinary tract was first reported by Morales in 1992. He recorded an infectious disease characterized by the erosion and calcification of the renal pelvis as well as the calyceal mucosa. It was then called encrusted pyelitis, a condition not reported in China until now. In recent years, with the development of endoscopic technology and the clarification of the etiology of the disease, we now have a deeper understanding of the progression, outcome, and treatment of the condition.
The principal idea is that the cause of the disease is closely related to a urinary tract infection caused by urease-producing bacteria. There are more than 40 species of urease-producing bacteria known to cause urothelial encrusted disease, with Corynebacterium urealyticum being the most important[5-7]. Urease has a strong enzymatic activity and can make the urine alkaline and decompose urea to produce ammonia. On one hand, ammonium ions can destroy the epithelial cells of the glycosaminoglycans layer and increase bacterial adhesion to the mucous membrane. On the other hand, alkaline urine can promote the supersaturation and precipitation of organic salts such as magnesium ammonia phosphate and calcium phosphate from urine, thus forming calcifications and encrustations within the urothelial tissues, ultimately leading to the development of the disease[8]. Other urease-producing bacteria, including Proteus, Pseudomonas, Klebsiella, Staphylococcus, Ureaplasma, and Urealyticum, are also pathogenic factors of the disease. In this study, all three cases had urinary tract infections, with the cultures detecting the presence of Staphylococcus and Flavobacterium, all urease-producing bacteria, in two cases, further supporting the mainstream view. Moreover, other risk factors include: (1) Significantly reduced or suppressed immunity such as cases with rheumatic immune system diseases or immunosuppressant therapies after kidney transplantation, AIDS, serious mental diseases, and drug abuse; (2) Injuries of the urothelium as well as acute or chronic diseases such as various levels of urinary tract infection, urinary tract tumors, and conditions requiring long-term oral or perfusion of therapeutic drugs; and (3) Surgical treatments or invasive procedures such as various endoscopic urological surgeries, diversion of urine flow, as well as long-term indwelling catheter or stent placement[9-12]. In our cases, all three patients originally had compromised immunity, with the presence of vasculitis and dermatomyositis and the continuous use of immunosuppressants. Additionally, all received endoscopic treatments and had indwelling stents or renal catheters placed at the initially visited hospitals.
The typical clinical manifestations of the disease are mainly characterized by different levels of obstructions and infections of the upper urinary tract. Imaging examinations are often suggestive of hydronephrosis of the upper urinary tract, and CT or KUB scan can show diffuse or focal irregular annular high-density areas of the renal pelvis and ureter, as well as calcifications[13]. CT angiography can also reveal the severity of the obstruction and the extent of the calcification. In this study, the first patient’s NCCT and KUB scans showed irregular circular high-density shadows and calcifications within the right renal pelvis. The calcification was concentrated in the upper left ureter. The third patient’s NCCT scan showed annular high-density shadows at the junction between the urinoma and the left ureter. For the second patient, the KUB scans showed hollow tubular high-density shadows within both upper ureters. The imaging features of these three patients were significantly different from those of traditional urinary calculi and are more consistent with the traditional manifestations of urothelium encrusted disease. Further intraoperative findings confirmed that the observed annular encrusted lesions were spread through the segmental urothelium.
The combination of the patients’ past medical history, their clinical manifestations, laboratory examinations, and radiographic features led to the preliminary diagnosis of the disease. However, the exclusion of differential diagnoses, for example, urothelial neoplasms, is necessary since these conditions can also develop partial calcifications on the surface of tumors as they progress. Urothelial calcifications may also occur after urinary tuberculosis, leukoplasia, and intravenous injection of formaldehyde or cyclophosphamide. The calcified layer in cases of the encrusted disease is mainly composed of carbonate apatite and magnesium ammonia phosphate, with a small amount of matrix such as proteins[14]. The lesions are composed of three layers, with the first being calcified tissue on the surface, the second an inflammatory response layer located in the middle, and normal mucosal tissue in the innermost layer[15]. A variety of inflammatory cells can infiltrate the layers and create thick granules, leading to the formation of granulomas. The pathophysiological changes after a Corynebacterium infection lead to the activation of cytokines that promote the differentiation of urinary epithelial cells into osteoclasts and trigger the synthesis of typical osteoid proteins, further aggravating tissue damage and cicatrix-repair processes, with the end-game being the formation of encrusted tissues. Such type of fibrotic pathological change makes local tissues tough and further complicates their surgical removal. The calcification analysis and histopathology of our three cases were consistent with the relevant studies.
In cases of a urothelial encrusted disease, there are two main therapeutic goals, with the first being the clearance of the encrustation as much as needed to relieve the obstruction and the second being the effective control of the urinary tract infection. Additional specific measures include surgical treatments, sensitive antibiotic treatments, and acidification of the urine. de Perre et al[16] emphasized the necessity of surgical removal of the lesions, which might require repeated operations. Extra precautions should also be taken during the operation not only to avoid excessive procedures and unnecessary injuries but also to prevent postoperative bleeding, ureteral stricture, and other complications. Based on our hospital’s experience in treating and following similar conditions, we believe that early surgical removal of the lesions is key. In our cases, according to the extent and position of the lesions, we used a combination of minimally invasive endoscopy with anterograde percutaneous nephroscopy and retrograde ureteroscopy, coupled with pneumatic lithotripsy and stone forceps to achieve favorable results. Pneumatic lithotripsy was primarily used to smash the surface layer in cases with a thick calcification. The layers were then removed using stone grippers or baskets while controlling the strength and vibrational intensity of the handle to avoid excessive damage or perforation of the mucosal layer. The surface calcification is usually brittle and easy to remove. In contrast, the deep encrustations are hard and compact, and should be dealt with gently to prevent tissue coloboma and ischemia. However, deep encrustations that are particularly difficult to remove may be partially retained. Additionally, secondary endoscopic examinations performed in such conditions have shown that most of the remaining encrusted tissues might fall off spontaneously after the self-repair process of the mucosal layer. The process often lasts half a year after the surgery and can potentially lead to a normal restoration. Moreover, for lesions with thicker and wider encrustation areas, which are harder to operate during the first stage, it is preferable to unblock the lumen and place a stent in preparation for the second-stage operation. However, it has been shown that the treatment effect and prognosis are often poor in such cases, and reconstruction and repair surgeries might be needed. Usually, an anti-infection therapy should be used during the early stages before the surgery, according to the urine culture. Corynebacterium is particularly sensitive to glycopeptides[17]. Since the initial operation cannot always completely remove the encrustation and the remaining necrotic tissues probably contain bacterial colonies, we recommend an appropriate extension of the duration of the medication and regular urine bacteriological tests after patient discharge. Additional treatments such as the acidification of the urine are considered effective in removing residual calcification and preventing encrustations through inhibition of struvite and carbonate apatite supersaturation[18,19]. The most commonly used oral drugs are acetohydroxamic acid and methionine. Meanwhile, perfusions via renal catheters or retrograde ureteral catheters can be performed using Thomas C24 and Suby's G solutions. These solutions have bactericidal effects and can induce the formation of a calcium citrate complex to prevent crystallization[20,21]. In this study, two of the patients had a urine pH < 6 and did not receive a urine acidification treatment. The remaining patient was given oral acetohydroxamic acid (15 mg/kg, < 1000 mg/d) but failed to continue the treatment due to adverse gastrointestinal symptoms and nervous system reactions. Another crucial component of the treatment is a close follow-up after the surgery. Indeed, the postoperative repair process involves a constant shedding of necrotic tissues which can easily obstruct the stent. Moreover, the intensity and duration of oral antibiotics should be adjusted according to the urine test after patient discharge, and intravenous antibiotics should be considered if necessary. The second patient in our study can be considered as a typical example of the need for such measures since he developed a persistent fever and hydronephrosis about 4 mo after the operation. We subsequently performed a stent replacement procedure and treated him with an intravenous antibiotic regimen. He recovered within 1 wk. Another effective therapeutic option is to actively treat the primary disease to reduce the intensity and dosage of the used immunosuppressive drug.
From 1990 to 2022, a total of 34 articles about encrusted pyelitis or encrusted ureteritis were retrieved, including 27 case reports, 2 retrospective studies, and 4 reviews, involving a total of 42 cases. There were 19 cases of renal transplantation or immunosuppressive status (45.2%), 8 cases of urological surgery (19%), 12 cases of urinary tract invasive operation (28.5), 11 cases of urinary tract non-infectious diseases (26.2%), 11 cases of urinary infection or other infectious diseases (26.2%), and 7 cases of rheumatic immune diseases (16.7). The positive rate of urine culture was 85.7%; Corynebacterium was detected in 65.5% of cases, urealyticum (ureaplasma) in 17.2%, Escherichia coli in 3.4%, Staphylococcus epidermidis in 1.7%, and uncertain and negative results were obtained in 10.3%. Treatment measures included anti-infective therapy (83.3%), urine acidification therapy (oral or local perfusion; 92.8%), and surgical therapy (16.6%). The overall prognoses were as follows: Only 6 cases were cured (14.3%), 24 cases were improved (57.1%), 14 cases aggravated (33.3%), and 5 cases were uncertain or lost to follow-up (11.9%). According to the above statistics, it can be seen that the current treatment of this disease is difficult: The experience of surgical treatment is little, the prognoses are poor, and there is no definite treatment standard. In our center, endoscopic surgery as the main means of treatment has achieved good efficacy. In the three patients reported here, who had six lesions, five lesions basically reached the cured level, which provides some valuable reference for the treatment of this disease.
At present, there has been no unified diagnostic standard for this disease. Due to its particularity, the early stage of this disease is prone to misdiagnosis. Based on this study and literature reports, we preliminarily propose several diagnostic criteria for urinary epithelial encrusted diseases. Major diagnostic indicators include: (1) Previous autoimmune disease or chronic kidney disease with immunosuppressive drug therapy; (2) Annular or tubular high-density nidus of the collecting system observed on preoperative imaging examination; and (3) Diffuse or segmentary calcification (scab) lesions of the urothelium in the lumen. Secondary diagnostic indicators include: (1) Urinary tract infection or positive urine culture (urease-producing bacteria); (2) Previous history of urological surgery or endoscopic procedure; and (3) Calcification analysis indicates infectious stones. The disease can be initially diagnosed if ≥ 2 primary indicators and ≥ 1 secondary indicator are met.
Urothelial encrusted pyelo-ureteritis disease is rare in clinical settings. This work shows that the minimally invasive endoscopic treatment of an upper urinary tract obstruction caused by this diseases is effective. Moreover, patients can benefit from an early diagnosis, rational methods to clear the encrustation and relieve obstructions, and the active control of both the infection and the primary disease. Furthermore, long-term follow-up is also necessary for the assessment of the efficacy and effectiveness of the treatment and adjuvant measures.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bellini MI, Italy; Nakamura K, Japan S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Fu JG, Xie KJ. Successful treatment of encrusted cystitis: A case report and review of literature. World J Clin Cases. 2020;8:4234-4244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 2. | Morales JM, Aguado JM, Diaz-Gonzalez R, Salto E, Andres A, Campo C, Praga M, Martinez MA, Leiva O, Rodriguez-Noriega A. Alkaline-encrusted pyelitis/cystitis and urinary tract infection due to corynebacterium urealyticum: a new severe complication after renal transplantation. Transplant Proc. 1992;24:81-82. [PubMed] |
| 3. | Mhiri MN, Rebai T, Hammami A. Encrusted pyelo-ureteritis. Ann Urol (Paris). 1992;26:340-343. [PubMed] |
| 4. | François JJ. La cystite incrustée. [cited 20 September 2022]. Available from: https://www.semanticscholar.org/paper/La-cystite-incrust%C3%A9e-Clinique-Lefi/69b188d36309cf50a22fdf1da7202f67d8efd51b. |
| 5. | Meria P, Desgrippes A, Arfi C, Le Duc A. Encrusted cystitis and pyelitis. J Urol. 1998;160:3-9. [PubMed] |
| 6. | Thoumas D, Darmallaicq C, Pfister C, Savoye-Collet C, Sibert L, Grise P, Lemaitre L, Benozio M. Imaging characteristics of alkaline-encrusted cystitis and pyelitis. AJR Am J Roentgenol. 2002;178:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Ito M, Kanno T, Kawase N, Taki Y. Encrusted cystitis with ammonium acid urate calculi: a case report. Hinyokika Kiyo. 2002;48:221-224. [PubMed] |
| 8. | Soriano F, Tauch A. Microbiological and clinical features of Corynebacterium urealyticum: urinary tract stones and genomics as the Rosetta Stone. Clin Microbiol Infect. 2008;14:632-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Pagnoux C, Berezne A, Damade R, Paillot J, Aouizerate J, Le Guern V, Salmon D, Guillevin L. Encrusting cystitis due to Corynebacterium urealyticum in a patient with ANCA-associated vasculitis: case report and review of the literature. Semin Arthritis Rheum. 2011;41:297-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Favre G, García-Marchiñena P, Bergero M, Dourado L, González MI, Tejerizo J, Damia O. Diagnóstico y tratamiento de la cistitis incrustante [Diagnosis and treatment of the encrusted cystitis]. Actas Urol Esp. 2010;34: 477-478. [PubMed] |
| 11. | Johnson M, Perkins SQ, Leavitt D. Alkaline-Encrusted Pyelitis Causing Renal Failure in a Transplant Kidney: Treatment with Percutaneous Nephrolithotomy and Urinary Acidification. J Endourol Case Rep. 2020;6:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Sakhi H, Join-Lambert O, Goujon A, Culty T, Loubet P, Dang J, Drouot S, de Bayser H, Michaud C, Ghislain L, Stehlé T, Legendre C, Joly D, Meria P, Zaidan M. Encrusted Urinary Tract Infections Due to Corynebacteria Species. Kidney Int Rep. 2021;6:179-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Semaan A, Tayeh GA, Chebel JA, Hallit R, Matta M, Hajj P. Arcanobacterium pyogenes and encrusted pyelitis. Future Sci OA. 2019;6:FSO430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zheng J, Wang G, He W, Jiang N, Jiang H. Imaging characteristics of alkaline-encrusted cystitis. Urol Int. 2010;85:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Vergura M, Carosi I, Ercolino G, Palladino D, Prencipe M, Scarlatella A, Aucella F. [Encrusted Pyelitis during a case of Thrombotic Thrombocytopenic Purpura]. G Ital Nefrol. 2018;35. [PubMed] |
| 16. | Van de Perre E, Reichman G, De Geyter D, Geers C, Wissing KM, Letavernier E. Encrusted Uropathy: A Comprehensive Overview-To the Bottom of the Crust. Front Med (Lausanne). 2021;7:609024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Riar SK, Purandare AV, Koenig JF. More Than Dysuria: Corynebacterial Encrusted Cystitis. Urology. 2021;149:e18-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Saljoghi R, Lipsker A, Caillet K, Malaterre J, Le Roux F, Pignot G, Saint F. Encrusted Uretero-pyelitis: Case Report. Urol Case Rep. 2016;7:58-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sabiote L, Emiliani E, Kanashiro AK, Balañà J, Mosquera L, Sánchez-Martín FM, Millán F, Alonso C, Palou J, Angerri O. Oral Acidification with l-Methionine as a Noninvasive Treatment for Encrusted Uropathy. J Endourol Case Rep. 2020;6:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ito K, Takahashi T, Kanno T, Okada T, Higashi Y, Yamada H. Renal failure due to encrusted cystitis and pyelitis. IJU Case Rep. 2020;3:112-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Loghmari A, Bouassida K, Belkacem O, Othmane MB, Hmida W, Jaidane M. The importance of surgical treatment in encrusted cystitis and pyelitis: A case report. Int J Surg Case Rep. 2020;77:392-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |