Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12946
Peer-review started: August 24, 2022
First decision: September 25, 2022
Revised: October 10, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 16, 2022
Processing time: 111 Days and 14 Hours
As operative techniques and mortality rates of pancreatectomy have improved, there has been a shift in focus to maintaining and improving the nutritional status of these patients as we continue to learn more about post-operative complications. Although pancreatic endocrine and exocrine insufficiencies are known complications of pancreatectomy, increased longevity of these patients has also led to a higher incidence of de novo fatty liver disease which differs from traditional fatty liver disease given the lack of metabolic syndrome.
To identify and summarize patterns and risk factors of post-pancreatectomy de novo fatty liver disease to guide future management.
We performed a database search on PubMed selecting papers published between 2001 and 2022 in the English language. PubMed was last accessed 1 June 2022.
Various factors influence the development of de novo fatty liver including indication for surgery (benign vs malignant), type of pancreatectomy, amount of pancreas remnant, and peri-operative nutritional status. With an incidence rate up to 75%, de novo non-alcoholic fatty liver disease (NAFLD) can develop within 12 mo after pancreatectomy and various risk factors have been established including pancreatic resection line and remnant pancreas volume, peri-operative malnutrition and weight loss, pancreatic exocrine insufficiency (EPI), malignancy as the indication for surgery, and postmenopausal status.
Since majority of risk factors leads to EPI and malnutrition, peri-operative focus on nutrition and enzymes replacement is key in preventing and treating de novo NAFLD after pancreatectomy.
Core Tip: As surgical techniques for pancreatectomy have improved, patients have had an improvement in mortality which has allowed further investigation into the metabolic changes after surgery. Currently there are no guidelines on the management or prevention of de novo non-alcoholic fatty liver disease (NAFLD) after pancreatectomy. In this review, we synthesize both the patterns and risk factors of de novo NAFLD to help guide management.
- Citation: Shah P, Patel V, Ashkar M. De novo non-alcoholic fatty liver disease after pancreatectomy: A systematic review. World J Clin Cases 2022; 10(35): 12946-12958
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12946.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12946
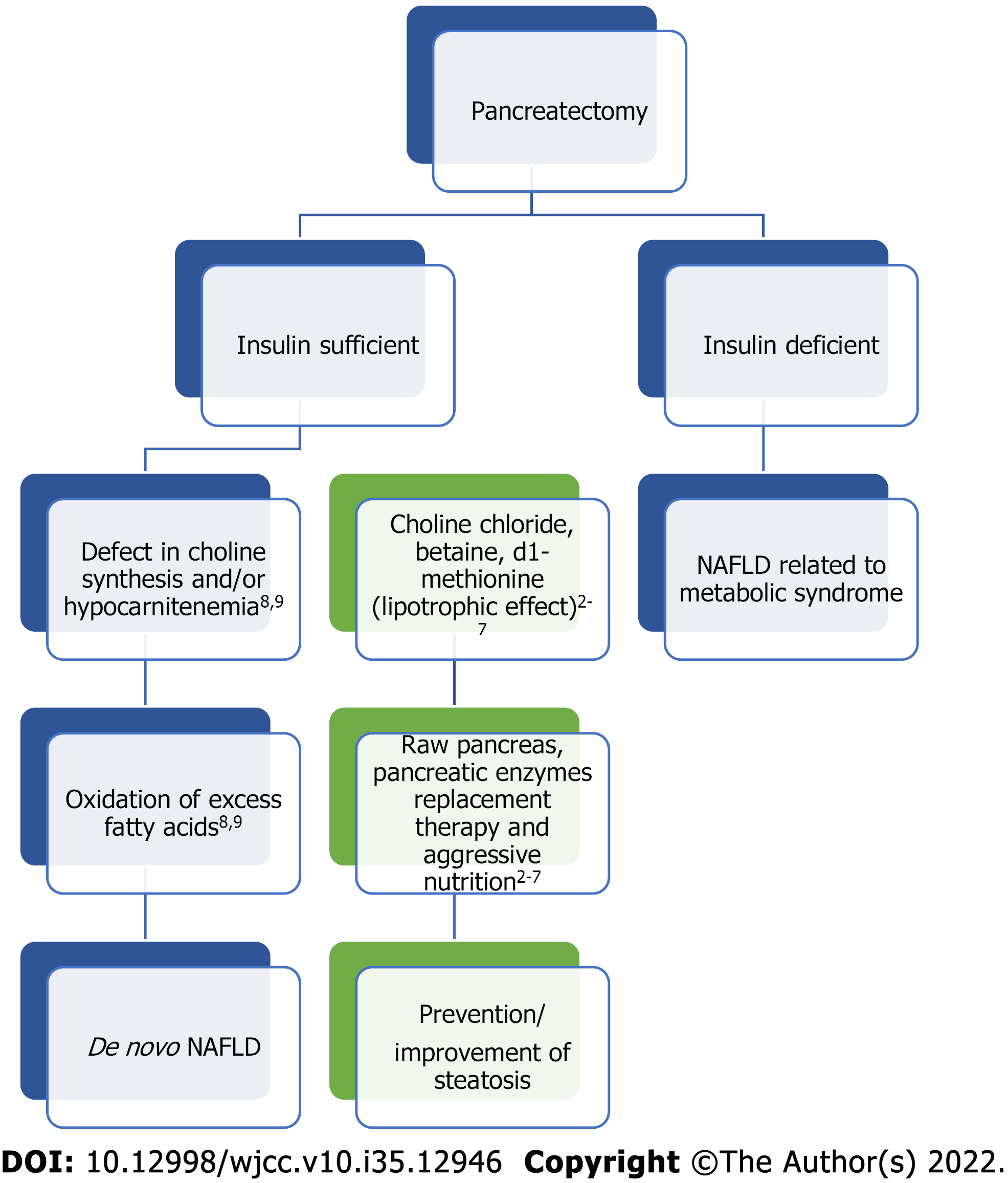
The study of de novo non-alcoholic fatty liver disease (NAFLD) after pancreatectomy began as early as the 1930’s in de-pancreatized dogs that were initially used as the animal prototype for studying diabetes[1]. Two types of fatty liver following pancreatectomy were described. The first had an early onset and due to insulin deficiency whereas the second was slower in onset and developed regardless of control with insulin. Early studies showed that the latter could be prevented, and survival improved by adding raw pancreas to the diet[2-7]. The authors postulated that raw pancreas exerted a lipotrophic effect by preventing and reversing fatty liver disease. This suggested that there may be another pancreatic hormone other than insulin playing a role, there may be a deficiency in the external secretions of the pancreas, or there was an essential factor in raw pancreas that led to improvement. In fact, dogs deprived of pancreatic external secretion by pancreatectomy or ligation of the external pancreatic ducts resulted in fatty liver, which could be prevented by dietary addition of raw pancreas[4]. Other animal studies suggested that feeding choline chloride, betaine, or d1-methionine separately exerted a lipotrophic effect as well suggesting that a defect in choline synthesis may be responsible for de novo NAFLD development[4-7]. Follow up studies showed that deficiencies of lipotrophic agents, after pancreatectomy, interfere with normal formation of low-density lipoproteins and block fat transport out of the liver[4-7]. Hence, there may be a benefit of pancreatic enzymes replacement therapy (PERT) in preventing or treating de novo NAFLD after pancreatectomy.
Findings from animal studies led to investigating the pathophysiology of hepatic steatosis in humans who had undergone pancreatectomy. One study showed that 61.9% of patients that had pancreatectomy developed hypocarnitenemia with a subsequent high ratio of acyl to free carnitine, which was associated with hepatic steatosis[8]. Carnitine has an essential role in lipid metabolism by transporting fatty acids from the cytosol to the mitochondria[9]. Fatty acids usually form acyl-CoA which is conjugated with carnitine and forms acylcarnitine. This then becomes oxidized and is one of the primary pathways of lipid metabolism[9]. Therefore, total carnitine deficiency or an increased ratio of acyl to free carnitine may reflect mitochondrial dysfunction leading to decreased fatty acid oxidation and impaired lipid metabolism leading to hepatic steatosis. Figure 1 illustrates the potential relationship and mechanism of de novo NAFLD after pancreatectomy along with the effect of certain treatments.
Starting in the 1980’s, due to increased awareness of de novo NAFLD, providers began noticing the development of hepatic steatosis in patients who had undergone pancreatectomy[10]. Progression to non-alcoholic steatohepatitis (NASH), advanced fibrosis, and hepatocellular carcinoma were also documented. Therefore, the impact and prevention of hepatic steatosis began to be investigated. Early studies reported incidence of post-pancreatectomy de novo NAFLD up to 37% with a mix of benign and malignant etiologies[10,11]. Other studies reported broader incidence from 8% to 37% with a majority occurring within 12 mo after pancreatectomy[12-18]. Clinical features of de novo NAFLD after pancreatectomy include non-obesity, absence of metabolic syndrome, and malnutrition. Factors such as etiology of disease, pancreatic resection line, functional capacity of remnant tissue, reconstruction methods altering intestinal physiology, gender, pancreatic endocrine and exocrine insufficiency, and post-operative decrease in body mass index (BMI) have been influential in the development of hepatic steatosis[10,11]. Therefore, management has been focused on treating pancreatic insufficiency and maintaining or improving post-operative nutritional status. We aim to summarize the various surgical techniques and risk factors that lead to development of de novo NAFLD after pancreatectomy along with emerging management and treatment options.
A literature search was performed in PubMed using the following key words: pancreatectomy, de novo fatty liver, fatty liver, nonalcoholic fatty liver, pancreaticoduodenectomy, Total pancreatectomy (TP), distal pancreatectomy (DP), pancreatic insufficiency, and malnutrition. Reference lists from studies found were also searched for further relevant studies. All study designs from 2001 to January 2022 that suggested de novo NAFLD after pancreatectomy regardless of method of evaluation were included, such as case reports, case series, and literature reviews. A total of 76 references were included. The results are reported in different paragraphs, containing a brief introduction followed by a summary of previously published data.
Residual pancreas volume: One of the main concerns after pancreatectomy stems from the ability of the residual pancreas to maintain endocrine and exocrine function. Depending on the type of surgical technique and etiology of disease, patients will have various remnant pancreas volume. When comparing different types of pancreatectomy (total, distal, pancreaticoduodenectomy), the pancreatic resection line was found to be an independent risk factor for development of hepatic steatosis, which also relates to the degree of exocrine pancreatic insufficiency (EPI) due to reduced amounts of pancreatic parenchyma[10]. In other studies, residual pancreas volume ratio, harder pancreatic texture, and advanced pancreatic cancer were found to be strong predictors of post-operative EPI after DP and subsequent de novo NAFLD[19-22]. Patients with low residual pancreas volume may have more atrophy in the remnant pancreas as suggested by histologic loss of pancreatic acinar cells in the pancreatic stump which was associated with post-operative EPI[19]. The same study also noted hardened pancreatic texture from severe atrophy that potentially contributed to the risk of post-operative EPI and subsequent hepatic steatosis. Notably, PERT was effective in treating post-operative EPI, advocating for early therapy to prevent de novo NAFLD[19].
The minimal amount of functioning pancreas necessary for fat assimilation to maintain appropriate weight and nutrition post pancreatectomy is unknown. Therefore, residual pancreas volume becomes very important in risk stratifying patients who may become malnourished after surgery.
Pancreatic glandular dysfunction: (1) Pancreatic exocrine insufficiency: Post-operative EPI may result from insufficient pancreas tissue after resection or inadequate secretion of endogenous exocrine enzymes. This has been associated with development of de novo NAFLD after pancreatectomy in the setting of diarrhea and malabsorption leading to weight loss and malnutrition. Specifically, malabsorption of amino acids leads to decreased synthesis of hepatic lipoprotein (including lipoprotein B) along with decreased delivery of very low-density lipoprotein (VLDL)[17,23]. Free fatty acids then accumulate in hepatocytes and hepatic steatosis develops. Over time, increased lipid oxidation and activation of reactive oxygen damage hepatocytes and induce NASH with subsequent fibrosis[17,23].
(2) Pancreatic endocrine insufficiency and insulin resistance: Pancreatic endocrine insufficiency can lead to de novo NAFLD through disruption of regulatory pathways despite absence of traditional risk factors for metabolic syndrome. Pancreatic resection related imbalance of insulin, glucagon, and pancreatic polypeptide may have an effect on post-operative hepatic steatosis[14]. This promotes insulin resistance-related hyperinsulinemia and hyperglycemia by increased hepatic glucose production and decreased glucose disposal[14,17]. The extra glucose gets converted to fatty acids which enters the Krebs cycle which becomes a substrate for lipogenesis in hepatocytes[17,23-25]. Insulin resistance also increases lipase which promotes adipose tissue lipolysis and creates triglycerides and free fatty acids with subsequent increased hepatic fat accumulation[17,23-25].
Although hepatic steatosis is relatively uncommon in patients with type 1 diabetes mellitus compared to those with type 2, the mechanism of de novo NAFLD after pancreatectomy appears similar to that of type 1 diabetes, due to inadequate levels of glucoregulatory hormones in the setting of a pancreatectomy; this can be described as pancreatogenic diabetes (type 3C)[14,26-28].
Benign disease: A recent systematic review and meta-analysis evaluated metabolic changes after pancreatoduodenectomy (PD), pancreatic left resection (PLR), and parenchyma-sparing local resections for benign tumors[29]. After PD, 14.1% of patients developed new onset diabetes after a mean of 27.9 mo while 44.9% developed EPI after a mean of 32 mo. Patients who underwent PLR had a higher frequency of glandular dysfunction when compared to those who underwent pancreatic middle segment resection. After duodenum-preserving pancreatic head resection (DPPHR), 5% developed diabetes and 6.7% developed EPI. Patients who underwent tumor enucleation developed diabetes and EPI in 5.7% and 4.3% of cases respectively. On the other hand, pylorus preserving PD led to diabetes and EPI in 19.7% and 54.4% of patients respectively. Regarding development of de novo NAFLD, 23.8% of patients had radiologic evidence of NAFLD after a mean of 30.4 mo of follow up time but some of these included malignancies. There were otherwise a few studies that reported de novo NAFLD in 16%-26% of patients with benign tumors who underwent PD[29-31]. The odds ratio of development of de novo NAFLD after DPPHR compared to pylorus-sparing PD was 0.11 (95%CI 0.03-0.49)[29].
Regardless of the type of surgery, pancreatectomy for benign disease results in metabolic derangement. As described earlier, pancreatic insufficiency is related to the extent of tissue resection as reinforced by these results. The data further suggest that traditional surgical techniques for benign disease lead to considerable morbidity and parenchyma-sparing techniques may be more favorable. Additionally, out of the patients that developed de novo NAFLD, a majority occurred within the first year and were also related to traditional surgical techniques such as PD[29].
Malignancy: Due to various comorbidities and diseases processes, the reason for pancreatectomy was also found to be linked to the development of de novo NAFLD due to higher rates of malnutrition in patients with malignancy[32]. Since pancreatic malignancy often required larger resections for clear margins, there is often less residual pancreatic volume which can lead to EPI and subsequent hepatic steatosis through pathways mentioned previously[10,13,18,32,33]. These patients also tended to undergo chemotherapy or radiation therapy which increases the risk for pancreatic atrophy, fibrosis, and subsequent pancreatic insufficiency. Additionally, pancreatic cancer of the head was also linked to higher rates of EPI possibly due to secondary obstruction of the pancreatic duct which causes fibrosis and atrophy of the pancreas[33]. On average, these patients had lower BMIs and more malnutrition due to the underlying process of malignancy and related treatments and effects[10,13,18,32,33].
Surgical techniques: Due to improvement in surgical techniques and peri-operative management, there has been a decline in mortality rates after different types of pancreatectomy[19]. The location of the pancreas resection line and subsequent reconstruction may explain the difference between the rates of pancreatic insufficiency and de novo NAFLD. Along with resection of pancreatic parenchyma, the loss of duodenum, altered gastric emptying, and intestinal transit of biliary and pancreatic fluids likely contribute to impaired pancreatic function[11]. Since the duodenum is involved in fat digestion and absorption by exogenous enzymes, any modification can cause a disconnect between gastric emptying and endogenous pancreatic enzymes which leads to maldigestion[34-36]. Additionally, the pancreatic anastomosis can become obstructed with tumor or chemoradiation-induced fibrosis and atrophy which can lead to impaired exocrine function[11].
(1) Total Pancreatectomy: TP can be performed to treat both benign and malignant diseases, but due to increased risk for endocrine and exocrine insufficiency, this surgical approach has been limited[37]. TP has been established as an option for pancreatic ductal adenocarcinoma (PDAC), invasive or multifocal intraductal papillary mucinous neoplasms, multiple pancreatic neuroendocrine tumors, chronic pancreatitis with severe pain, or even pancreatic fistulas or hemorrhage after PD[38-40]. The gallbladder, common bile duct, duodenum, part of the stomach, spleen, and portal vein are often removed as well.
Early studies of patients that had a regional TP that included a regional lymph node dissection, sympathectomy, subtotal gastrectomy, duodenectomy, splenectomy, and cholecystectomy noted a significant amount of diarrhea, malabsorption, and weight loss in the post-operative period. These symptoms were likely due to altered anatomy, EPI, and impaired small bowel motility from loss of inhibitory effect in the setting of sympathectomy[32,37]. Those patients were treated with bile acid sequestrants, somatostatin analogues, and alpha-2 adrenergic agonists which improved symptoms. Also, the addition of PERT, calcium supplements, multivitamins, and multiple calorie dense meals per day led to successful weight gain or maintenance[37]. However, despite adequate diabetic control and caloric restoration, the incidence of de novo NAFLD ranged from 19.6%-37% with both benign and malignant indications[32,41]. Similarly, studies investigating outcomes after residual TP (due to local recurrence or new primary cancer) vs one stage TP showed a prevalence of 50%-74% of de novo NAFLD at 12 mo post-operatively[42]. These patients had well-controlled post-operative glucose levels and hemoglobin A1c values, but those with PDAC experienced more severe post-operative weight loss and worse outcomes.
These findings were also supported by another study of patients that underwent TP including the pylorus, half antrum, duodenum, spleen, gallbladder, extrahepatic bile duct, and a short segment of the proximal jejunum[32]. Thirty seven percent developed steatosis in correlation with decline in BMI indicating a state of malnutrition. Additionally, EPI and females were also found to increase risk of post-operative de novo NAFLD.
Advancements in islet auto-transplantation have led to more patients receiving a TP (TPIAT). Interestingly, 63% of patients who received TPIAT developed de novo NAFLD within a mean of 27 mo[43]. Those patients were also noted to have an atypical pattern of fat deposition likely arising from local fat deposition from functioning islet cells producing insulin and fat which is different from the homogenous steatosis seen with metabolic syndrome[44-46].
(2) Pancreaticoduodenectomy: PD involves resection of the head of the pancreas, distal stomach, duodenum, and proximal jejunum. The effects on digestion are different compared to DP or TP given the asynchronous delivery of nutrients and digestive enzymes that results in severe malnutrition[47]. The reduction in islet and acinar cells coupled with malabsorption can lead to EPI and de novo NAFLD. Early studies of PD for periampullary cancer documented post-operative EPI and weight loss in all patients despite not having residual cancer[47]. Seventy-five percent of patients were noted to have de novo NAFLD and none of them carried a diagnosis of diabetes. Despite PERT, they were unable to regain weight which emphasizes the importance of monitoring metabolic derangements and risk factors including weight changes and EPI to prevent de novo NAFLD.
A similar pattern was seen in patients with PDAC in the pancreatic head since it can cause obstruction of the main pancreatic duct and atrophic changes in the pancreatic body and tail[32]. After PD, the atrophic remnant leads to decreased exocrine and endocrine function[10,13-15,18,33]. Additionally, PD often requires a major surgery to obtain free margins with nerve dissection which increases the risk for malabsorption[16,48,49]. These risk factors all play a role in the development of de novo NAFLD, but those who receive PERT within 2 wk of surgery had lower incidence of hepatic steatosis[32]. Notably in those studies, older females with post-operative hepatic steatosis did not respond as well to PERT. This confirms observations from other studies that suggest post-menopausal women have an increased risk of insulin resistance, hyperlipidemia, and visceral fat accumulation which are all risk factors for development of conventional hepatic steatosis due to imbalance of sex hormones metabolism[50-53].
Multiple subsequent studies noted an incidence of de novo NAFLD from 15.6% to 66% within 12 mo depending on the indication for surgery and peri-operative risk factors[13,47,50]. Those with benign surgical indications had a lower incidence of de novo NAFLD whereas patients with malignancy had higher incidence. This observation is likely explained by the correlation between decline in BMI and de novo NAFLD suggesting that the pathophysiology is similar to fatty infiltration in malnutrition[50-53]. Additionally, patients that underwent subtotal stomach preserving PD with Billroth II reconstruction were at increased risk of EPI and de novo NAFLD if they were female or had a BMI greater than 22.5 kg/m2[54]. Obese patients may be more susceptible to steatosis due to dietary limitation and rapid weight loss from the antrectomy whereas the gender difference may be explained by a majority of these women being post-menopausal so they may not have the protective effect of estrogen on fatty liver disease that has been demonstrated in prior studies[50-53]. Additionally, atrophy of the pancreas remnant was a significant risk factor for development of steatosis, which improved in a majority of these patients after administration of high dose PERT[47].
Eventually, studies were also done in other countries with populations that did not have high prevalence of traditional risk factors for NAFLD. For example, studies from Japan included patients who were not obese and did not have metabolic syndrome, yet 23%-37% developed de novo NAFLD within 6 mo in the setting of weight loss[13,14,33]. These patients also had lower serum albumin, total cholesterol, apolipoprotein B, and fasting insulin concentrations which improved after PERT suggesting EPI as a main pathway for de novo NAFLD[33]. These clinical features were similar to rodents in a prior study with methionine and choline deficient induced NASH[55,56]. Therefore, malabsorption of essential amino acids and fat soluble nutrients such as choline could lead to development of de novo NAFLD after PD. Similarly, these subjects also had low apolipoprotein B levels which helps secrete triglycerides from hepatocytes as VLDL. Serum zinc levels were also lower in patients with de novo NAFLD and studies have shown a protective role of zinc against alcoholic fatty liver disease[57,58]. Despite aggressive supplementation and administration of PERT within 1 wk of PD, de novo NAFLD prevalence was as high as 41.3% with a higher rate in the group with lower doses of PERT[12].
(3) Distal Pancreatectomy: DP is the surgical procedure of choice for benign or malignant lesions in the body or tail of the pancreas and does not include the duodenum or distal bile duct. Longevity after DP has been better for benign disease than it is for cancer, making evaluation of metabolic consequences very important, but complications are similar compared to other techniques for pancreatectomy[22,59].
A large retrospective analysis demonstrated that 20% of patients developed endocrine insufficiency within 20.8 mo after DP[11]. Independent risk factors included male gender, higher BMI, tobacco use, and family or personal history of diabetes. EPI was also present in 36% of patients with lower BMI, family history of diabetes, steatorrhea, and elevated total bilirubin leading to an increased risk with mean time to onset of 14.2 mo. These findings suggest that although traditional risk factors for metabolic syndrome place post-DP patients at higher risk of endocrine and exocrine insufficiency, those with lower BMI and risk for malnutrition with less pancreas remnant are more at risk for EPI which has been more associated with de novo NAFLD[34,35,60].
Additional studies also confirmed these findings and noted pancreatic endocrine insufficiency after DP in 31% of patients[36,61,62]. These changes after DP were thought to be due to heterogeneity of islet distribution in the setting of a higher islet volume density in the tail than in the head and body of the pancreas. EPI was noted in 17% of patients and pre-operative jaundice was noted to be a risk factor, indicating ductal obstruction with possible atrophy and fibrosis[63].
When investigating malignancy as an etiology for surgery, patients that underwent DP had more adjuvant chemotherapy and higher rates of PERT use compared to those that underwent PD[16]. De novo NAFLD developed in 17%-55% of patients without metabolic syndrome within 6-12 mo[50,64]. The presence of PDAC, peri-operative blood loss, and EPI affected development of hepatic steatosis likely due to pancreatic volume loss/ischemia leading to malnutrition and weight loss. These patients may also have less pre-operative reserve which predisposes them to post-operative malnutrition and steatosis.
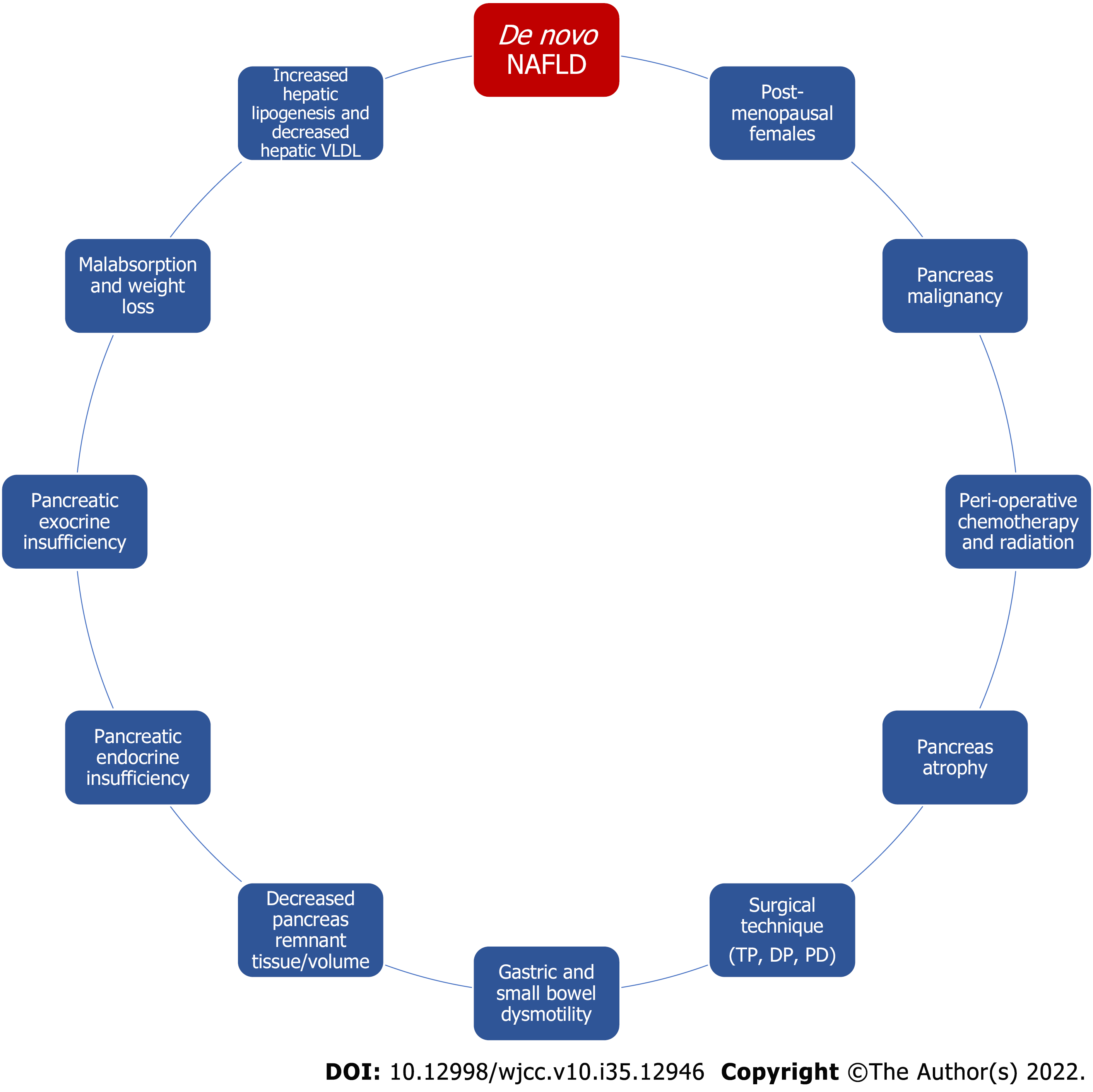
Several factors have been linked to the development of hepatic steatosis mainly through pancreatic insufficiency, malabsorption, and subsequent weight loss as depicted in Figure 2. The common thread between these risk factors and development of de novo NAFLD appears to be malnutrition. In fact, survival rates and development of de novo NAFLD have improved with appropriate nutrition so these patients should be treated aggressively with insulin and nutritional management[10]. This can be achieved by treating EPI adequately, which biochemically manifests with low vitamin A, D, E, K, selenium, zinc, magnesium, calcium, phosphate, potassium, and elevated parathyroid hormone (likely due to low vitamin D)[65-68]. While clinically, these patients manifest with weight loss, sarcopenia, weakness, and even anorexia due to concern for triggering diarrhea.
Specifically, PERT plays a key role in minimizing risk of malabsorption post-pancreatectomy. There are several forms of PERT including enteric coated microspheres, tablets, granules, and powdered forms. These enzymes should be taken with all meals and snacks, but dose depends on each individual and meal content. Patients on enteric feeding should be on a semi-elemental peptide, medium chain triglycerides-based feed, and PERT may need to be given along with these feeds[68]. If unable to provide PERT for these patients, a strict elemental formula can be used instead. All patients receiving PERT should be taught how to self-adjust doses based on meal content and duration. Additionally, gastric acid suppression may be of benefit to prevent denaturing of the enzymes since they require a pH > 5.5 for activation but are denatured in very acidic environments[68]. Notably, each PERT product has its own activation time and ideal pH but there are limited trials comparing products in different clinical situations. If a patient does not experience benefit with one brand, switching to another brand may be beneficial.
To further assess the effect of PERT, studies have investigated administering enzymes based on risk factors vs prophylactically to all patients who undergo pancreatectomy. One study administered PERT prophylactically after PD and demonstrated an incidence of de novo NAFLD of 27% compared to 43% in patients who did not receive PERT[69]. Recently, another study was published regarding the effect of early post-operative administration of delayed-release high dose pancrealipase for patients that underwent pancreatectomy (other than TP)[70]. Patients were given PERT starting post-operative day 3 vs 3 mo after surgery in the control group. Starting at 3 mo and going up to 6 mo after surgery, prevalence of de novo NAFLD was not significantly different between the 2 groups, but BMI and nutrition indices were higher in the group that was administered PERT earlier. These findings support aggressive and early focus on post-operative PERT to improve nutritional status and decrease risk of subsequent de novo NAFLD after pancreatectomy.
Historically, malabsorption has been also treated with a low-fat diet which minimizes symptoms, but this does not always correct nutritional deficiencies[65-68]. Some clinical experts advocate for more balanced and not low-fat diets due to concern for a potential negative impact on nutritional status. Instead, patients are instructed to eat high energy meals with nutritional supplements under the guidance of a dietician. Since the duodenum is also removed in some forms of pancreatectomy, there is less absorption of vitamins and minerals on top of increased metabolic demand which leads to worsening micronutrient deficiencies. Therefore, fat soluble vitamins and trace elements are recommended following surgery.
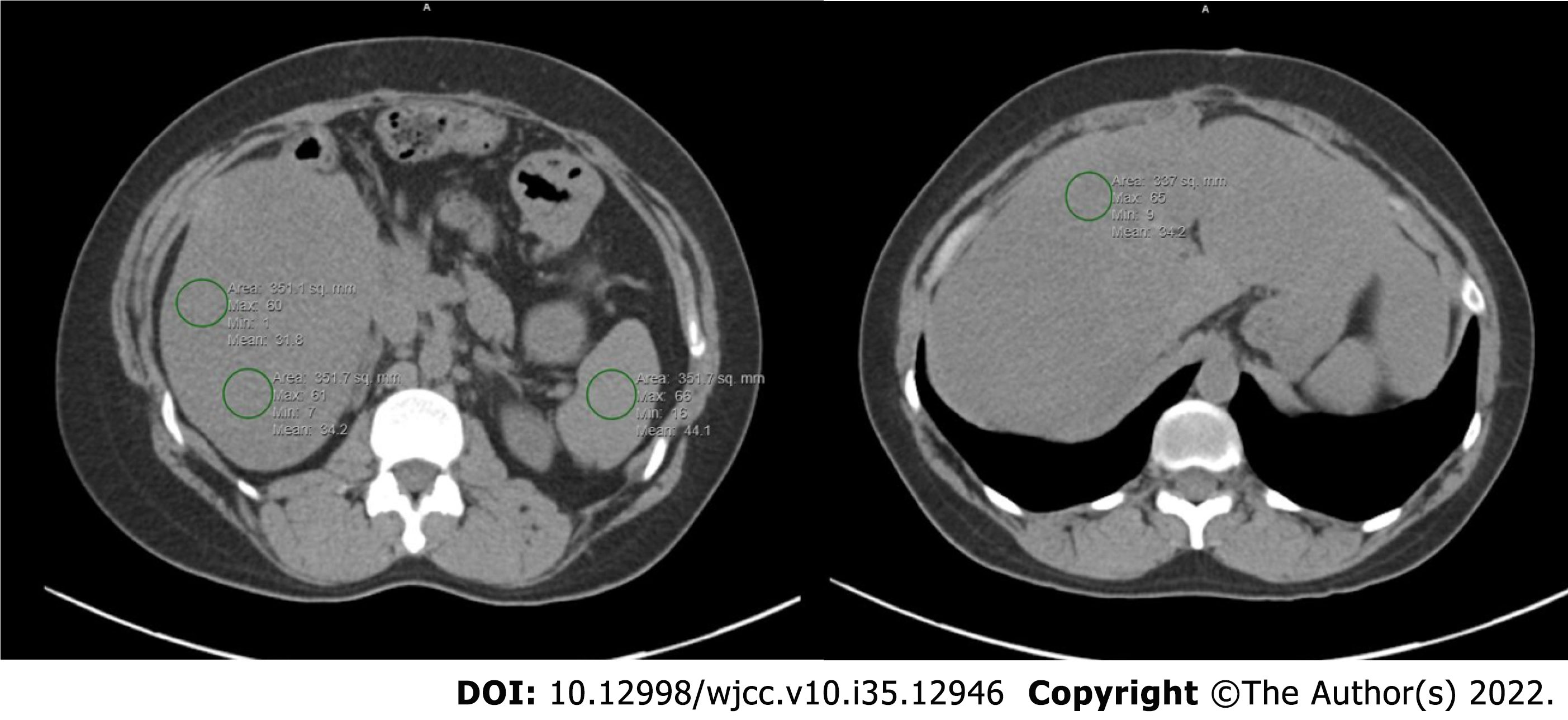
Although liver biopsy for histological examination is the most accurate diagnostic method for hepatic steatosis, it is invasive with the possibility of complications[71-76]. While abdominal ultrasound can show liver hyperechogenicity, it cannot quantify and detect mild steatosis. Magnetic resonance imaging has good sensitivity to detect hepatic steatosis but limited by the test expensive cost, which makes it less used at small institutions. Therefore, many studies optioned to determine the presence or severity of hepatic steatosis using hepatic attenuation with Hounsfield units (HU) on computed tomography (CT) images. As seen in Figure 3, regions of interests are drawn and hepatic steatosis can be defined as an absolute CT attenuation value of less than 40 HU or a relative attenuation of at least 10 HU less than that of the spleen[71-76]. Another method involves using the liver to spleen ratio of HU with a ratio of 0.9 or less to diagnose hepatic steatosis. These methods on a non-contrast CT have good correlation to pathologic findings of at least moderate steatosis and are more convenient and available[71-76].
Current data is limited mainly due to the heterogeneity of the patient population studied. Prior studies have included patients with both benign and malignant diseases along with different surgical techniques. Patients that also undergo chemotherapy or radiation therapy are at risk for recurrent or metastatic disease have also been included and may confound results. These patients also often have a history of alcohol abuse or underlying metabolic disorder that are not always accounted for. Additionally, when diagnosing patients with non-invasive techniques, only moderate and severe steatosis are captured given the limitations of CT scans which may under-report true values. Some studies also used CT scans with contrast which is not ideal due to lack of regulation of the type and amount of contrast injected, injection timing, and scan parameters which can influence the diagnosis of steatosis. Diagnosis of EPI is also difficult without objective data which many studies do not provide, and this limits the ability to make extrapolations. Lastly, although many studies incorporated treatment with PERT, there is no way to compare them due to the various formulations of enzymes that are available based on regions. This would be important to study in the setting of patients with and without pre-operative NAFLD to follow the disease course.
The key to differentiating between de novo NAFLD after pancreatectomy and classic NAFLD is the absence of metabolic syndrome. Many studies have tried to investigate the pathogenesis and risk factors for development of de novo NAFLD to avoid the morbidity and mortality associated with chronic liver disease by increasing awareness of the underlying process. Early animal models have suggested that a defect in choline synthesis leading to oxidation of fatty acids may play a key role in development of NAFLD after pancreatectomy[2-7]. These studies also showed benefit in supplementation with choline, raw pancreas, and pancreatic enzymes which led to further investigational trials.
As synthesized in this review, various factors have been linked to increased risk for development of NAFLD after pancreatectomy. Although these studies often included a heterogenous population making comparison difficult, certain factors such as pancreatic endocrine and exocrine dysfunction in the setting of decreased residual pancreas volume were consistent. These factors lead to malnutrition and weight loss which appears to be the driving force in the pathogenesis of de novo NAFLD through increased hepatic lipogenesis and decreased hepatic VLDL. Therefore, emphasis should be placed on peri-operative nutritional optimization to not only avoid but also potentially treat de novo NAFLD.
Future directions should investigate specific peri-operative dietary techniques including calorie counts and supplements while trending changes in weight and development or improvement of hepatic steatosis. Similarly, further studies are needed with regard to testing the appropriate dosage and formulation of PERT to provide uniform guidance on treatment along with appropriate timing of administration.
As surgical techniques for pancreatectomy have improved, patients have had an improvement in mortality which has allowed further investigation into the metabolic changes after surgery. With an incidence rate up to 75% (Table 1), de novo NAFLD can develop within 12 mo after pancreatectomy and various risk factors have been established including pancreatic resection line and remnant pancreas volume, peri-operative malnutrition and weight loss, EPI, pancreatic endocrine insufficiency, malignancy as the indication for surgery, and postmenopausal status. Since a majority of risk factors leads to EPI and malnutrition, peri-operative focus on nutrition and enzymes replacement is key in preventing and treating de novo NAFLD after pancreatectomy.
Pancreatic surgery for various disease processes (including both malignancy and benign disease) have been described for many years but only recently have techniques improved making certain metabolic consequences more known. De novo non-alcoholic fatty liver disease (NAFLD) has been associated with pancreatectomy after about 12 mo but risk factors are not well known given the heterogeneous populations that have been studied thus far.
Given the improvement in surgical techniques used for pancreatectomy, mortality has improved which has led to increased awareness of downstream metabolic effects. One of those effects, the development of de novo NAFLD, is especially interesting given the lack of traditional metabolic risk factors that are often associated with fatty liver disease. Therefore, there is more importance in defining risk factors that could lead to development of de novo NAFLD so that prevention and treatment can also be better defined.
To summarize the various per-operative risk factors that lead to development of de novo NAFLD after pancreatectomy along with potential management and treatment options.
A literature search from 2001 to 2022 was done and all study designs that investigated de novo NAFLD were included.
With an incidence rate up to 75%, de novo NAFLD can develop within 12 mo after pancreatectomy. Various risk factors have been established including pancreatic resection line and remnant pancreas volume, peri-operative malnutrition and weight loss, EPI, pancreatic endocrine insufficiency, malignancy as the indication for surgery, and postmenopausal status.
Since a majority of risk factors leads to exocrine pancreatic insufficiency and malnutrition, peri-operative focus on nutrition and enzymes replacement is key in preventing and treating de novo NAFLD after pancreatectomy.
Future studies should focus on per-operative management of nutrition including pancreatic enzyme replacement therapy while monitoring weight change and development, prevention, or improvement of de novo fatty liver disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Maher H, China; Matsuo Y, Japan S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Bennett LL. What causes fatty liver after pancreatectomy--an unresolved and forgotten controversy. The 1936-1954 years of controversy. Perspect Biol Med. 1983;26:595-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Chaikoff IL, Kaplan A. The blood lipids in completely depancreatized dogs maintained with insulin. J Biol Chem. 1934;106:267-279. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Kaplan A, Chaikoff, IL. Liver lipids in completely depancreatized dogs maintained with insulin. J Biol Chem. 1935;108:201-216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Chaikoff IL, Kaplan A. The influence of the ingestion of raw pancreas upon die blood lipids of completely depancreatized dogs maintained with insulin. J Biol Chem. 1935;112:155-165. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Kaplan A, Chaikoff, IL. Effect of autoclaved pancreas upon lipids of blood and liver in depancreatized dogs maintained with insulin. Exp Biol Med. 1936;34:606-607. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Van Prohaska J, Dragstedt LR, Harms HP. The relation of pancreatic juice to the fatty infiltration and degeneration of the liver in the depancreatized dog. Am J Physiol. 1936;117:166-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Dragstedt LR, Van Prohaska J, Harms HP. Observations on a substance in pancreas (a fat metabolizing hormone) which permits survival and prevents liver changes in depancreatized dogs. Am J Physiol. 1936;117:175-181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Nakamura M, Nakata K, Matsumoto H, Ohtsuka T, Yoshida K, Tokunaga S, Hino K. Acyl/free carnitine ratio is a risk factor for hepatic steatosis after pancreatoduodenectomy and total pancreatectomy. Pancreatology. 2017;17:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Ishikawa H, Takaki A, Tsuzaki R, Yasunaka T, Koike K, Shimomura Y, Seki H, Matsushita H, Miyake Y, Ikeda F, Shiraha H, Nouso K, Yamamoto K. L-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS One. 2014;9:e100627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Okamura Y, Sugimoto H, Yamada S, Fujii T, Nomoto S, Takeda S, Kodera Y, Nakao A. Risk factors for hepatic steatosis after pancreatectomy: a retrospective observational cohort study of the importance of nutritional management. Pancreas. 2012;41:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kusakabe J, Anderson B, Liu J, Williams GA, Chapman WC, Doyle MMB, Khan AS, Sanford DE, Hammill CW, Strasberg SM, Hawkins WG, Fields RC. Long-Term Endocrine and Exocrine Insufficiency After Pancreatectomy. J Gastrointest Surg. 2019;23:1604-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Yasukawa K, Shimizu A, Yokoyama T, Kubota K, Notake T, Seki H, Kobayashi A, Soejima Y. Preventive Effect of High-Dose Digestive Enzyme Management on Development of Nonalcoholic Fatty Liver Disease after Pancreaticoduodenectomy: A Randomized Controlled Clinical Trial. J Am Coll Surg. 2020;231:658-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Kato H, Isaji S, Azumi Y, Kishiwada M, Hamada T, Mizuno S, Usui M, Sakurai H, Tabata M. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Nomura R, Ishizaki Y, Suzuki K, Kawasaki S. Development of hepatic steatosis after pancreatoduodenectomy. AJR Am J Roentgenol. 2007;189:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Nakagawa N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Kondo N, Sasaki H, Okano K, Sueda T. Nonalcoholic fatty liver disease after pancreatoduodenectomy is closely associated with postoperative pancreatic exocrine insufficiency. J Surg Oncol. 2014;110:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Nagai M, Sho M, Satoi S, Toyokawa H, Akahori T, Yanagimoto H, Yamamoto T, Hirooka S, Yamaki S, Kinoshita S, Nishiwada S, Ikeda N, Kwon AH, Nakajima Y. Effects of pancrelipase on nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yu HH, Shan YS, Lin PW. Effect of pancreaticoduodenectomy on the course of hepatic steatosis. World J Surg. 2010;34:2122-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Sato R, Kishiwada M, Kuriyama N, Azumi Y, Mizuno S, Usui M, Sakurai H, Tabata M, Yamada T, Isaji S. Paradoxical impact of the remnant pancreatic volume and infectious complications on the development of nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Miyamoto R, Inagaki Y, Ikeda N, Oda T. Three-dimensional remnant pancreatic volume ratio indicates postoperative pancreatic exocrine insufficiency in pancreatic cancer patients after distal pancreatectomy. Pancreatology. 2020;20:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Okano K, Murakami Y, Nakagawa N, Uemura K, Sudo T, Hashimoto Y, Kondo N, Takahashi S, Sueda T. Remnant pancreatic parenchymal volume predicts postoperative pancreatic exocrine insufficiency after pancreatectomy. Surgery. 2016;159:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 21. | Iizawa Y, Kato H, Kishiwada M, Hayasaki A, Tanemura A, Murata Y, Azumi Y, Kuriyama N, Mizuno S, Usui M, Sakurai H, Isaji S. Long-term outcomes after pancreaticoduodenectomy using pair-watch suturing technique: Different roles of pancreatic duct dilatation and remnant pancreatic volume for the development of pancreatic endocrine and exocrine dysfunction. Pancreatology. 2017;17:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kalser MH, Leite CA, Warren WD. Fat assimilation after massive distal pancreatectomy. N Engl J Med. 1968;279:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 35] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 809] [Article Influence: 161.8] [Reference Citation Analysis (1)] |
| 24. | Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411:21-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Yang S, Chen J, Su Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front Endocrinol (Lausanne). 2018;9:802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 26. | Anderson DK, Brunicardi FC. Pancreatic anatomy and physiology. In: Greenfield LJ, ed. Surgery: scientific principles and practice, 2nd ed. Philadelphia: Lippincott-Raven, 1997: 857-874. |
| 27. | Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 205] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Chatila R, West AB. Hepatomegaly and abnormal liver tests due to glycogenosis in adults with diabetes. Medicine (Baltimore). 1996;75:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 126] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 29. | Beger HG, Mayer B, Vasilescu C, Poch B. Long-term Metabolic Morbidity and Steatohepatosis Following Standard Pancreatic Resections and Parenchyma-sparing, Local Extirpations for Benign Tumor: A Systematic Review and Meta-analysis. Ann Surg. 2022;275:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 30. | Ooshima N, Hatori T, Suzuki S, Kimijima A, Furukawa T, Shimizu K, Taniai M, Tokushige K, Hashimoto E, Shiratori K, Yamamoto M. Does duodenum-preserivng pancreatectomy prevent severe postoperative fatty liver? Pancreatology. 2013;13. [DOI] [Full Text] |
| 31. | Mackay TM, Genç CG, Takkenberg RB, Besselink MG, Somers I, Nieveen van Dijkum EJM. New onset non-alcoholic fatty liver disease after resection of pancreatic neuroendocrine tumors. J Surg Oncol. 2018;117:1548-1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Hata T, Ishida M, Motoi F, Sakata N, Yoshimatsu G, Naitoh T, Katayose Y, Egawa S, Unno M. Clinical Characteristics and Risk Factors for the Development of Postoperative Hepatic Steatosis After Total Pancreatectomy. Pancreas. 2016;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Tanaka N, Horiuchi A, Yokoyama T, Kaneko G, Horigome N, Yamaura T, Nagaya T, Komatsu M, Sano K, Miyagawa S, Aoyama T, Tanaka E. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology. 2015;15:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Seiler CM, Izbicki J, Varga-Szabó L, Czakó L, Fiók J, Sperti C, Lerch MM, Pezzilli R, Vasileva G, Pap Á, Varga M, Friess H. Randomised clinical trial: a 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment Pharm Ther. 2013;37:691-702. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 36. | Kwon JH, Kim SC, Shim IK, Song KB, Lee JH, Hwang DW, Park KM, Lee YJ. Factors Affecting the Development of Diabetes Mellitus After Pancreatic Resection. Pancreas. 2015;44:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Scholten L, Stoop TF, Del Chiaro M, Busch OR, van Eijck C, Molenaar IQ, de Vries JH, Besselink MG; Dutch Pancreatic Cancer Group. Systematic review of functional outcome and quality of life after total pancreatectomy. Br J Surg. 2019;106:1735-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Del Chiaro M, Rangelova E, Segersvärd R, Arnelo U. Are there still indications for total pancreatectomy? Updates Surg. 2016;68:257-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D, D'Ambra M, Pezzilli R, Calculli L, Santini D, Minni F. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg. 2010;62:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 41. | Kato H, Kamei K, Suto H, Misawa T, Unno M, Nitta H, Satoi S, Kawabata Y, Ohtsuka M, Rikiyama T, Sudo T, Matsumoto I, Okano K, Suzuki Y, Sata N, Isaji S, Sugiyama M, Takeyama Y. Incidence and risk factors of nonalcoholic fatty liver disease after total pancreatectomy: A first multicenter prospective study in Japan. J Hepatobiliary Pancreat Sci. 2022;29:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Hashimoto D, Chikamoto A, Taki K, Arima K, Yamashita Y, Ohmuraya M, Hirota M, Baba H. Residual total pancreatectomy: Short- and long-term outcomes. Pancreatology. 2016;16:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Robbins AJ, Ritter S, Markese M, Skube ME, McEachron KR, Bellin MD, Stice MJ, Beilman GJ, Schat R, Spilseth B. Atypical Hepatic Steatosis Patterns on MRI After Total Pancreatectomy With Islet Autotransplant. AJR Am J Roentgenol. 2021;217:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Idilman IS, Ozdeniz I, Karcaaltincaba M. Hepatic Steatosis: Etiology, Patterns, and Quantification. Semin Ultrasound CT MR. 2016;37:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Takeshita A, Yamamoto K, Fujita A, Hanafusa T, Yasuda E, Shibayama Y. Focal hepatic steatosis surrounding a metastatic insulinoma. Pathol Int. 2008;58:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Sohn J, Siegelman E, Osiason A. Unusual patterns of hepatic steatosis caused by the local effect of insulin revealed on chemical shift MR imaging. AJR Am J Roentgenol. 2001;176:471-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Luu C, Thapa R, Rose T, Woo K, Jeong D, Thomas K, Chen DT, Friedman M, Malafa MP, Hodul PJ. Identification of nonalcoholic fatty liver disease following pancreatectomy for noninvasive intraductal papillary mucinous neoplasm. Int J Surg. 2018;58:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Song SC, Choi SH, Choi DW, Heo JS, Kim WS, Kim MJ. Potential risk factors for nonalcoholic steatohepatitis related to pancreatic secretions following pancreaticoduodenectomy. World J Gastroenterol. 2011;17:3716-3723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Satoh D, Yagi T, Nagasaka T, Shinoura S, Umeda Y, Yoshida R, Utsumi M, Tanaka T, Sadamori H, Fujiwara T. CD14 upregulation as a distinct feature of non-alcoholic fatty liver disease after pancreatoduodenectomy. World J Hepatol. 2013;5:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Olefson S, Jackson M, Grand DJ, Charpentier KP, Makwana N, Promrat K. Identification of Nonalcoholic Fatty Liver Disease following Pancreatic Surgery in a Western Cohort Using a Novel Radiographic Technique. J Clin Transl Hepatol. 2015;3:246-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 51. | Gilsanz V, Chung SA, Kaplowitz N. Differential effect of gender on hepatic fat. Pediatr Radiol. 2011;41:1146-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood). 2004;229:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 266] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6 Suppl 1:60-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 654] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 54. | Takemura N, Saiura A, Koga R, Yamamoto J, Yamaguchi T. Risk Factors for and Management of Postpancreatectomy Hepatic Steatosis. Scand J Surg. 2017;106:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol. 2004;40:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 355] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 56. | Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 57. | Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 59. | Kleeff J, Diener MK, Z'graggen K, Hinz U, Wagner M, Bachmann J, Zehetner J, Müller MW, Friess H, Büchler MW. Distal pancreatectomy: risk factors for surgical failure in 302 consecutive cases. Ann Surg. 2007;245:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 60. | Halloran CM, Cox TF, Chauhan S, Raraty MG, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: a prospective study. Pancreatology. 2011;11:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Gersell DJ, Gingerich RL, Greider MH. Regional distribution and concentration of pancreatic polypeptide in the human and canine pancreas. Diabetes. 1979;28:11-15. [PubMed] |
| 62. | Henquin JC, Ibrahim MM, Rahier J. Insulin, glucagon and somatostatin stores in the pancreas of subjects with type-2 diabetes and their lean and obese non-diabetic controls. Sci Rep. 2017;7:11015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Kachare SD, Fitzgerald TL, Schuth O, Vohra NA, Zervos EE. The impact of pancreatic resection on exocrine homeostasis. Am Surg. 2014;80:704-709. [PubMed] |
| 64. | Ivanics T, Sanjeevi S, Ansorge C, Andrén-Sandberg Å. Hepatic Steatosis Following Pancreatic Surgery: A Swedish Centers Experience with Demographics, Risks and Outcome. JOP. 2015;16:527-532. |
| 65. | Brennan GT, Saif MW. Pancreatic Enzyme Replacement Therapy: A Concise Review. JOP. 2019;20:121-125. [PubMed] |
| 66. | Layer P, Keller J, Lankisch PG. Pancreatic enzyme replacement therapy. Curr Gastroenterol Rep. 2001;3:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | Ferrie S, Graham C, Hoyle M. Pancreatic enzyme supplementation for patients receiving enteral feeds. Nutr Clin Pract. 2011;26:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Phillips ME. Pancreatic resection nutritional implications and long term follow up. Nutrition Issues in Gastroenterology. Practical Gastroenterol. 2016;40. |
| 69. | Sato T, Matsuo Y, Shiga K, Morimoto M, Miyai H, Takeyama H. Factors that predict the occurrence of and recovery from non-alcoholic fatty liver disease after pancreatoduodenectomy. Surgery. 2016;160:318-330. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Maehira H, Iida H, Mori H, Nitta N, Maekawa T, Tokuda A, Takebayashi K, Kaida S, Miyake T, Tani M. Aggressive Intervention of Pancrelipase After Pancreatectomy Prevents Deterioration of Postoperative Nutritional Status. Pancreas. 2022;51:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 71. | Kim JM, Ha SY, Joh JW, Sinn DH, Jeong WK, Choi GS, Gwak GY, Kwon CHD, Kim YK, Paik YH, Lee JH, Lee WJ, Lee SK, Park CK. Predicting Hepatic Steatosis in Living Liver Donors via Noninvasive Methods. Medicine (Baltimore). 2016;95:e2718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Jacobs JE, Birnbaum BA, Shapiro MA, Langlotz CP, Slosman F, Rubesin SE, Horii SC. Diagnostic criteria for fatty infiltration of the liver on contrast-enhanced helical CT. AJR Am J Roentgenol. 1998;171:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Kawamoto S, Soyer PA, Fishman EK, Bluemke DA. Nonneoplastic liver disease: evaluation with CT and MR imaging. Radiographics. 1998;18:827-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Park YS, Park SH, Lee SS, Kim DY, Shin YM, Lee W, Lee SG, Yu ES. Biopsy-proven nonsteatotic liver in adults: estimation of reference range for difference in attenuation between the liver and the spleen at nonenhanced CT. Radiology. 2011;258:760-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 75. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Méndez-Sánchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:212-220. [PubMed] |
| 76. | Fraum TJ, Ludwig DR, Kilian S, Curtis WA, Pilgram TK, Sirlin CB, Fowler KJ. Epidemiology of Hepatic Steatosis at a Tertiary Care Center: An MRI-based Analysis. Acad Radiol. 2018;25:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |