Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12936
Peer-review started: October 19, 2022
First decision: November 4, 2022
Revised: November 8, 2022
Accepted: November 14, 2022
Article in press: November 14, 2022
Published online: December 16, 2022
Processing time: 55 Days and 21.4 Hours
Hypertensive cerebral hemorrhage (HICH) is a common clinical cerebrovascular disease and one of the most serious complications of hypertension. Early warning of the occurrence of infection during treatment and timely anti-infective treatment are of great significance for the early prevention and treatment of postoperative infection in patients with HICH. Changes in the levels of inflammatory mediators, which are closely related to the occurrence and development of postoperative infection, and procalcitonin (PCT), which is a sensitive indicator for diagnosing bacterial infections, are widely used in clinical practice.
To explore the application value of inflammatory mediator profiles and PCT in predicting postoperative infection in patients with HICH.
A total of 271 patients who underwent HICH surgery at our hospital between March 2019 and March 2021 were selected and divided into the infection (n = 80) and non-infection (n = 191) groups according to whether postoperative infection occurred. The postoperative infection status and etiological characteristics of the infective pathogens in the infection group were analyzed. Changes in inflammatory mediator profile indices and PCT levels were compared between the two groups, pre- and postoperatively.
A total of 109 strains of pathogenic bacteria were detected in the infection group, including 67 strains (61.47%) of gram-negative bacteria, 32 strains (29.36%) of gram-positive bacteria, and 10 strains (9.17%) of fungi. The main infection site of the patients in the infection group was the respiratory system (63.75%). Preoperative interleukin (IL)-4, IL-6, IL-10, tumor necrosis factor-α, interferon-γ, and PCT levels were higher in the infection group than in the non-infection group (P < 0.05), and there were no significant differences in the IL-2 Levels between the two groups (P > 0.05). The inflammatory mediator profile indices and PCT levels were higher in the two groups of patients on the first postoperative day than preoperatively (P < 0.05), and were higher than those in the non-infection group (P < 0.05). Logistic regression analysis showed that preoperative IL-6 and PCT levels correlated with postoperative infection (P < 0.05). Operating characteristic curve analysis results showed that the area under the curve (AUC) values of preoperative IL-6 and PCT levels in predicting postoperative infection in patients with HICH were 0.755 and 0.824, respectively. The AUC value of joint detection was 0.866, which was significantly higher than that of the single index (P < 0.05).
Preoperative IL-6 and PCT levels are correlated with postoperative infection in patients with HICH. Their detection is clinically significant for early identification of patients at high risk for postoperative infection.
Core Tip: Early warning of the occurrence of infection during treatment and timely anti-infective treatment are of great significance for the early prevention and treatment of postoperative infection in patients with hypertensive cerebral hemorrhage. Changes in the levels of inflammatory mediators are closely related to the occurrence and development of postoperative infection, and procalcitonin is a sensitive indicator for diagnosing bacterial infections and is widely used in clinical practice.
- Citation: Yin RH, Zhang B, Zhou XH, Cao LP, Li M. Value of inflammatory mediator profiles and procalcitonin in predicting postoperative infection in patients with hypertensive cerebral hemorrhage. World J Clin Cases 2022; 10(35): 12936-12945
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12936.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12936
Hypertensive cerebral hemorrhage (HICH) is a common clinical cerebrovascular disease and one of the most serious complications of hypertension. In China, the incidence of HICH is approximately (12–15)/100000 person-years, and the 30 d case fatality rate after onset can reach 35%–52%, which seriously affects the patients’ quality of life[1,2]. Surgery is an effective method for the clinical treatment of HICH, with good results in bleeding control, intracranial pressure reduction, and hematoma removal[3,4]. However, most patients with HICH are elderly, have a lower immune function than that of the general population, and due to surgical stress and prolonged postoperative bed rest, are prone to infection-related complications, which prolong hospital stay and adversely affect their prognosis[5,6]. Therefore, early warning of the occurrence of infection during treatment and timely anti-infective treatment are of great significance for the early prevention and treatment of postoperative infection in patients with HICH[7]. Previous studies have found that changes in the levels of inflammatory mediators are closely related to the occurrence and development of postoperative infection[8](Wu JY, Prentice H. Potential new therapeutic intervention for ischemic stroke.
A total of 271 patients who underwent HICH surgery at our hospital between March 2019 and March 2021 were included. Inclusion criteria: (1) Clinical examination conformed to the diagnostic criteria of HICH[11], all patients were diagnosed using imaging examination, and the hematoma location was clear; (2) all patients were first onset, and the onset time was less than 72 h; (3) all patients met the indications for surgical treatment, which they received; (4) the sex of the patients was not limited, and the age was more than 18 years; and (5) the patients and their families provided signed informed consent.
Exclusion criteria: patients with (1) cerebral hemorrhage caused by other reasons; (2) an infection before diagnosis or with a history of using antibacterial drugs and immunomodulatory agents 3 d before enrollment; (3) mixed malignant tumors, with dysfunction of important organs, blood diseases or self-regulatory diseases patients with immune diseases; and (4) a history of cerebral infarction or cerebral hemorrhage. This study was approved by the hospital ethics committee and conducted in accordance with the Declaration of Helsinki (Test registration number: ChiCTR2200062199).
After patients were admitted to the hospital, relevant laboratory examinations were improved, and the changes in the vital signs of the patients were closely monitored during the treatment.
The "Diagnostic Criteria for Nosocomial Infection"[12] was used as a reference for diagnosing whether patients had postoperative infections: (1) The presence of clinical symptoms, such as postoperative high fever, headache, nausea, chills, and cough; (2) a positive bacterial culture result; and (3) an increased white blood cell count and proportion of neutrophils. Patients were divided into the infected and non-infected groups according to their infection status. Pharyngeal swabs, sputum, urine, feces, wound secretions, bone marrow, and other clinical specimens of infected patients were collected, and complete bacterial culture were submitted for analysis. Strains were identified using a fully automatic strain identification instrument (VITEK 2 Compact, BioMérieux, France).
General clinical data, such as sex, age, body mass index (BMI), underlying diseases, smoking history, Glasgow coma scale (GCS), bleeding site, and bleeding volume were collected.
Fasting venous blood (5 mL) was preoperatively collected from patients (the morning after admission) and 1 d postoperatively, centrifuged at 3000 r/min for 10 min, and the supernatant was separated and stored at a low temperature for testing. An enzyme-linked immunosorbent assay was used to detect the level of inflammatory mediator profile indicators using an automatic biochemical analyzer (Model 7600-020, Hitachi, Ltd., Japan), including interleukin (IL)-2, IL-4, IL-6, interleukin-10 IL-10, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). PCT levels were detected using electrochemiluminescence immunoassay. The kit was a matching kit for the detection instrument, and detection was performed in strict accordance with the requirements of the instrument operation and use of the kit.
SPSS 20.0 statistical software was used to analyze the data. Measurement data are expressed as mean ± SD, an independent sample t-test was used for intergroup comparisons, and a paired t test was used for pre- and postoperative comparisons in the same group. Enumeration data are expressed as n (%), and the χ2 test or Fisher's exact probability test were used. Correlations were analyzed using a multivariate logistic regression model. The receiver operating characteristic (ROC) curve was used to evaluate the predictive value of inflammatory mediator profile indices and PCT levels in postoperative complications in patients with HICH; inspection level α = 0.05.
Of the 271 patients, 80 (29.52%) had postoperative infections; the infection site was mainly the respiratory system, followed by the surgical incision and urinary system, as shown in Table 1.
| Infection site | Cases (n = 80) | Proportion (%) |
| Respiratory system | 51 | 63.75 |
| Surgical incision | 15 | 18.75 |
| Urinary system | 9 | 11.25 |
| Intracranial | 5 | 6.25 |
Among the 80 patients in the infection group, 109 strains of pathogenic bacteria were detected, including 67 strains of gram-negative bacteria (61.47%), 32 strains of gram-positive bacteria (29.36%), and 10 strains of fungi (9.17%). The proportions are listed in Table 2.
| Pathogens | Strains (n) | Proportion (%) |
| Gram-negative bacteria | 67 | 61.47 |
| Klebsiella pneumoniae | 24 | 22.02 |
| Escherichia coli | 18 | 16.51 |
| Pseudomonas aeruginosa | 13 | 11.93 |
| Acinetobacter baumannii | 8 | 7.34 |
| Enterobacter cloacae | 4 | 3.67 |
| Gram-positive bacteria | 32 | 29.36 |
| Staphylococcus aureus | 13 | 11.93 |
| Staphylococcus epidermidis | 9 | 8.26 |
| coagulase-negative staphylococci | 7 | 6.42 |
| Enterococcus | 3 | 2.75 |
| Fungus | 10 | 9.17 |
| Total | 109 | 100.00 |
The proportion of patients with underlying diseases and volume of bleeding in the infection group were higher than those in the non-infection group (P < 0.05). There were no significant differences in general clinical data, such as sex, age, BMI, smoking history, GCS score, and bleeding site between the two groups (P > 0.05), as shown in Table 3.
| Index | Infection group (n = 80) | Non-infection group (n = 191) | t/χ2 value | P value |
| Sex | 0.586 | 0.444 | ||
| Male | 57 (71.25) | 127 (66.49) | ||
| Female | 23 (28.75) | 64 (33.51) | ||
| Age (yr) | 68.49 ± 9.67 | 67.52 ± 10.31 | 0.719 | 0.473 |
| BMI (kg/m2) | 23.67 ± 2.51 | 24.06 ± 2.74 | 1.095 | 0.275 |
| Underlying diseases | 4.844 | 0.028 | ||
| Yes | 49 (61.25) | 89 (46.60) | ||
| No | 31 (38.75) | 102 (53.40) | ||
| Smoking history | 0.865 | 0.352 | ||
| Yes | 35 (43.75) | 72 (37.70) | ||
| No | 45 (56.25) | 119 (62.30) | ||
| GCS score (points) | 6.85 ± 1.26 | 7.24 ± 1.68 | 1.867 | 0.063 |
| Bleeding site | - | 0.613 | ||
| Lobe | 49 (61.25) | 103 (53.93) | ||
| Thalamus | 19 (23.75) | 53 (27.75) | ||
| Basal Ganglia | 7 (8.75) | 25 (13.09) | ||
| Brain stem | 5 (6.25) | 10 (5.24) | ||
| Bleeding (mL) | 50.46 ± 10.63 | 48.35 ± 9.78 | 2.327 | 0.021 |
Preoperative IL-4, IL-6, IL-10, TNF-α, IFN-γ, and PCT levels were higher in the infection group than in the non-infection group (P < 0.05), and there were no significant differences in the IL-2 Levels between the two groups (P > 0.05). The inflammatory mediator profile indices and PCT levels were higher in the two groups of patients on the first postoperative day than preoperatively (P < 0.05), and were higher than those in the non-infection group (P < 0.05), as shown in Table 4.
| Index | Infection group (n = 80) | Non-infection group (n = 191) | t/χ2 value | P value |
| IL-2 (ng/L) | ||||
| Preoperative | 1.28 ± 0.35 | 1.23 ± 0.24 | 1.356 | 0.176 |
| POD | 2.62 ± 0.87a | 1.78 ± 0.53a | 9.725 | < 0.001 |
| IL-4 (ng/L) | ||||
| Preoperative | 1.06 ± 0.31 | 0.98 ± 0.26 | 2.179 | 0.030 |
| POD | 3.68 ± 1.01a | 2.35 ± 0.76a | 11.873 | < 0.001 |
| IL-6 (ng/L) | ||||
| Preoperative | 3.21 ± 1.94 | 2.87 ± 0.88 | 3.447 | 0.001 |
| POD | 11.28 ± 3.27a | 8.64 ± 2.13a | 7.870 | < 0.001 |
| IL-10 (ng/L) | ||||
| Preoperative | 2.75 ± 0.89 | 2.53 ± 0.68 | 2.209 | 0.028 |
| POD | 8.72 ± 1.94a | 6.53 ± 1.85a | 8.762 | < 0.001 |
| TNF-α (ng/L) | ||||
| Preoperative | 1.73 ± 0.46 | 1.58 ± 0.51 | 2.272 | 0.024 |
| POD | 6.58 ± 2.04 | 3.91 ± 0.94 | 14.755 | < 0.001 |
| IFN-γ (ng/L) | ||||
| Preoperative | 0.86 ± 0.25 | 0.80 ± 0.19 | 2.151 | 0.032 |
| POD | 3.18 ± 0.97 | 2.76 ± 0.84 | 3.583 | < 0.001 |
| PCT (μg/L) | ||||
| Preoperative | 1.55 ± 0.42 | 0.51 ± 0.19 | 28.087 | < 0.001 |
| POD | 1.83 ± 0.56a | 0.63 ± 0.21a | 25.667 | < 0.001 |
Underlying diseases; blood loss; and preoperative IL-4, IL-6, IL-10, TNF-α, IFN-γ, and PCT levels were included in multivariate logistic regression analysis. The results showed that preoperative IL-6 and PCT levels were correlated with postoperative infection in patients with HICH and were independent risk factors for postoperative infection in patients with HICH (P < 0.05), as shown in Table 5.
| Factors | β | SE | Wald χ2 | OR | 95%CI | P value |
| Underlying diseases | 0.615 | 0.377 | 2.661 | 1.850 | 0.883-3.873 | 0.104 |
| Bleeding | 0.096 | 0.068 | 1.993 | 1.101 | 0.963-1.258 | 0.159 |
| IL-4 | 0.876 | 0.478 | 3.359 | 2.401 | 0.941-6.128 | 0.068 |
| IL-6 | 0.893 | 0.321 | 7.739 | 2.442 | 1.302-4.582 | 0.006 |
| IL-10 | 0.483 | 0.282 | 2.934 | 1.621 | 0.933-2.817 | 0.087 |
| TNF-α | 0.473 | 0.289 | 2.679 | 1.605 | 0.911-2.828 | 0.102 |
| IFN-γ | 0.469 | 0.248 | 3.576 | 1.598 | 0.983-2.599 | 0.059 |
| PCT | 1.457 | 0.391 | 13.886 | 4.293 | 1.995-9.238 | < 0.001 |
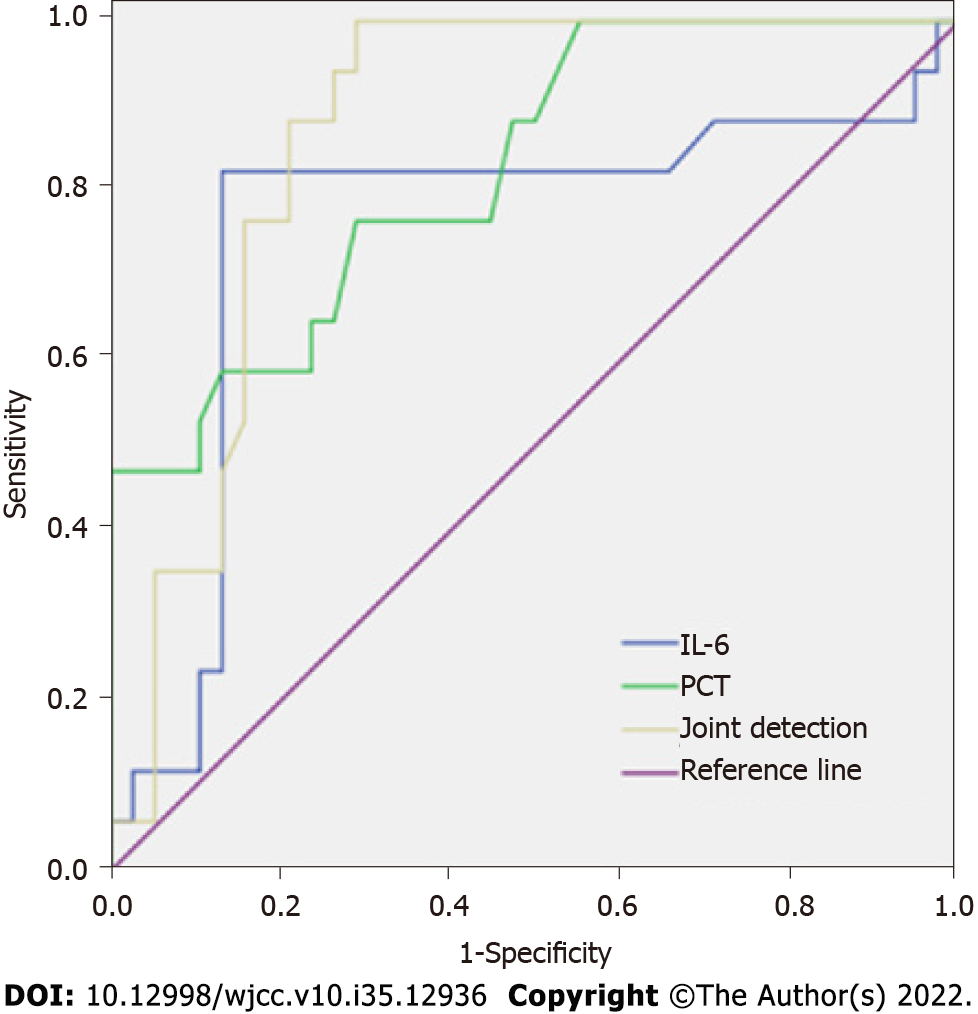
The results of the ROC curve analysis showed that the area under the curve (AUC) values of preoperative IL-6 and PCT levels in predicting postoperative infection in patients with HICH were 0.755 and 0.824, respectively, and were statistically significant (P < 0.05). The AUC value of combined detection for predicting postoperative infection in patients with HICH was 0.866, which was significantly higher than the single index of IL-6 and PCT (Z = 4.152, 2.253, P = 0.014, 0.036). The cutoff value, Younden's index, and the sensitivity and specificity of each index are shown in Table 6 and Figure 1.
| Index | AUC | P value | Cutoff value | Younden’s Index | Sensitivity | Specificity |
| IL-6 | 0.755 | 0.003 | 3.10 ng/L | 0.475 | 76.47 | 71.05 |
| PCT | 0.824 | < 0.001 | 0.96 μg/L | 0.692 | 82.35 | 86.84 |
| Joint detection | 0.866 | < 0.001 | - | 0.711 | 90.00 | 81.05 |
Long-term hypertension causes pathological changes, such as kidney injury[13], ischemic necrosis of small arteries in the brain and the formation of tiny aneurysms. When the blood pressure is further increased, small blood vessels rupture and bleed, ultimately causing HICH[14]. Hematoma removal and decompressive craniectomy are commonly used clinical treatments for HICH, and can effectively remove hematoma, reduce intracranial pressure, and reduce hematoma damage to the brain tissue. There is an inevitable risk of postoperative infection[15,16]. Therefore, early diagnosis of postoperative HICH infection and enhanced analysis of the etiological characteristics of bacterial infection are significant for guiding rational clinical drug use and improving patient prognosis. Although traditional pharyngeal swab, sputum, urine, and other clinical specimen culture test results have high accuracy, challenges, such as easy contamination of specimens and long-time consumption, are not conducive to the formulation and implementation of diagnosis and treatment plans. Recent studies have shown that changes in the levels of serum inflammatory markers can reflect the infection status of the body and are significant to the diagnosis and assessment of infectious diseases[17]. In this study, we analyzed the changes of inflammatory mediator profile indices and PCT levels in patients with HICH with postoperative infections and compared them with uninfected patients. Inflammatory mediator profile indices and PCT levels were correlated with postoperative infection in patients with HICH.
This study found that among the 271 patients, 80 were complicated with postoperative infection, and the main infection site was the respiratory system (63.75%). Patients with HICH require prolonged postoperative bed rest, and have a poor nutritional status. The barrier function of the respiratory mucosa and ability to expel sputum are weakened, which allows pathogen invasion and increases the probability of respiratory system infections[18]. A total of 109 strains of pathogenic bacteria were detected in the infection group, including 67 (61.47%) of gram-negative bacteria, 32 (29.36%) of gram-positive bacteria, and 10 (9.17%) of fungi. Jiang et al[19] analyzed the etiological characteristics and influencing factors of postoperative infection in patients with HICH, and found that postoperative infection after HICH was primarily respiratory tract infection, and the infective pathogens were primarily gram-negative bacteria, which is similar to the results of this study. It is believed that with the abuse of antibacterial drugs, the resistance of strains has been increasing, the pathogenic bacteria profiles of infection have changed, and the proportion of gram-negative bacteria has gradually increased.
Inflammation is considered the pathophysiological basis for the occurrence and development of infection. After infection, pathogenic bacteria and their endotoxins enter the blood, triggering a cascade of inflammatory mediators, leading to abnormal increases in the levels of various cytokines, such as IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ. These factors further expand the inflammatory response and induce cytotoxicity, leading to brain tissue damage. IL-2 and IL-4 are cytokines produced by activated T cells, and changes in their levels reflect the immune function of the body[20]. IL-6 is a cytokine with a variety of biological activities, mainly produced by mononuclear macrophages, and Th2 cells, which can regulate the immune and inflammatory responses of the body[21]. IL-10 is an anti-inflammatory factor and important negative regulator of the body’s immune response, and its level imbalance can increase the risk of immune diseases[22]. TNF-α is a response factor for the initiation of inflammation and can be produced in large quantities under the stimulation of bacterial endotoxin, which can lead to severe microcirculation disturbance by altering vascular endothelial function[23]. IFN-γ is mainly secreted by Th1 cells, which can activate neutrophils and induce the body’s inflammatory response in a hyperactive state[24]. PCT is the precursor of calcitonin, and its serum level is extremely low under normal circumstances; when the body is infected, its concentration can be rapidly increased within 2–4 h under the induction of cytokines, such as endotoxins. Oxidative stress injury can be exacerbated by binding to glycoprotein ligands in infected patients[25]. The results of this study showed that the inflammatory mediator profile indices and PCT levels were higher in the two groups of patients on the first postoperative day than preoperatively, and the IL-4, IL-6, IL-10, TNF-α, IFN-γ, and PCT levels in the infection group were higher than those in the non-infection group before and 1 d after surgery. Logistic regression analysis showed that preoperative IL-6 and PCT levels were independent risk factors for postoperative infection in patients with HICH, suggesting that the infection group had preoperative immune disorders, and surgical treatment would further aggravate the immune disorder and increase the risk of postoperative infection in patients. As a strong stressor, surgery can trigger an inflammatory response, significantly increase the levels of inflammatory factors, such as IL-6 and TNF-α, and initiate a cascade of inflammatory mediators, resulting in a continuous increase in the levels of a large number of inflammatory factors to increase the severity of infection[26,27]. Therefore, in patients with HICH, elevated pre- and postoperative inflammatory mediator profiles or PCT levels require high vigilance for the occurrence of postoperative infection. Although the IL-2 Level in the infection group was higher than that in the non-infection group 1 d postoperatively in this study, there were no significant differences in the preoperative IL-2 Levels between the two groups. The inhibitory effects of IL-10 on IL-2 production and macrophage activity[28] may be related to the small number of subjects included in this study.
Further analysis of the value of preoperative IL-6 and PCT levels in predicting postoperative infection in patients with HICH revealed that the AUC values of preoperative IL-6 and PCT levels in predicting postoperative infection in patients with HICH were all greater than 0.500. Combined detection can further improve the predictive value, suggesting that the combined detection of inflammatory mediator profiles and PCT levels is helpful for the early diagnosis of postoperative infection in patients with HICH. Therefore, clinical risk stratification of patients with HICH may be performed by monitoring the inflammatory mediator profiles and PCT levels before and after surgery, and provide reference for the prevention, diagnosis, and treatment of postoperative infection in patients.
In conclusion, preoperative IL-6 and PCT levels were correlated with the postoperative infection in patients with HICH. The combined detection of inflammatory mediator profiles and PCT levels is helpful for the early diagnosis of postoperative infection in patients with HICH, and has guiding significance for the early identification of patients with high risk of postoperative infection in clinical practice.
Hypertensive cerebral hemorrhage (HICH) is a common clinical cerebrovascular disease and one of the most serious complications of hypertension. Early warning of the occurrence of infection during treatment and timely anti-infective treatment are of great significance for the early prevention and treatment of postoperative infection in patients with HICH. Changes in the levels of inflammatory mediators, which are closely related to the occurrence and development of postoperative infection, and procalcitonin (PCT), which is a sensitive indicator for diagnosing bacterial infections, are widely used in clinical practice.
In this study, the authors found that the preoperative interleukin (IL)-6 and PCT levels were correlated with the postoperative infection in patients with HICH.
This study aimed to explore the application value of inflammatory mediator profiles and PCT in predicting postoperative infection in patients with HICH.
A total of 271 patients who underwent HICH surgery were selected and divided into the infection (n = 80) and non-infection (n = 191) groups according to whether postoperative infection occurred. The postoperative infection status and etiological characteristics of the infective pathogens in the infection group were analyzed. Changes in inflammatory mediator profile indices and PCT levels were compared between the two groups, pre- and postoperatively.
The main infection site of the patients in the infection group was the respiratory system. Preoperative IL-4, IL-6, IL-10, tumor necrosis factor-α, interferon-γ, and PCT levels were higher in the infection group than in the non-infection group. The inflammatory mediator profile indices and PCT levels were higher in the two groups of patients on the first postoperative day than preoperatively, and were higher than those in the non-infection group. Logistic regression analysis showed that preoperative IL-6 and PCT levels correlated with postoperative infection.
Preoperative IL-6 and PCT levels are correlated with postoperative infection in patients with HICH. Their detection is clinically significant for early identification of patients at high risk for postoperative infection
We will continue to study HICH and hypertension in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Knieling A, Romania; Yoshimura T, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Wang X, Chen Y, Wang Z, Qian M. Clinical Research of Early Hyperbaric Oxygen Therapy on Patients with Hypertensive Cerebral Hemorrhage After Craniotomy. Turk Neurosurg. 2020;30:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Gong W, Zhang S, Li X, Shi L. Dexmedetomidine is superior to midazolam for sedation and cerebral protection in postoperative hypertensive intracerebral hemorrhage patients: a retrospective study. J Int Med Res. 2020;48:300060520957554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Sun G, Li X, Chen X, Zhang Y, Xu Z. Comparison of keyhole endoscopy and craniotomy for the treatment of patients with hypertensive cerebral hemorrhage. Medicine (Baltimore). 2019;98:e14123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Li BZ, Bao JZ, Yang C, Liu LK, Lu ZC, Qi B. Effects of neuroendoscopy and craniotomy on matrix metalloproteinase-9 and serum chitinase protein 40 in patients with hypertensive cerebral hemorrhage and their relationship with cerebral edema. Zhonghua Shiyan Waike Zazhi. 2021;38:1378-1379. [DOI] [Full Text] |
| 5. | Zhang S, Zhang X, Ling Y, Li A. Predicting Recurrent Hypertensive Intracerebral Hemorrhage: Derivation and Validation of a Risk-Scoring Model Based on Clinical Characteristics. World Neurosurg. 2019;127:e162-e171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Qin MY, Zhang WT, Ling YH, Li KH, Li LH, Yu HY. Incidence of and Risk Factors for Early Intracranial Infection after Invasive Intracranial Pressure Monitoring for Severe Traumatic Brain Injury or Hypertensive Intracranial Hemorrhage. Zhongguo Yike Daxue Xuebao. 2019;48:786-790. [DOI] [Full Text] |
| 7. | Jeong TS, Yee GT. Prospective Multicenter Surveillance Study of Surgical Site Infection after Intracranial Procedures in Korea : A Preliminary Study. J Korean Neurosurg Soc. 2018;61:645-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Yan L, Wang S, Xu L, Zhang Z, Liao P. Procalcitonin as a prognostic marker of patients with acute ischemic stroke. J Clin Lab Anal. 2020;34:e23301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Ding RD, Zhang HJ. Effect of linezolid on serum PCT, ESR, and CRP in patients with pulmonary tuberculosis and pneumonia. Medicine (Baltimore). 2018;97:e12177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Zhang JZ, Zhang QC, Liu C, Li K, Zhang L, Zheng YZ. Changes and significance of serum inflammatory markers and copeptin in patients with hypertensive cerebral hemorrhage. Zhonghua Yiyuan Ganranxue Zazhi. 2019;29:1811-1814. |
| 11. | Neurology Branch of Chinese Medical Association; Cerebrovascular Disease Group of Neurology Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of cerebral hemorrhage in China (2019). Zhonghua Shenjingke Zazhi. 2019;52:994-1005. [DOI] [Full Text] |
| 12. | Ministry of Health of the People's Republic of China. Diagnostic criteria for nosocomial infection (trial). Zhonghua Yixue Zazhi. 81:314-320. [DOI] [Full Text] |
| 13. | Sun D, Wang J, Shao W, Yao L, Li Z, Ohno S. Pathogenesis and Damage Targets of Hypertensive Kidney Injury. J Transl Int Med. 2020;8:205-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392:1257-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 15. | Hayashi T, Karibe H, Akamatsu Y, Narisawa A, Shoji T, Sasaki T, Kameyama M, Tominaga T. Endoscopic Hematoma Evacuation for Intracerebral Hemorrhage Under Local Anesthesia: Factors That Affect the Hematoma Removal Rate. World Neurosurg. 2019;126:e1330-e1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. 2018;114:42-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | McSorley ST, Tham A, Dolan RD, Steele CW, Ramsingh J, Roxburgh C, Horgan PG, McMillan DC. Perioperative Blood Transfusion is Associated with Postoperative Systemic Inflammatory Response and Poorer Outcomes Following Surgery for Colorectal Cancer. Ann Surg Oncol. 2020;27:833-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | López-de-Andrés A, Perez-Farinos N, de Miguel-Díez J, Hernández-Barrera V, Jiménez-Trujillo I, Méndez-Bailón M, de Miguel-Yanes JM, Jiménez-García R. Type 2 diabetes and postoperative pneumonia: An observational, population-based study using the Spanish Hospital Discharge Database, 2001-2015. PLoS One. 2019;14:e0211230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Jiang L, Li K, Wang H, Guo X, Yan FF, Zhang HM. Influencing factors of postoperative infection in patients with hypertensive cerebral hemorrhage and nursing preventive interventions. Zhonghua Yiyuan Ganranxue Zazhi. 2019;29:905-908. |
| 20. | Soyoz M, Pehlivan M, Tatar E, Cerci B, Coven HIK, Ayna TK. Consideration of IL-2, IFN-γ and IL-4 expression and methylation levels in CD4+ T cells as a predictor of rejection in kidney transplant. Transpl Immunol. 2021;68:101414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Winslow S, Odqvist L, Diver S, Riise R, Abdillahi S, Wingren C, Lindmark H, Wellner A, Lundin S, Yrlid L, Ax E, Djukanovic R, Sridhar S, Higham A, Singh D, Southworth T, Brightling CE, Olsson HK, Jevnikar Z. Multi-omics links IL-6 trans-signalling with neutrophil extracellular trap formation and Haemophilus infection in COPD. Eur Respir J. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Ouyang W, O'Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50:871-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 723] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 23. | Belenguer G, Duart-Abadia P, Jordán-Pla A, Domingo-Muelas A, Blasco-Chamarro L, Ferrón SR, Morante-Redolat JM, Fariñas I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem Cell. 2021;28:285-299.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 24. | Kim EY, Ner-Gaon H, Varon J, Cullen AM, Guo J, Choi J, Barragan-Bradford D, Higuera A, Pinilla-Vera M, Short SA, Arciniegas-Rubio A, Tamura T, Leaf DE, Baron RM, Shay T, Brenner MB. Post-sepsis immunosuppression depends on NKT cell regulation of mTOR/IFN-γ in NK cells. J Clin Invest. 2020;130:3238-3252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 25. | Gai L, Tong Y, Yan BQ. Research on the diagnostic effect of PCT level in serum on patients with sepsis due to different pathogenic causes. Eur Rev Med Pharmacol Sci. 2018;22:4238-4242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Wang F, Yu GQ, Wang XQ, Wang XJ, Yao ST. Research progress on risk factors, diagnosis and prevention of postoperative pneumonia complicated by hypertensive cerebral hemorrhage. Shandong Yiyao. 2019;59:81-84. |
| 27. | Chao J, Cui S, Liu C, Liu S, Han Y, Gao Y, Ge D, Yu A, Yang R. Detection of Early Cytokine Storm in Patients with Septic Shock After Abdominal Surgery. J Transl Int Med. 2020;8:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Zhu K, Hill WG, Li F, Shi B, Chai TC. Early Increased Urinary IL-2 and IL-10 Levels Were Associated With Development of Chronic UTI in a Murine Model. Urology. 2020;141:188.e1-188.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |