Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12959
Peer-review started: August 16, 2022
First decision: September 26, 2022
Revised: October 8, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: December 16, 2022
Processing time: 120 Days and 3 Hours
As a first-line treatment regimen for Helicobacter pylori (H. pylori) infection, antibiotic therapy is widely used worldwide. However, the question of increasing antibiotic resistance must be considered. Given this issue, we need to find ways to reduce drug resistance. This study examined all currently available first-line regimens and compared them with standard triple treatment through a network meta-analysis of randomized controlled trials (RCTs).
To compare first-line treatment regimens for eradication of antibiotic-resistant H. pylori strains.
To compare the effectiveness of the first-line regimens for treating H. pylori infection, a Bayesian network meta-analysis was applied to process data extracted from RCTs. The plausible ranking for each regimen was assessed by the surface under the cumulative ranking curve (SUCRA). In addition, we conducted a relevant search by reference citation analysis.
Twenty-five RCTs involving 12029 participants [including 1602 infected with clarithromycin (CAM)-resistant strains and 1716 infected with metronidazole (MNZ)-resistant strains] were included, in which a total of seven regimens were used for H. pylori eradication. The results showed that dual therapy containing a high-dose proton pump inhibitor (HDDT) [odds ratio (OR): 4.20, 95% confidence interval (CI): 2.29-8.13] was superior to other therapies for all patients, including those with CAM/MNZ-resistant H. pylori infection. In the comparative effectiveness ranking, for CAM-resistant H. pylori, HDDT (OR: 96.80, 95%CI: 22.46-521.9) had the best results, whereas standard triple therapy ranked last (SUCRA: 98.7% vs 0.3%). In the subgroup of high cure rates (≥ 90%), HDDT was also generally better than other therapies.
For the eradication of CAM- and MNZ-resistant H. pylori strains, HDDT exhibited considerable advantages. The studies of CAM-resistant H. pylori were based on small samples due to a lack of antibiotic sensitivity tests in many RCTs, but the results showed that all patients, including those with CAM-resistant H. pylori infection, had a concordant trend. Overall, HDDT may be a reference for RCTs and other studies of H. pylori eradication.
Core Tip: This is the first study to compare currently available first-line treatment regimens for eradication of antibiotic-resistant Helicobacter pylori strains. For clarithromycin-resistant and metronidazole-resistant strains, dual therapy containing a high-dose proton pump inhibitor (HDDT) shows an absolute advantage over other first-line therapies. There was a difference in the effectiveness of HDDT between all patients and patients with clarithromycin-resistant Helicobacter pylori infection. In the subgroup of high cure rates (≥ 90%), HDDT was also generally better than other therapies. The use of fewer antibiotics may be better to prevent global antibiotic resistance effectively.
- Citation: Zou SP, Cheng Q, Feng CY, Xu C, Sun MH. Comparative effectiveness of first-line therapies for eradication of antibiotic-resistant Helicobacter pylori strains: A network meta-analysis. World J Clin Cases 2022; 10(35): 12959-12970
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12959.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12959
Helicobacter pylori (H. pylori) infection is a severe health problem affecting almost half of the global population; the organism not only causes gastric acid-related diseases but is also closely associated with gastric malignancies[1,2]. In addition, the World Health Organization classified H. pylori as a Group 1 carcinogen. For example, in Asia, the percentage of H. pylori-infected population varies across countries: 79% in India, 75% in Vietnam, 60% in South Korea, and 58% in China[3]. In 2020, the Taipei Global Consensus highlighted that eradication of H. pylori could beat the target of reducing death rates from gastric cancer[4]. It is extremely important to determine the best eradication treatment for H. pylori to prevent gastric cancer. However, the eradication rate achieved by the traditional first-line regimen consisting of clarithromycin (CAM), amoxicillin (AMX), and proton pump inhibitors (PPIs) has recently declined due to an increase in CAM-resistant H. pylori strains[5]. Univariate and multivariate analyses have identified resistant bacteria, inadequate gastric acid inhibition, and traditional triple therapy as risk factors for eradication failure[6,7]. Additionally, the calculation of total eradication rate considered both antibiotic susceptible and resistant H. pylori strains. Depending on drug-resistant bacteria, several treatments have been tried, including sequential therapy, traditional quadruple therapy, combination therapy, and dual therapy containing a high dose PPI (HDDT)[8]. In a meta-analysis performed by Zhu et al[9], 15 randomized control trials (RCTs) with 3818 patients were eligible for inclusion. Trial sequential analysis showed reliable evidence that HDDT was equivalent to the recommended regimens, including standard triple therapy, bismuth quadruple therapy (BQT), and non-BQT[9]. Network meta-analysis (NWM) blends direct and indirect evidence in various RCTs and provides a relative and referable result among three or more therapeutic interventions[10]. Although there are recent pairwise meta-analyses including NWM, none have compared the current therapeutic interventions for antibiotic-resistant H. pylori strains. The purpose of our current study was to compare the effectiveness of vonoprazan (VPZ)-based and PPI-based first-line treatment regimens using NWM and to rank the treatments. To reach reliable conclusions, we only included RCTs with a minimal risk of bias.
The scheme of the study was successfully registered at PROSPERO (registration number: 42022326460). The quality of evidence and data derived from NWM was evaluated using the Grading of Recommendations Assessment, Development and Evaluation and Cochrane Handbook (Version 6.3, 2022).
PubMed, EMBASE, Web of Science, OVID, Cochrane Library (all years up to March 2022), and Cochrane Central Register of Controlled Trials (CENTRAL, all years up to March 2022) were searched using the following keywords: (“vonoprazan”, “VPZ”, “potassium-competitive acid blocker”, “P-CAB”, “TAK438”, or “TAK-438”) OR (“PPI”, “proton pump inhibitor”, “PPIs”) AND (“Helicobacter pylori”, “H. pylori”, “HP”). We also manually searched the references of all identified trials, relevant review articles, and conference abstracts about antibiotic-resistant strains. In addition, we conducted a relevant search by reference citation analysis.
We formulated the inclusion and exclusion criteria before conducting study searches. Additionally, the latest relevant studies were searched. Appropriate RCTs were included in the NWM according to the following criteria: (1) Adult patients with Helicobacter pylori infection; (2) studies reported in English; (3) treatment including VPZ or PPIs; (4) cases stratified by antibiotic susceptibility; (5) H. pylori infection before and after treatment confirmed by one or more of the following methodologies: The rapid urease test, culture, the 13C-urea breath test, and the stool H. pylori antigen test; (6) RCTs with first-line therapy (except levofloxacin-containing treatments); and (7) human studies[11].
Studies were excluded based on the following criteria: (1) Non-RCTs and observational studies; (2) lack of antibiotic sensitivity testing; and (3) animal studies.
Two investigators (Chan X and Cheng Q) skimmed the literature independently, and standards-compliant studies were extracted and recorded. When a disagreement arose, a consensus was reached by discussion with other investigators.
Two reviewers (Zou S and Feng C) independently used processed data forms to extract the data from the eligible studies. The following information was extracted: First author, study title, year of pub-lication, study design, participants, study period, trial number, treatment period, criteria of eradication, eradication rate (intention-to-treat [ITT]), and other details[11].
In the latest review, Graham et al suggested that to be clinically relevant, the primary regimen being tested should achieve a cure rate of ≥ 90% unless it is impossible to achieve with an optimized regimen[12]. To achieve high cure rates, we performed a subgroup analysis of the RCTs in the high cure rate group (≥ 90%).
For binary NWM and heterogeneity estimation with Bayesian analysis, we followed the approach described by the Cochrane Handbook and evaluated inconsistencies by node splitting. The NWM accounted for heterogeneity utilizing the random-effects model. The surface under the cumulative ranking curve (SUCRA) metric was used to rank the effectiveness of each treatment and identify the best treatment[10,13]. R (version 4.2.0) and Stata (version 14.0) were used for statistical analyses. All P values were two-tailed, and a P value < 0.05 represented significant differences for all measurements[10].
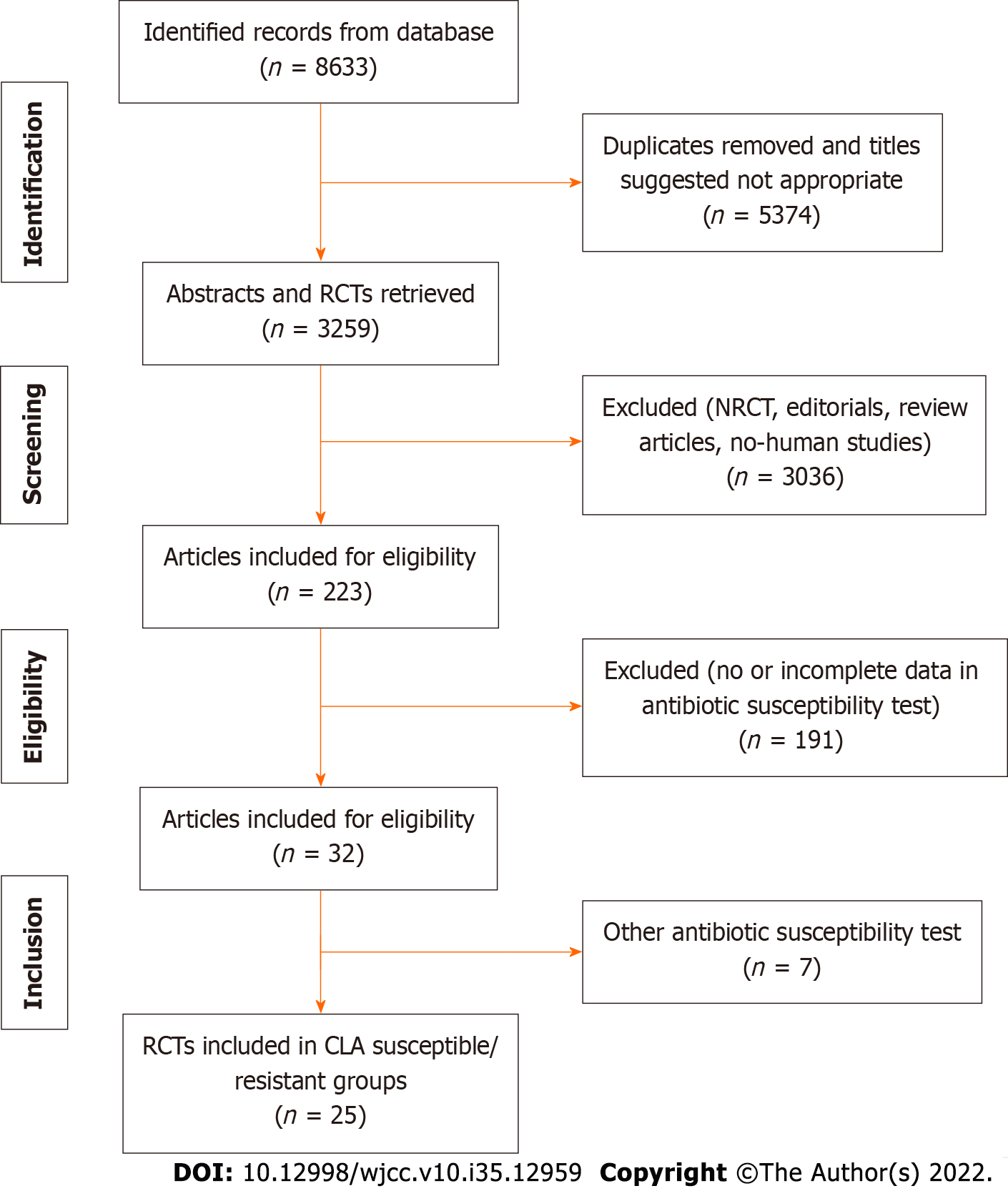
A flow diagram of the study selection, including inclusion and exclusion criteria, is shown in Figure 1. As shown in Supplementary Table 1, among 25 studies, 5 RCTs for VPZ and 19 RCTs for PPIs were applied to clarithromycin-susceptible and -resistant H. pylori strains[14-38]. Other studies used metronidazole (MNZ) resistance for qualitative examination. A total of 12029 participants (including 1602 participants for CAM resistance) from RCTs were analyzed. The rate of CAM resistance was 13.3% in our network meta-analysis. There were 19 two-arm RCTs and 5 three-arm RCTs, including 12 paired comparisons in total and 9 indirect comparisons in NWM. The characteristics of the above RCTs, such as study ID, type of article, trial number, study design, participants, and treatment duration, are shown in Supplementary Table 1. The seven first-line treatment regimens used were: (1) VPZ dual therapy (Vono-dual therapy at conventional dose); (2) VPZ triple therapy (Vono-triple therapy); (3) sequential therapy (PPI-based therapy); (4) HDDT; (5) PPI-bismuth QT; (6) PPI-nonbismuth QT; and (7) PPI-triple therapy.
The network map of 25 studies from the databases is depicted in Figure 2. The network included direct and indirect comparisons. The inconsistent and consistent model of the network showed no significant difference (P = 0.864, > 0.05). In the map, the node size and edge thickness reflected the number of patients allocated to each regimen. Data were pooled using the random-effects model.
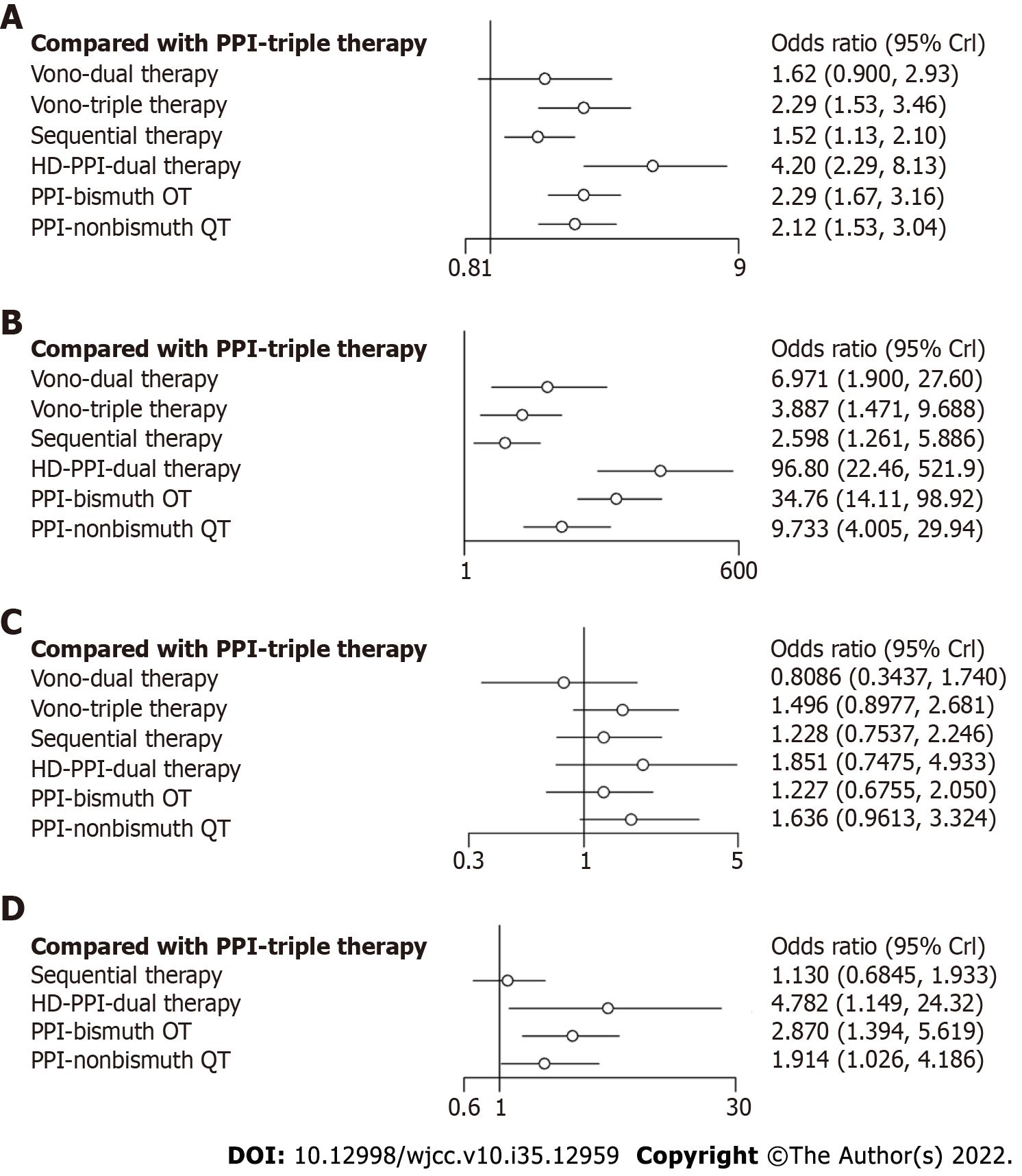
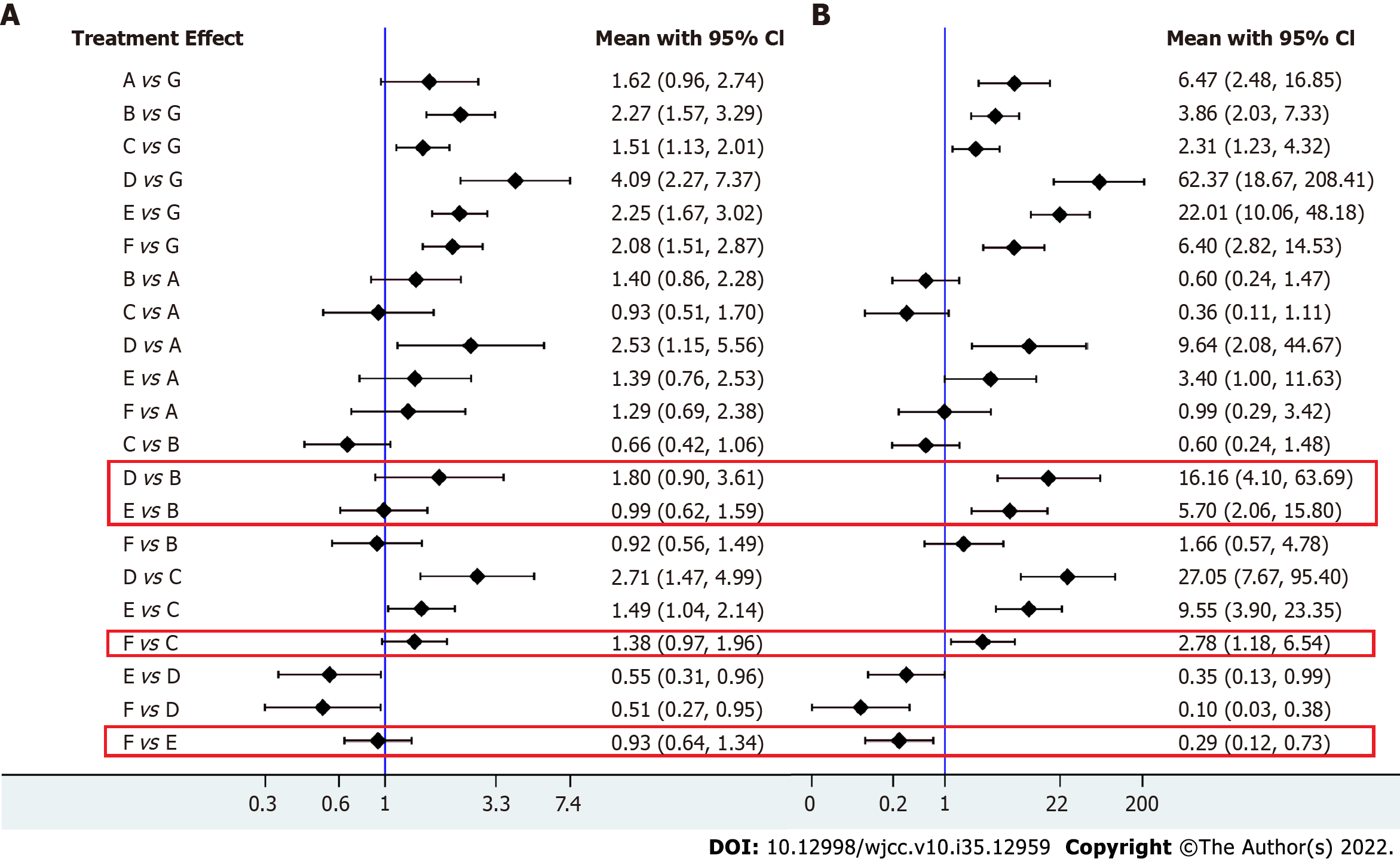
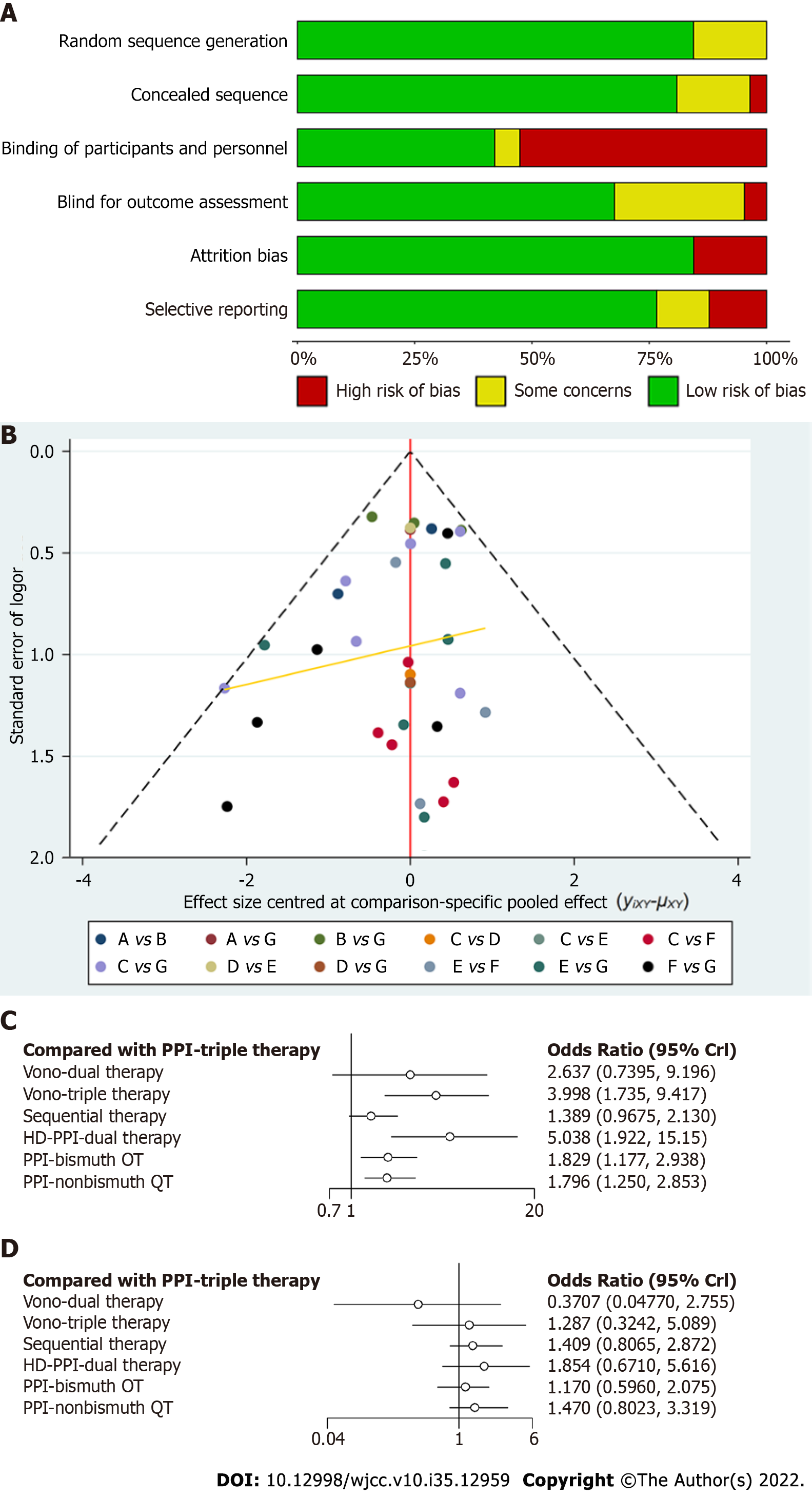
In our NWM, all therapies for CAM-resistant H. pylori were compared with PPI triple therapy. The efficacy of other treatments for all patients and patients with CAM-resistant H. pylori infection is depicted in Figure 3A and B. Twenty-five individual direct pair comparisons grouped into 12 pairwise regimens and the heterogeneity of meta-analyses are shown in Supplementary Figure 1. In Figure 3A and B, the comparisons of HDDT vs PPI-triple therapy (odds ratio [OR]: 96.80, 95%CI: 22.46-521.9), PPI-BQT vs PPI-triple therapy (OR: 34.76, 95%CI: 14.11-98.92), PPI-nonbismuth QT vs PPI-triple therapy (OR: 9.73, 95%CI: 4.01-29.94), Vono-dual therapy vs PPI-triple therapy (OR: 6.97, 95%CI: 1.90-27.6), Vono-triple therapy vs PPI-triple therapy (OR: 3.89, 95%CI: 1.47-9.69), and sequential therapy vs PPI-triple therapy (OR: 2.60, 95%CI: 1.26-5.89) all yielded significant results for CAM-resistant H. pylori and were consistent with the results on all patients. In Figure 4, the network forest plot of the league matrix illustrates all 21 pair network comparisons of regimens included in the RCTs. Furthermore, the comparisons of Vono-dual therapy vs sequential therapy (OR: 2.69, 95%CI: 0.56-12.4), HDDT vs PPI-nonbismuth QT (OR: 10.0, 95%CI: 1.74-53.7), and PPI-bismuth QT vs PPI-nonbismuth QT (OR: 3.56, 95%CI: 1.08-10.08) yielded significant results. In Supplementary Figure 3, the node-splitting analysis was non-significant for all results (P > 0.05), meaning that indirect comparisons of our NWM were consistent with direct comparisons. On the other hand, the loop-specific heterogeneity showed that each loop of NWM was congruent, as shown in Supplementary Figures 4 and 5.
For CAM-resistant H. pylori, we found apparent differences compared with all patients in Figure 4A and B: PPI-nonbismuth QT vs PPI-bismuth QT (OR: 0.29, 95%CI: 0.12-0.73), HDDT therapy vs Vono-triple therapy (OR: 16.16, 95%CI: 4.10-63.69), PPI-bismuth QT vs Vono-triple therapy (OR: 5.7, 95%CI: 2.06-15.80), and PPI-nonbismuth QT vs sequential therapy (OR: 2.78, 95%CI: 1.18-6.54).
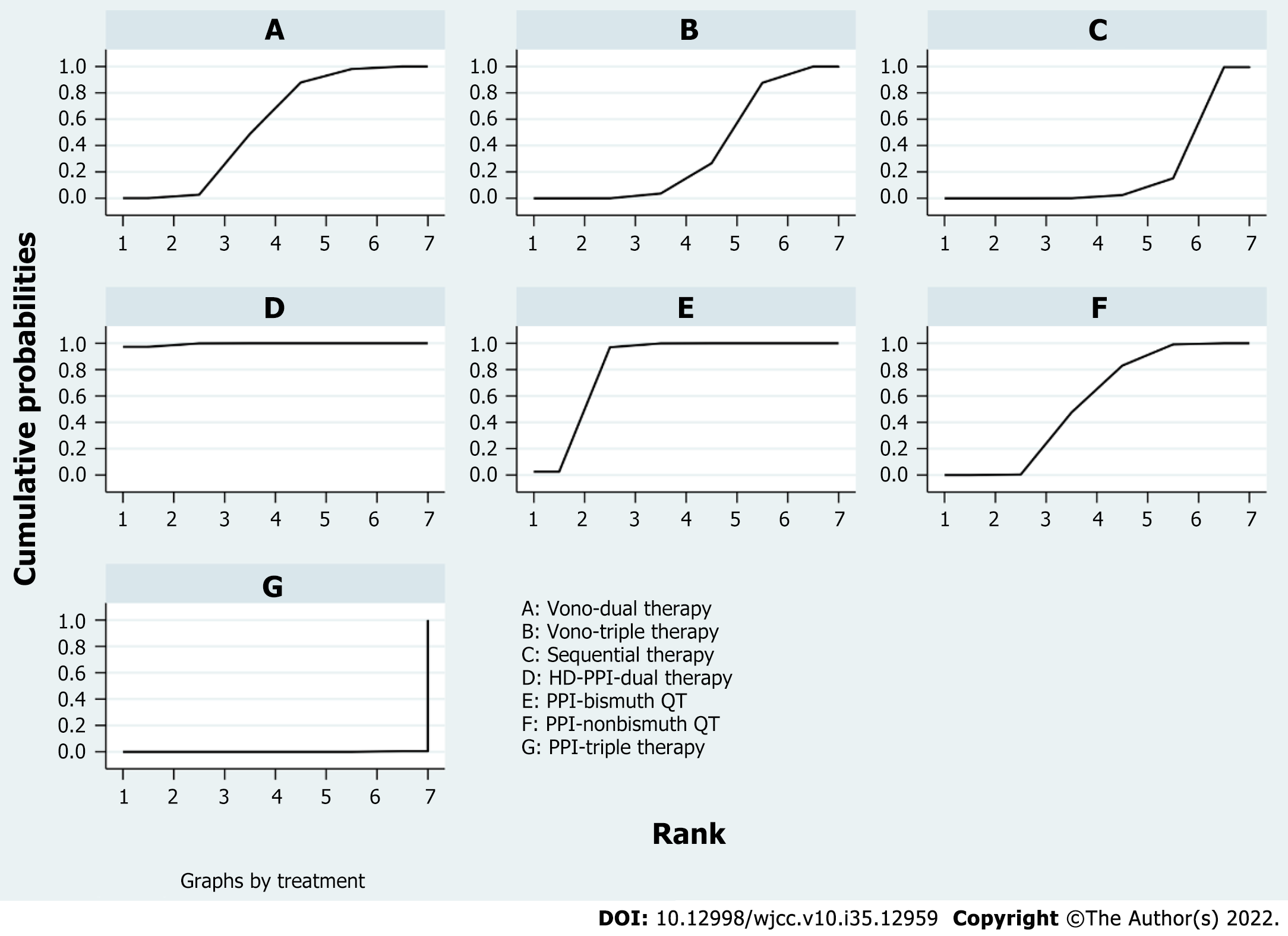
According to the league matrix and the SUCRA in NWM, the comparative efficacies of the seven regimens are shown in Figure 5. The results might be unexpected; nevertheless, they were reliable. The SUCRA value of HDDT as the best treatment was 98.7% and that of PPI-triple therapy as the worst treatment was 0.3%. Remarkably, the number of VPZ-related studies was not so considerable.
In the quality evaluation of the included RCTs, the risk of bias is shown in Supplementary Figure 6 and Figure 6A. According to the risk of bias tool (Cochrane Handbook, 2.0), blinding of participants and personnel was the main source of potential bias[2]. This was the result of fifteen studies using an open-label design, whereas seven studies were double-blinded and three studies were indeterminate. In Figure 6B, the relevant funnel plot showed perfect symmetry, and there was no evidence of publication bias.
For CAM-susceptible strains, the network forest plot is displayed in Figure 3C. The results showed that the seven different regimens were almost coincident in confidence intervals. Overall, the comparisons of HDDT vs PPI-triple therapy (OR: 1.85, 95%CI: 0.75-4.93) and PPI-nonbismuth QT vs PPI-triple therapy (OR: 1.64, 95%CI: 0.96-3.32) yielded significant results.
Figure 3D shows the eradication of MNZ-resistant strains. Twenty-two included studies only contained PPI-based researches. Therefore, there were only efficacy comparisons between C, D, E, F, and G. Compared with PPI-triple therapy, HDDT (OR: 4.78, 95%CI: 1.15-24.32) and PPI-bismuth QT (OR: 2.87, 95%CI: 1.39-5.62) showed obvious curative effects. Other antibiotic-resistant strains, including levofloxacin and amoxicillin, were not analyzed in our study because few RCTs completed antibiotic sensitivity tests.
As shown in Figure 6C, the network forest plot shows that HDDT (OR: 5.04, 95%CI: 1.92-15.15) and Vono-based therapy (OR: 4.00, 95%CI: 1.74-9.42) had substantial advantages over PPI-triple therapy (≥ 85%) in all patients (Figure 6C). In Figure 6D, the network forest plot shows that HDDT was slightly superior to PPI-triple therapy (≥ 85%) in CAM-susceptible H. pylori strains.
In the present network study, the random model that we used showed great convergence diagnostics, as shown in Supplementary Figure 5. Some differences between CAM-resistant and other H. pylori strains were found (Figure 4). Compared with PPI-triple therapy, HDDT showed tremendous advantages. PPI-bismuth QT (OR: 2.29, 95%CI: 1.67-3.16) and PPI-nonbismuth QT (OR: 2.12, 95%CI: 1.53-3.04) also yielded significant results in all patients. Compared to PPI-triple therapy in patients infected with CAM-resistant H. pylori strains, Vono-based therapy was unlikely to be better than other PPI-based therapies. However, the number of Vono-based RCTs was much less than that of PPI-based RCTs in our study. Similarly, sequential therapy also failed to achieve the desired result, because it included different antibiotics and methods. In CAM-susceptible strains, the curative effects of other treatments might be similar to those of PPI-triple therapy. The results also showed that HDDT probably had the best effect. H. pylori can cause peptic ulcers and finally result in stomach cancer in certain conditions[39]. According to relevant recommendations for the diagnosis from the European and American College of Gastroenterology, people with H. pylori infection are suggested to receive eradication therapy[40]. Because antibiotic resistance is increasing worldwide, we need to find ways to reduce drug resistance. The standard triple therapy for H. pylori eradication, including PPI, AMX, and CAM, has been used as the first-line therapy[41]. As both primary and secondary resistance to amoxicillin remain rare in most countries, HDDT may be an accessible and reasonable option for eradicating H. pylori. Moreover, HDDT, which uses fewer antibiotics than other eradication regimens, restrains the development of resistance[42]. Furthermore, the dose frequency is essential for efficacy of PPI-amoxicillin dual therapy[43]. In a subgroup analysis of HDDT, a more significant effect was observed in trials dosing four times daily in comparison with trials dosing three times daily[9].
The primary problem of H. pylori eradication is the increasing antimicrobial-resistant strains[44]. Unregulated use of antibiotics will only result in serious drug-resistant consequences[42]. Therefore, intelligent use of antibiotics may be one of the best and most effective ways to solve the problem[44]. HDDT adopts a high dose of PPI and AMX and does not include more antibiotics. In 2015, a multicenter randomized controlled trial by Yang et al reported the use of dual therapy with rabeprazole (20 mg, qid) and AMX (750 mg, qid) for H. pylori eradication in Taiwan[22]. Their results showed an evident advantage (95.3% in ITT and 96.6% in per-protocol [PP] analyses), even in CAM-resistant strains (95.7% in PP analyses). HDDT would be superior to standard first-line therapy and can be used as a rescue therapy for H. pylori infection[42]. However, Hu et al[45] showed no satisfactory H. pylori eradication rates (81.6% in ITT and 83.5% in PP analyses) achieved by HDDT in China. In 2019, Yang et al[46] in a single-center randomized controlled study, compared 14 d dual therapy with bismuth-containing quadruple therapy for H. pylori eradication and found an advantage of 14 d achieved by dual therapy. In addition, VPZ-based therapy is superior to conventional PPI-based therapy in many studies[39,47,48].
Our findings have the following limitations: (1) VPZ-based therapy and HDDT was used in only two RCTs, respectively, and the results might be underrated or overrated because of the lack of antibiotic sensitivity tests in many studies; (2) VPZ was only recommended as a first-line regimen in Japan, and there were few reported applications of VPZ-based therapy for H. pylori eradication in other countries; (3) in our network study, we did not consider the therapy duration of H. pylori eradication; (4) to ensure the consistency of first-line treatments, we excluded levofloxacin-based therapy and other line regimens; and (5) little data was obtained in children, which limited the generalizability of our findings.
In conclusion, this is the first study to compare first-line treatments for eradication of antibiotic-resistant H. pylori strains. The therapeutic effect of VPZ-based therapy for eradicating CAM-susceptible H. pylori strains is nearly the same as that of PPI-based therapy. However, for CAM-resistant and MNZ-resistant strains, HDDT shows absolute advantages over PPI-triple therapy. According to included RCTs, HDDT is superior to major PPI- and VPZ-based therapies for eradication of CAM-resistant H. pylori strains. In our study, we can observe the immense potential of HDDT, which perhaps solves the problem of antibiotic resistance in H. pylori eradication. On the other hand, we found that many RCTs were excluded because of the lack of antibiotic sensitivity tests. Therefore, our study provides physicians and researchers with more options. In fact, H. pylori therapy should be based on its absolute cure rates and local conditions. However, additional multicenter studies are required to confirm this assumption and the conclusion.
Helicobacter pylori (H. pylori) bacteria can cause peptic ulcers and finally result in stomach cancer in certain conditions. Meanwhile, the question of increasing antibiotic resistance must be considered. Given this issue, we need to find ways to reduce drug resistance. This study examined all first-line regimens and compared them with standard triple treatment through a network meta-analysis of randomized controlled trials (RCTs).
To the best of our knowledge, there are no relevant network meta-analyses comparing first-line treatment regimens for eradication of antibiotic-resistant H. pylori strains.
To compare first-line treatment regimens for eradication of antibiotic-resistant H. pylori strains.
A comprehensive search was performed in databases such as PubMed, EMBASE, Web of Science, OVID, Cochrane Library (all years up to March 2022), and Cochrane Central Register of Controlled Trials (all years up to March 2022).
Twenty-five RCTs consisting of 12029 participants [including 1602 infected with clarithromycin (CAM)-resistant strains and 1716 infected with metronidazole (MNZ)-resistant strains] were included, in which seven regimens were used for H. pylori eradication. The results showed that dual therapy containing a high-dose proton pump inhibitor (HDDT) [odds ratio (OR): 4.20, 95% confidence interval (CI): 2.29-8.13] was superior to other therapies for all patients, including those infected with CAM/MNZ-resistant H. pylori strains. In the comparative effectiveness ranking, for CAM-resistant H. pylori strains, HDDT (OR: 96.80, 95%CI: 22.46-521.9) had the best results, whereas standard triple therapy ranked last (SUCRA: 98.7% vs 0.3%). In the subgroup of high cure rates (≥ 90%), HDDT was also generally better than other therapies.
For eradication of CAM- and MNZ-resistant H. pylori strains, HDDT have a considerable advantage. Overall, HDDT may be a reference for RCTs and other studies of H. pylori eradication.
Additional multicenter studies are required to confirm the conclusion of this study.
We thank all the technicians at the Department of Pharmacy, Tongji Hospital for their technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reshetnyak VI, Russia; Toyoshima O, Japan S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Chen YX
| 1. | Sue S, Shibata W, Sasaki T, Kaneko H, Irie K, Kondo M, Maeda S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Yang C, Li S, Huang T, Lin H, Jiang Z, He Y, Yuan J, An H. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: A meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2022;47:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, Watari J, Miwa H. Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter. 2018;23:e12495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 4. | Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM; Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 5. | Saito Y, Konno K, Sato M, Nakano M, Kato Y, Saito H, Serizawa H. Vonoprazan-Based Third-Line Therapy Has a Higher Eradication Rate against Sitafloxacin-Resistant Helicobacter pylori. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Shinmura T, Adachi K, Yamaguchi Y, Izawa S, Hijikata Y, Ebi M, Funaki Y, Ogasawara N, Sasaki M, Kasugai K. Vonoprazan-Based Triple-Therapy Could Improve Efficacy of the Tailored Therapy of Helicobacter pylori Infection. J Gastrointestin Liver Dis. 2019;28:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J Gastroenterol Hepatol. 2021;36:1159-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Okubo H, Akiyama J, Kobayakawa M, Kawazoe M, Mishima S, Takasaki Y, Nagata N, Shimada T, Yokoi C, Komori S, Kimura K, Hisada Y, Iwata E, Watanabe K, Yanagisawa N, Shiroma S, Shimomura A, Okahara K, Cho H, Uemura N. Vonoprazan-based triple therapy is effective for Helicobacter pylori eradication irrespective of clarithromycin susceptibility. J Gastroenterol. 2020;55:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Zhu YJ, Zhang Y, Wang TY, Zhao JT, Zhao Z, Zhu JR, Lan CH. High dose PPI-amoxicillin dual therapy for the treatment of Helicobacter pylori infection: a systematic review with meta-analysis. Therap Adv Gastroenterol. 2020;13:1756284820937115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Rokkas T, Gisbert JP, Malfertheiner P, Niv Y, Gasbarrini A, Leja M, Megraud F, O'Morain C, Graham DY. Comparative Effectiveness of Multiple Different First-Line Treatment Regimens for Helicobacter pylori Infection: A Network Meta-analysis. Gastroenterology. 2021;161:495-507.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 11. | Zhang M, Pang M, Zhang M. Efficacy and safety of potassium-competitive acid blockers versus proton pump inhibitors as Helicobacter pylori eradication therapy: a meta-analysis of randomized clinical trials. Clinics (Sao Paulo). 2022;77:100058. [PubMed] |
| 12. | Graham DY, Hernaez R, Rokkas T. Cross-roads for meta-analysis and network meta-analysis of H. pylori therapy. Gut. 2022;71:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 13. | Wang Z, Carter RE. Ranking of the most effective treatments for cardiovascular disease using SUCRA: Is it as sweet as it appears? Eur J Prev Cardiol. 2018;25:842-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, Ito H, Kawamura M, Ogata Y, Ohtaka M, Nakahara M, Kawabe K. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 15. | Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology. 2022;163:608-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (2)] |
| 16. | Tamaki H, Morita M, Omura A. An open-label, multicenter, randomized controlled trial of vonoprazan vs esomeprazole as part of first-line triple therapy for Helicobacter pylori infection. J Gastroen Hepatol. 2018;33:36. |
| 17. | Sue S, Ogushi M, Arima I, Kuwashima H, Nakao S, Naito M, Komatsu K, Kaneko H, Tamura T, Sasaki T, Kondo M, Shibata W, Maeda S. Vonoprazan-vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial. Helicobacter. 2018;23:e12456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439-1446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 19. | Song Z, Zhou L, Xue Y, Suo B, Tian X, Niu Z. A comparative study of 14-day dual therapy (esomeprazole and amoxicillin four times daily) and triple plus bismuth therapy for first-line Helicobacter pylori infection eradication: A randomized trial. Helicobacter. 2020;25:e12762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Liou JM, Fang YJ, Chen CC, Bair MJ, Chang CY, Lee YC, Chen MJ, Tseng CH, Hsu YC, Lee JY, Yang TH, Luo JC, Chang CC, Chen CY, Chen PY, Shun CT, Hsu WF, Hu WH, Chen YN, Sheu BS, Lin JT, Wu JY, El-Omar EM, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet. 2016;388:2355-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Tsay FW, Wu DC, Yu HC, Kao SS, Lin KH, Cheng JS, Wang HM, Chen WC, Sun WC, Tsai KW, Hsu PI. A Randomized Controlled Trial Shows that both 14-Day Hybrid and Bismuth Quadruple Therapies Cure Most Patients with Helicobacter pylori Infection in Populations with Moderate Antibiotic Resistance. Antimicrob Agents Chemother. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: current status and future concepts. World J Gastroenterol. 2014;20:5283-5293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 128] [Cited by in RCA: 155] [Article Influence: 14.1] [Reference Citation Analysis (6)] |
| 23. | Tai WC, Liang CM, Lee CH, Chiu CH, Hu ML, Lu LS, Kuo YH, Kuo CM, Yen YH, Kuo CH, Chiou SS, Wu KL, Chiu YC, Hu TH, Chuah SK. Seven-Day Nonbismuth Containing Quadruple Therapy Could Achieve a Grade "A" Success Rate for First-Line Helicobacter pylori Eradication. Biomed Res Int. 2015;2015:623732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, Kao SS, Lai KH, Chen A, Tsay FW. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother. 2014;58:5936-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | Wu DC, Hsu PI, Wu JY, Opekun AR, Kuo CH, Wu IC, Wang SS, Chen A, Hung WC, Graham DY. Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol. 2010;8:36-41.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Zheng Q, Chen WJ, Lu H, Sun QJ, Xiao SD. Comparison of the efficacy of triple versus quadruple therapy on the eradication of Helicobacter pylori and antibiotic resistance. J Dig Dis. 2010;11:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F; Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 373] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 31. | Huang YK, Wu MC, Wang SS, Kuo CH, Lee YC, Chang LL, Wang TH, Chen YH, Wang WM, Wu DC, Kuo FC. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J Dig Dis. 2012;13:232-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Kutluk G, Tutar E, Bayrak A, Volkan B, Akyon Y, Celikel C, Ertem D. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in children: any advantage in clarithromycin-resistant strains? Eur J Gastroenterol Hepatol. 2014;26:1202-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Chen KY, Lin TJ, Lin CL, Lee HC, Wang CK, Wu DC. Hybrid vs sequential therapy for eradication of Helicobacter pylori in Taiwan: A prospective randomized trial. World J Gastroenterol. 2015;21:10435-10442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Hsu PI, Kao SS, Wu DC, Chen WC, Peng NJ, Yu HC, Wang HM, Lai KH, Cheng JS, Chen A, Chuah SK, Tsay FW; Taiwan Acid-Related Disease (TARD) Study Group. A Randomized Controlled Study Comparing Reverse Hybrid Therapy and Standard Triple Therapy for Helicobacter pylori Infection. Medicine (Baltimore). 2015;94:e2104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Liou JM, Chen CC, Chang CY, Chen MJ, Fang YJ, Lee JY, Yang TH, Luo JC, Wu JY, Liou TC, Chang WH, Hsu YC, Tseng CH, Chang CC, Bair MJ, Liu TY, Hsieh CF, Tsao FY, Shun CT, Lin JT, Lee YC, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Sequential therapy for 10 days versus triple therapy for 14 days in the eradication of Helicobacter pylori in the community and hospital populations: a randomised trial. Gut. 2016;65:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Tepeš B, Vujasinović M, Šeruga M, Stefanovič M, Forte A, Jeverica S. Randomized clinical trial comparing 10-day sequential, 7-day concomitant and 7-day standard triple therapies for Helicobacter pylori eradication. Eur J Gastroenterol Hepatol. 2016;28:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Liou JM, Chen CC, Fang YJ, Chen PY, Chang CY, Chou CK, Chen MJ, Tseng CH, Lee JY, Yang TH, Chiu MC, Yu JJ, Kuo CC, Luo JC, Hsu WF, Hu WH, Tsai MH, Lin JT, Shun CT, Twu G, Lee YC, Bair MJ, Wu MS; Members of the Taiwan Gastrointestinal Disease and Helicobacter Consortium. 14 day sequential therapy versus 10 day bismuth quadruple therapy containing high-dose esomeprazole in the first-line and second-line treatment of Helicobacter pylori: a multicentre, non-inferiority, randomized trial. J Antimicrob Chemother. 2018;73:2510-2518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Hsu PI, Tsay FW, Graham DY, Tsai TJ, Tsai KW, Kao JY, Peng NJ, Kuo CH, Kao SS, Wang HM, Lin TF, Wu DC; Taiwan Acid-related Disease (TARD) Study Group. Equivalent Efficacies of Reverse Hybrid and Bismuth Quadruple Therapies in Eradication of Helicobacter pylori Infection in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2018;16:1427-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Zuberi BF, Ali FS, Rasheed T, Bader N, Hussain SM, Saleem A. Comparison of Vonoprazan and Amoxicillin Dual Therapy with Standard Triple Therapy with Proton Pump Inhibitor for Helicobacter Pylori eradication: A Randomized Control Trial. Pak J Med Sci. 2022;38:965-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 40. | Guan JL, Hu YL, An P, He Q, Long H, Zhou L, Chen ZF, Xiong JG, Wu SS, Ding XW, Luo HS, Li PY. Comparison of high-dose dual therapy with bismuth-containing quadruple therapy in Helicobacter pylori-infected treatment-naive patients: An open-label, multicenter, randomized controlled trial. Pharmacotherapy. 2022;42:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Zou Y, Qian X, Liu X, Song Y, Song C, Wu S, An Y, Yuan R, Wang Y, Xie Y. The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: A systematic review and meta-analysis. Helicobacter. 2020;25:e12714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Georgopoulos S, Papastergiou V. An update on current and advancing pharmacotherapy options for the treatment of H. pylori infection. Expert Opin Pharmacother. 2021;22:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori Infection. Gastroenterol Clin North Am. 2010;39:465-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 44. | Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The Ideal Helicobacter pylori Treatment for the Present and the Future. Digestion. 2022;103:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 45. | Hu JL, Yang J, Zhou YB, Li P, Han R, Fang DC. Optimized high-dose amoxicillin-proton-pump inhibitor dual therapies fail to achieve high cure rates in China. Saudi J Gastroenterol. 2017;23:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 46. | Yang J, Zhang Y, Fan L, Zhu YJ, Wang TY, Wang XW, Chen DF, Lan CH. Eradication Efficacy of Modified Dual Therapy Compared with Bismuth-Containing Quadruple Therapy as a First-Line Treatment of Helicobacter pylori. Am J Gastroenterol. 2019;114:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 47. | Gunaratne AW, Hamblin H, Clancy A, Magat AJMC, Dawson MVM, Tu J, Borody TJ. Combinations of antibiotics and vonoprazan for the treatment of Helicobacter pylori infections-Exploratory study. Helicobacter. 2021;26:e12830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Ban H, Inatomi O, Murata M, Otsuka T, Oi M, Matsumoto H, Bamba S, Andoh A. Vonoprazan vs lansoprazole for the treatment of artificial gastric ulcer after endoscopic submucosal dissection: a prospective randomized comparative study. J Clin Biochem Nutr. 2021;68:259-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |