ANTENATAL IMAGING WITH CASES
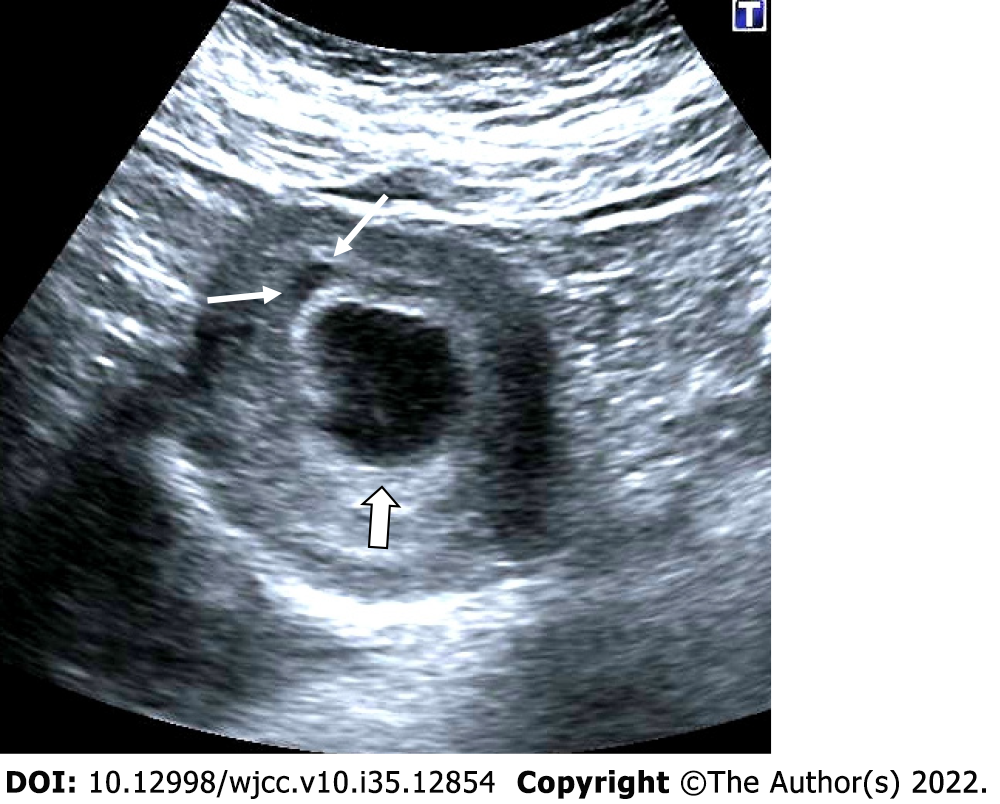
Anembryonic pregnancy
Anembryonic pregnancy, also known as the blighted ovum, is characterized by the failure of the embryo to develop despite the growth of the gestational sac. Diagnosis is made by seeing an empty gestational sac without an embryo on an ultrasonographic examination. In order to diagnose an anembryonic pregnancy, the mean sac diameter (MSD) in transvaginal ultrasonography must be at least 25 mm. In addition, if there is a yolk sac in the first examination, no embryo is found in the sonographic examination performed after 11 days or more, or if only the gestational sac is shown in the first examination, the diagnosis is made by the absence of embryo or yolk sac in the control sonography performed after 14 days. Early intrauterine pregnancy, ectopic pregnancy, pseudogestational sac, and gestational trophoblastic disease should be considered in differential diagnoses[20,21] (Figure 1).
Figure 1 Anembryonic pregnancy.
30-year-old, first pregnancy, complaining of vaginal bleeding. Ultrasonography revealed a gestational sac, slightly irregular border with a mean sac diameter of 28 mm (thick arrow). There was no yolk sac or embryo in the sac. There was also minimal perigestational hemorrhage in the superolateral of the sac (thin arrows).
Lower uterine segment gestation
Typically, the location of the gestational sac is in the middle of the uterine body segment. If the gestational sac is seen close to the cervix and located lower than normal, several pathologies should be considered. Impending or ongoing miscarriage, cervical pregnancy, cesarean scar ectopic pregnancy, cervico isthmic implantation, fundal fibroid or other mass compressing the sac downward should be considered in the differential diagnosis[22] (Figure 2).
Figure 2 Lower Uterine Segment Gestation.
25-year-old, first pregnancy. On ultrasonography, a 6-week well-circumscribed gestational sac is seen low located close to the cervix (Thick arrows: Cervix; thin arrow: Gestational sac).
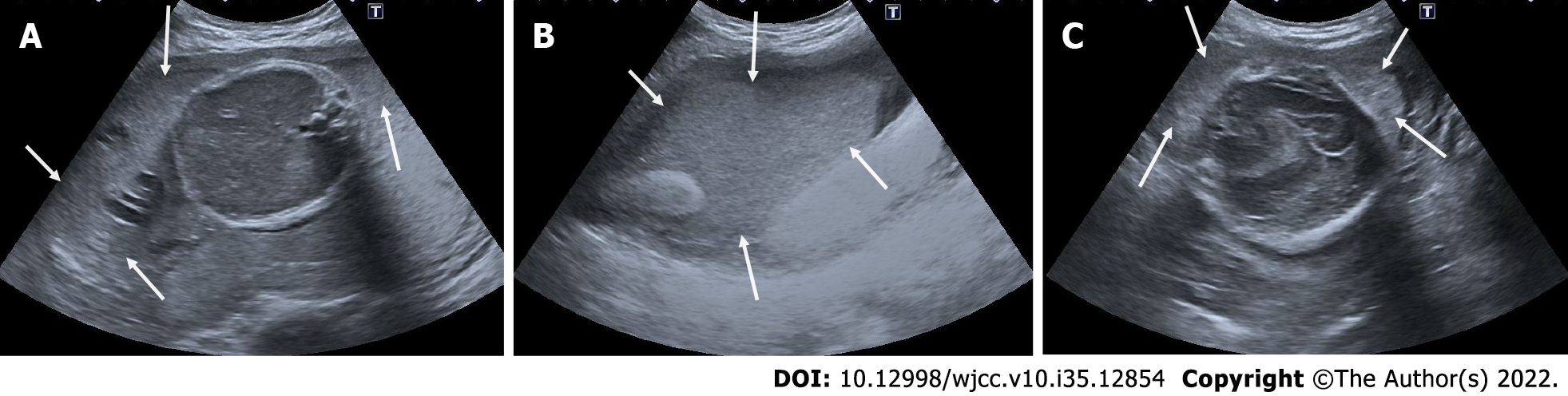
Abortus – miscarriage
Miscarriage is defined as the spontaneous termination of a pregnancy before 20 wk of gestation. A complete miscarriage is defined as the absence of retained fetal tissue or a gestational sac in the uterine cavity. A missed miscarriage is the absence of fetal heartbeat even though the CRL is greater than 7 mm. An empty sac miscarriage is the absence of the yolk sac or embryonic pol, although the MSD is greater than 25 mm[23]. Before diagnosing a total miscarriage, ectopic pregnancy, pregnancy too early to be detected and pregnancy of unknown location should be considered in the differential diagnosis (Figure 3).
Figure 3 Complete miscarriage.
A: 26-year-old, first pregnancy, 6 wk pregnant by date of the last menstrual period and complaining of vaginal bleeding. No gestational sac or fetal structure was seen in the endometrial cavity on axial section ultrasonography; There was enlargement in the cervical canal and hypoechoic collection at this level; B: Coronal section; C: Sagittal section, thin arrows. On ultrasonography performed 3 days ago, a gestational sac was seen in the uterine cavity (not shown in the figure).
Subamniotic - retroplacental hemorrhage
During pregnancy, bleeding may occur due to the separation of the placenta, chorion and amniotic membranes. If the bleeding area is posterior to the placenta, it is called retroplacental hemorrhage; if it is merely on the border of the placenta, it is called marginal subchorionic hemorrhage; and if it lies anterior to the placenta, it is called subamniotic hemorrhage. Subamniotic hemorrhage is usually limited to the umbilical cord. Although it is usually seen as oval-shaped hypoechoic areas on ultrasound, it can also be seen isoechoic or hyperechoic in cases of acute hemorrhage[24] (Figure 4).
Figure 4 Subamniotic and retroplacental hemorrhage.
A and B: Retroplacental hemorrhage. 18 wk pregnant, complaining of vaginal bleeding. On ultrasonography, there was a hypoechoic collection area (arrows) extending in a long segment posterior to the placenta (asterisk); C: Subamniotic hemorrhage, 12 wk pregnant, complaining of pain. On ultrasonography, there was a heterogeneous collection area (arrows) located anterior of the placenta, reaching 10 cm at its widest part (asterisk: Placenta); D: Subamniotic hemorrhage, 18 wk pregnant. On ultrasonography, there was a hypoechoic, minimally heterogeneous collection area (arrows) anterior to the placenta (asterisk: Placenta).
Intraamniotic hemorrhage
In the presence of a hematoma affecting the intrauterine membranes, blood may enter the amniotic space if the amniotic membrane ruptures. It usually limits itself after a while and causes no serious harm to the fetus. Primary intraamniotic hemorrhage most often develops secondary to trauma. It also often develops after amniocentesis. Intraamniotic fibrin materials and septa formations might be seen in the chronic process after active bleeding has ceased. In the amniotic fluid, echogenic particles resulting from bleeding are seen during ultrasonography. Ingestion of the free bleeding by the fetus may result in the appearance of a false mass in the stomach and increase in small intestine echogenicity[24] (Figure 5).
Figure 5 Intraamniotic hemorrhage.
A-C: 21 wk pregnant, on ultrasonography, there were diffuse moving echogenicities (arrows) filling the entire amniotic cavity.
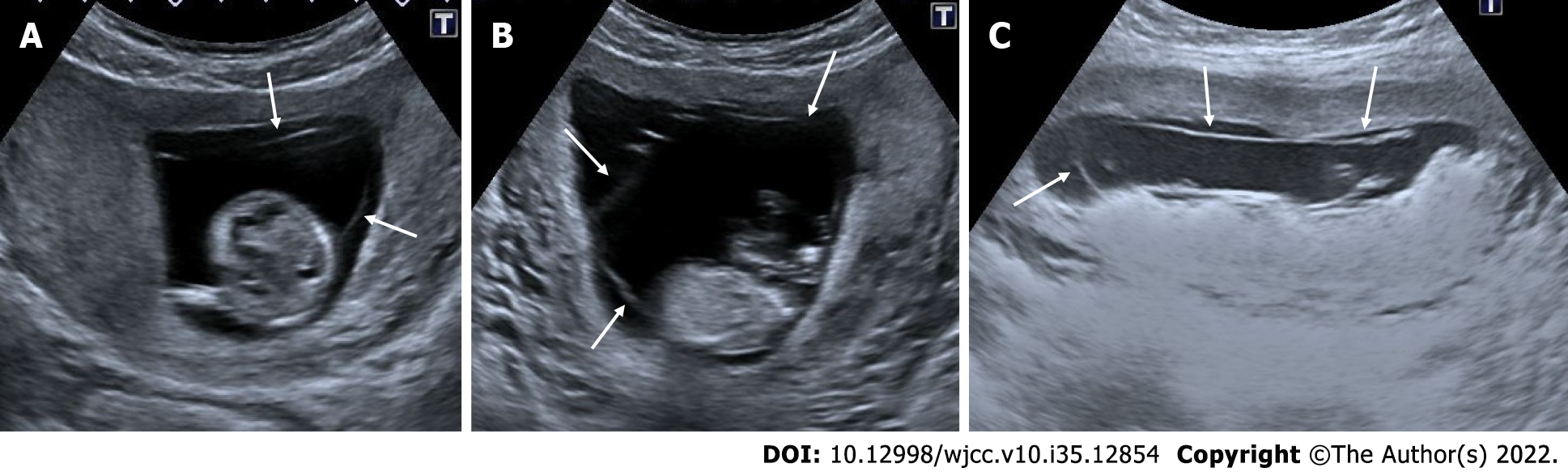
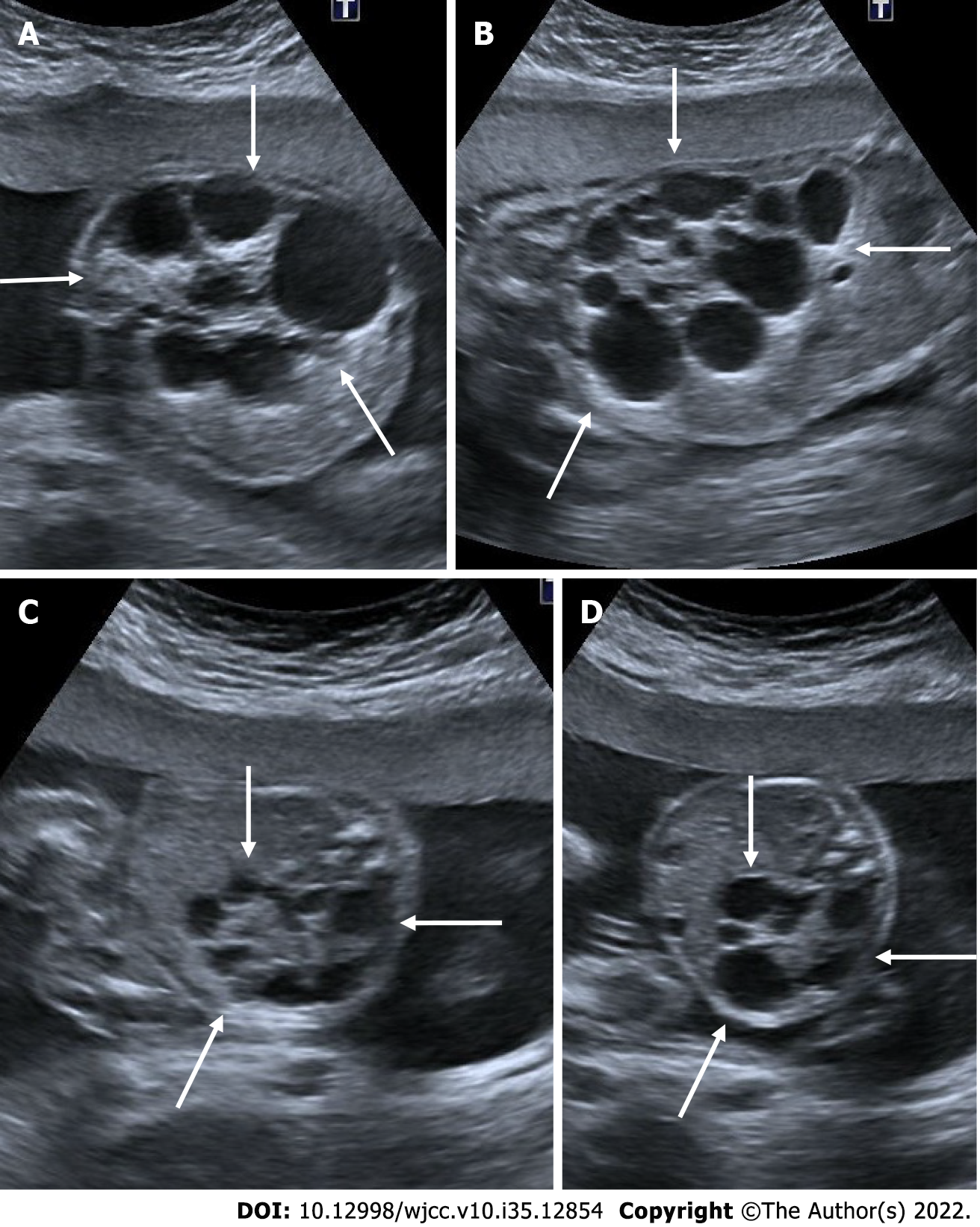
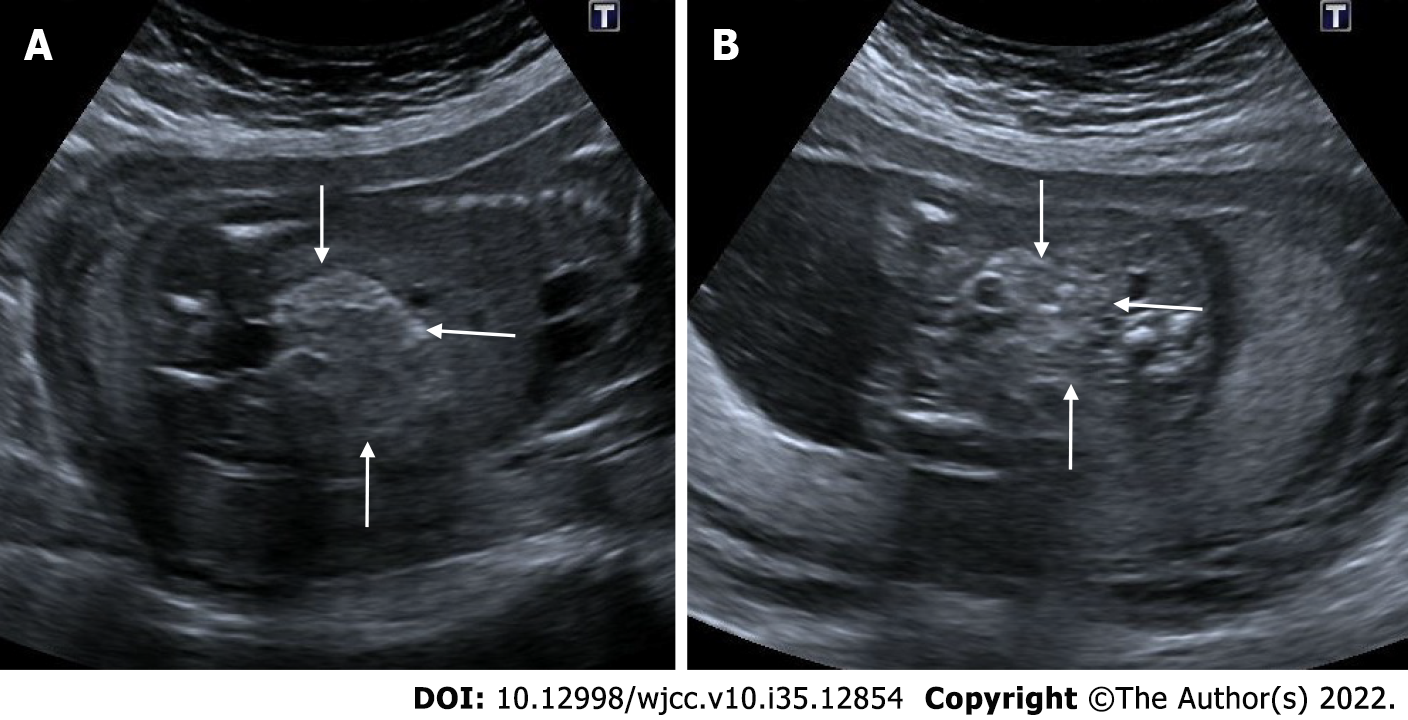
Molar pregnancy - gestational trophoblastic disease
Gestational trophoblastic disease is caused by the aberrant proliferation of trophoblastic tissue. The patient presents with findings such as abnormally elevated levels of beta - human chorionic gonadotropin (HCG), hyperemesis and hypertension. On ultrasonography, a complete hydatid mole appears as a complex multicystic heterogeneous echogenic mass that largely fills the intrauterine cavity, causes uterine enlargement, and contains multiple millimetric cystic areas. This view is defined as the "bunch of grapes" sign. These cystic regions are the result of hydropic chorionic villi. In addition to small cystic cavities, larger irregular fluid collections may also be seen. There is no fetus or fetal component in complete hydatiform mole. In subsequent weeks of pregnancy, the mass appearance and cystic areas become larger. In addition, theca lutein cysts can be seen in both ovaries due to beta HCG stimulation. In partial hydatiform mole, gestational sac and yolk sac can be seen. The placenta is abnormally large and contains numerous microcystic areas. Doppler ultrasonography shows low-resistance, high-velocity blood flow in both molar pregnancies[25,26] (Figure 6).
Figure 6 Partial hydatiform mole.
27-year-old, third pregnancy, 7 wk pregnant by date of the last menstrual period, complaining of pain, beta HCG value greater than 200000. A and C: On ultrasonography, there was a heterogeneous, echogenic mass appearance-enlarged multicystic placenta (thin arrows) that filled the endometrial cavity to a large extent, containing multiple millimetric cystic areas; B: The gestational sac was irregular and contained millimetric yolk sac (thick arrow).
Chorioamniotic separation
Before the 14th week of pregnancy, it is considered normal for the chorion and amnion to appear separate. Generally, chorioamniotic fusion occurs between the 14th and 16th weeks of pregnancy. It is uncommon and abnormal for it to appear separately after the 16th week of pregnancy. Chorioamniotic separation may occur spontaneously or iatrogenically as a result of interventions like amniocentesis, fetal surgery, or fetal blood sampling. Chorioamniotic separation has been linked to fetal aneuploidy, especially trisomy 21, premature membrane rupture, preterm birth, and fetal growth restriction[27,28] (Figure 7).
Figure 7 Chorioamniotic separation.
A-C: In 3 different pregnant women between 14th and 15th weeks, separation is seen in the chorion and amniotic membranes (arrows). In the follow-ups, fusion occurred and no persistence was observed in the chorioamniotic separation (not shown in the figure).
Cystic hygroma
Nuchal translucency (NT), defined as the normal fluid-filled subcutaneous space behind the fetal neck, is measured between 11 wk 3 days and 13 wk 6 days of pregnancy. Cystic hygroma can usually be detected during NT measurement in the first trimester ultrasonography. Septations are seen in the thickened NT in cystic hygroma. It can be seen as evidence of fetal anasarca or hydrops fetalis. The presence of septation is indicative of a poor prognosis. Compared to thickened NT, cystic hygroma has a higher risk of aneuploidy, cardiac anomaly and fetal demise (Figure 8).
Figure 8 Cystic hygroma.
24-year-old, first pregnancy, 12 wk of gestation. A: On ultrasonography, there was an increase in nuchal translucency (thin arrows); B: cystic areas of cystic hygroma (thick arrows) in the fetal cervical region.
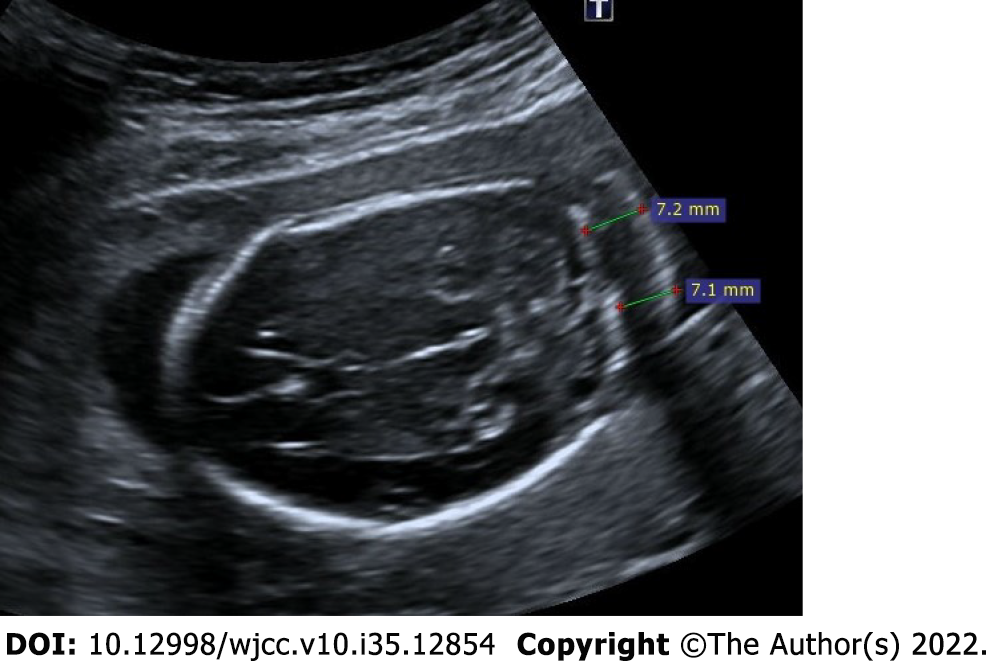
Thickened nuchal fold
The nuchal fold measurement is made between the outer edge of the occipital bone and the outer edge of the skin in the transcerebellar plane. The nuchal fold is measured in the 2nd trimester and greater than 6 mm is defined as the thickened nuchal fold. It is one of the most specific markers identified for trisomy 21. However, it can be detected in normal fetuses when detected in isolated. In addition, the presence of a thickened nuchal fold requires a thorough screening for congenital cardiac disease[29,30] (Figure 9).
Figure 9 Thickened nuchal fold.
25-year-old, first pregnancy, 19 wk of gestation. On ultrasonography, there was an increase in the thickness of the nuchal fold measuring 7.2 mm.
Single umbilical artery
Normally, the umbilical chord contains two umbilical arteries and one umbilical vein. If there is only one artery and one vein, it is referred to as a single umbilical artery. It is more common in twin pregnancies. The size of a single artery is typically larger than average and approaches that of the adjacent umbilical vein. It is best visualized in the axial plane of the umbilical cord. In addition, Doppler ultrasonography reveals a solitary artery at the level of the bladder in the fetal pelvis. When detected isolated single umbilical artery, it is generally no clinical significance, but there is an increased incidence of intrauterine growth restriction. When present with other fetal anomalies, it may be associated with chromosomal abnormalities such as trisomy 21, trisomy 18 and trisomy 13[31,32] (Figure 10).
Figure 10 Single umbilical artery.
29-years-old, first pregnancy, 21 wk of gestation. A: On ultrasonography, there was one artery (thin arrow) and one vein in the umbilical cord; B: Doppler ultrasonography reveals a solitary artery at the level of the bladder in the fetal pelvis (thick arrow).
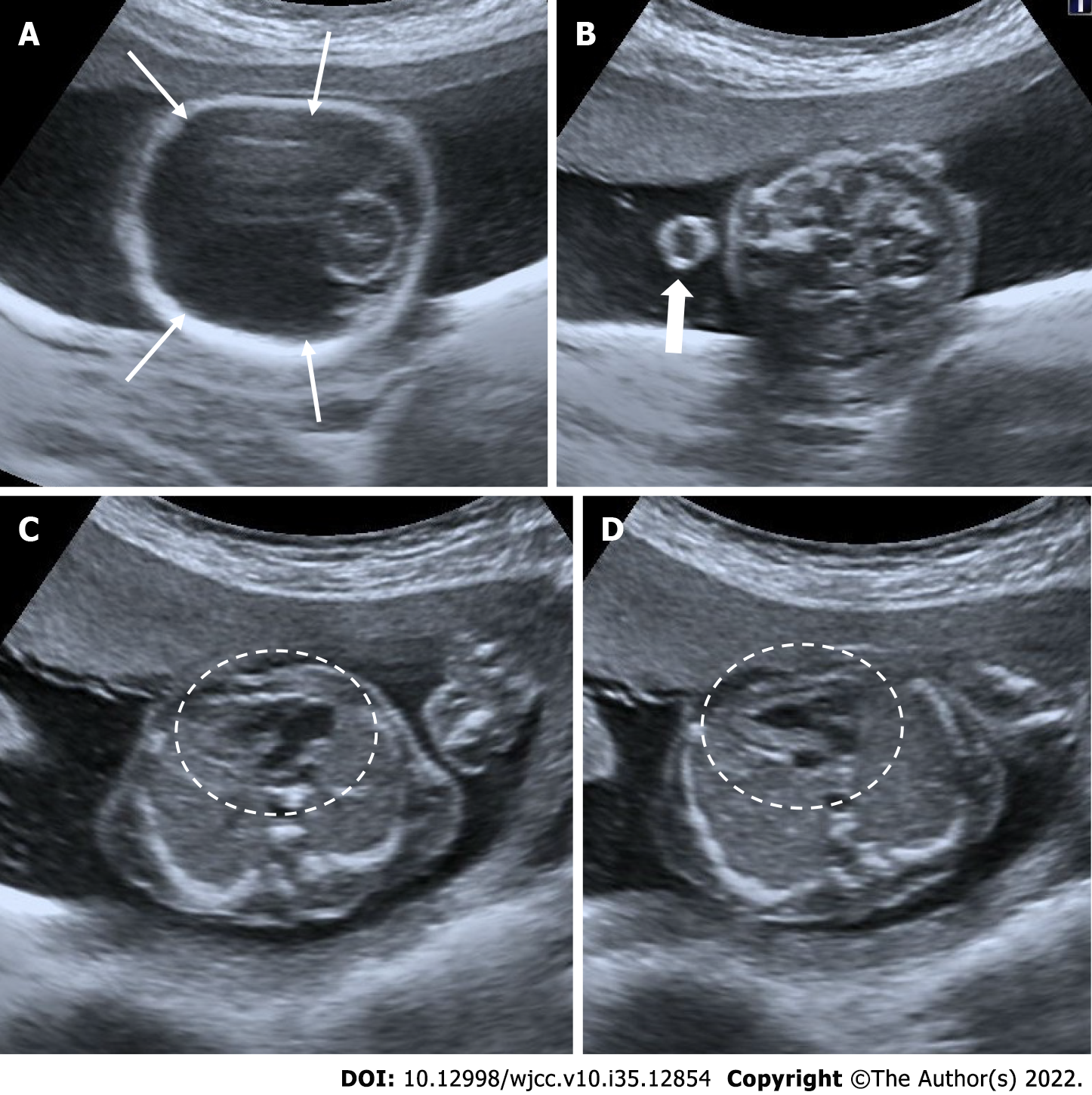
Acrania – exencephaly – anencephaly sequence
The fetal skull bones should have formed by 11 wk. Absence of fetal cranial bone structures in the first trimester ultrasonography scan performed between 11 wk and 13.6 wk is called acranii. Eccentric placement of fetal brain tissue due to the absence of cranial bone structures is called exancephaly. Unprotected fetal brain tissue degenerates over time due to friction, mechanical, and chemical traumas due to fetal movements in the amniotic fluid. Absence of fetal brain tissue is called anencephaly. These three interrelated pathological processes are called the acrania-exencephaly-anencephaly sequence[33,34] (Figures 11 and 12).
Figure 11 Acrania – exencephaly – anencephaly sequence.
21-year-old, pregnancy without follow-up, first examination. A-C: On ultrasonography, there was absence of fetal cranial bone structures (acrani) and exophytic extension of fetal cerebral tissue (exencephaly) (arrows).
Figure 12 Acrania – exencephaly – anencephaly sequence.
A and B: On ultrasonography, first case had absence of cranial bone structures (acrani) and cerebral tissue with exophytic extension (exencephaly) (arrows); C and D: There is another case had acrani and exencephaly (arrows) on ultrasonography.
Alobar holoprosencephaly
Alobar holoprosencephaly is a subtype of holoprosencephaly and is the most severe of the classic three subtypes. Other types of holoprosencephaly are semilobar and lobar holoprosencephaly. It is characterized by the failure of diencephalon and telencephalon transverse cleavage between 4-6 wk of gestation and the inability to separate the two hemispheres. Monoventricle, fused thalami, absent corpus callosum, absent interhemispheric fissure, absent cavum septi pellucidi, absence of 3rd ventricle, a single monoventricle that may communicate with a large dorsal cyst and severe facial malformations are seen in alober holoprosencephaly. Lateral and third ventricles are absent and single midline monoventricle appearance is seen. In addition, craniofacial anomalies such as proboscis, mono-orbit/cyclopia, midline facial anomalies including clefts, mononostril, hypotelorism, cebocephaly may also be seen. Holoprosencephaly may be associated with chromosomal anomalies, especially trisomy 13 and trisomy 18. Additionally, congenital renal and congenital cardiac anomalies may be detected[35,36] (Figure 13).
Figure 13 Alobar holoprosencephaly.
30-year-old, 21 wk of gestation. A: On ultrasonography, supratentorial cerebral structures are absent (thin arrows); B: There was bilateral hypotelorism-anophthalmia and a single orbital structure with exophytic extension from the midline (cyclopi) (thick arrow); C and D: The same patient also had a major cardiac anomaly (circle).
Hydrocephalus - ventriculomegaly
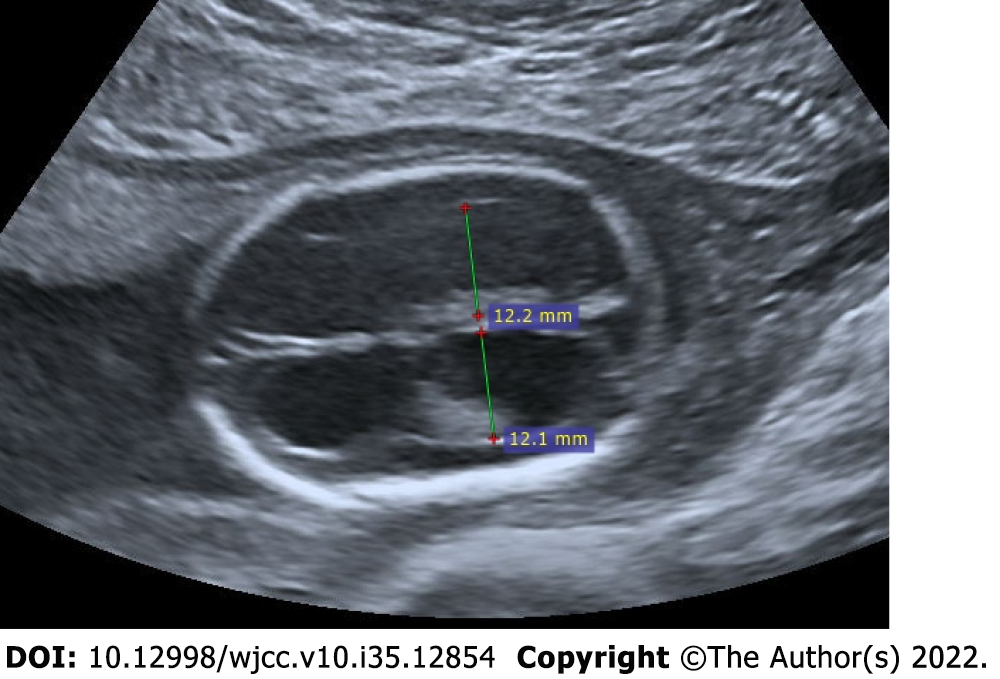
The enlargement of the cerebral ventricles is called ventriculomegaly. It is a significant finding because it is related to other anomalies of the central nervous system. The width of the lateral ventricle should be less than 10 mm. The width of the lateral ventricle between 10-12 mm is classified as mild ventriculomegaly, between 12-15 mm as moderate ventriculomegaly, and greater than 15 mm as severe ventriculomegaly-hydrocephalus. Even if there is no accompanying structural anomaly, the detection of mild ventriculomegaly is considered as a soft antenatal marker for underlying chromosomal abnormalities. Therefore, careful evaluation is required for other sonographic anomalies. The dangling choroid sign, a characteristic finding of ventriculomegaly, is the appearance of drooping choroid plexuses caused by the loss of their parallel extension to the ventricles as a result of the enlargement of the ventricles[37-39] (Figure 14).
Figure 14 Isolated bilateral mild ventriculomegaly.
22-year-old, first pregnancy, 19 wk of gestation. On ultrasonography, the measurements of both ventricles were 12 mm and larger than normal.
Choroid plexus cyst
Choroid plexus cysts are sonolucent cysts in the lateral ventricle, that are usually detected in the second trimester. It is considered as one of the soft ultrasound markers affecting the risk of aneuploidy. Choroid plexus cysts are hypoechoic, but the cyst wall can be seen as echogenic due to the surrounding choroid plexus tissue. Choroid plexus cysts usually disappear at 26-28 wk of pregnancy and in most cases are of no significance. However, if it is detected, the fetus should be carefully evaluated due to a slight increase in the risk of karyotype anomalies, particularly trisomy 18. If a choroid plexus cyst is greater than 1 centimeter, bilateral, multiple and there are other accompanying anomalies, it is cause for concern. If it is seen together with other anomalies, amniocentesis is recommended[29,40] (Figure 15).
Figure 15 Isolated choroid plexus cysts.
25-year-old, second pregnancy, 20 wk of gestation. A and B: On ultrasonography, there were anechoic choroid plexus cysts bilaterally reaching 1 cm diameter (diameters).
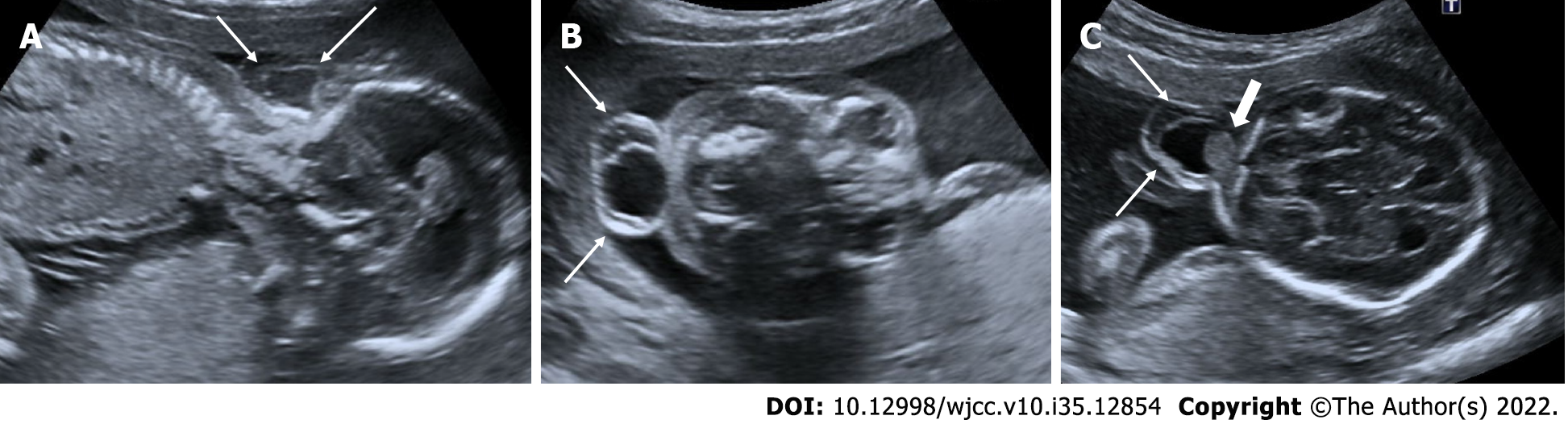
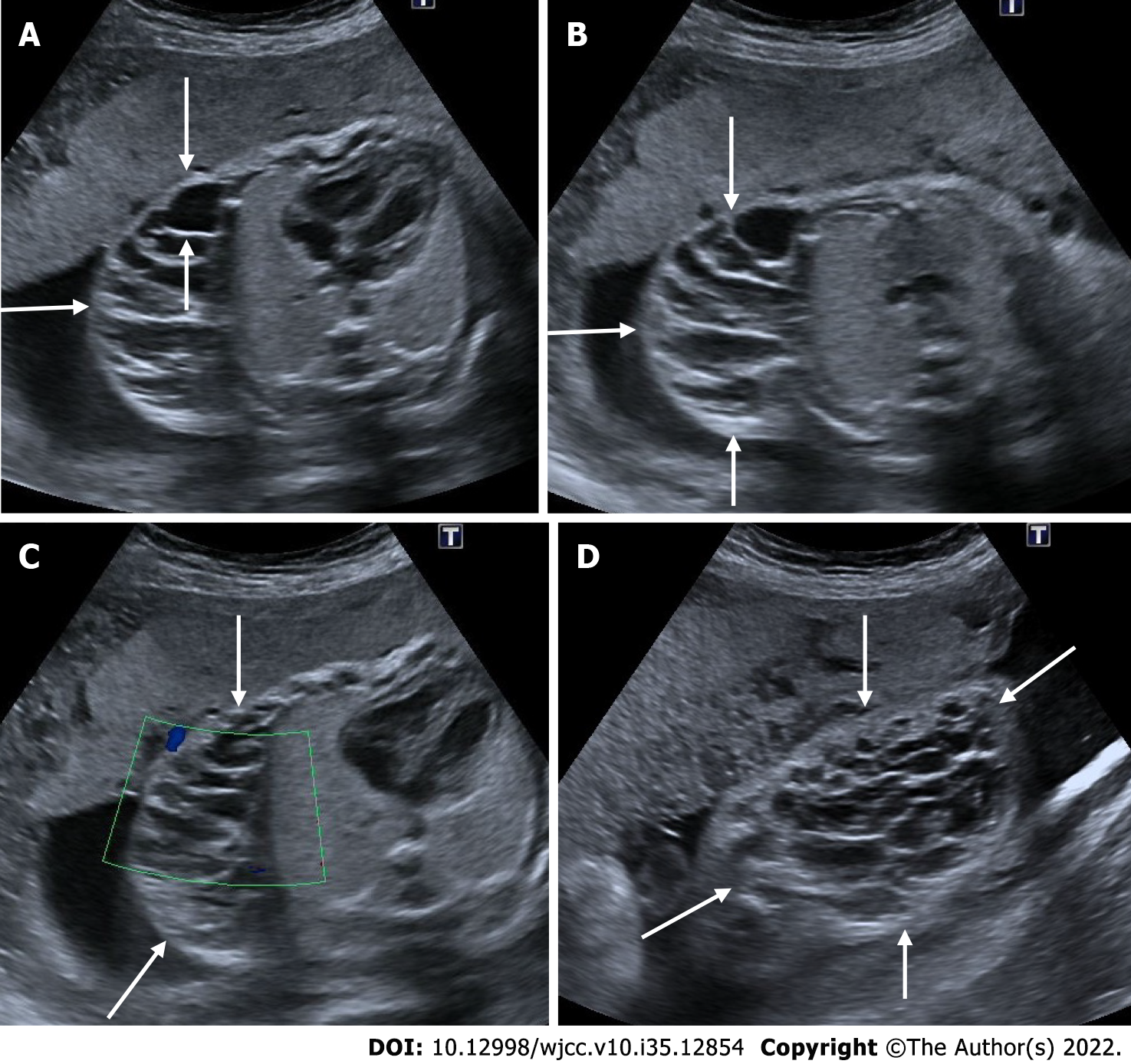
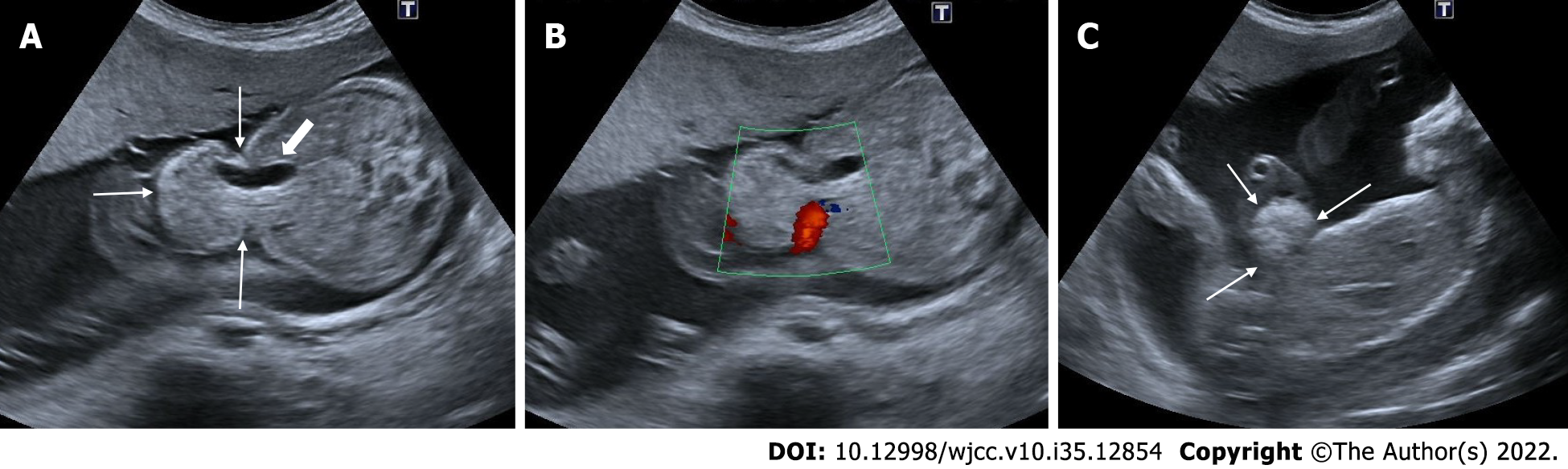
Spinal dysraphism, myelomeningocele, banana sign, lemon sign
Congenital malformations of the spine and spinal cord are commonly referred to as spinal dysraphism. Neural tube defects are the most common congenital anomaly after congenital heart diseases. It is most commonly seen in the lumbosacral region. They are divided into two categories as open and closed spinal dysraphism, depending on whether there is skin tissue or not. The usage of folic acid before and throughout pregnancy reduces its incidence. In addition, neurological damage can be reduced by intrauterine surgeries. With spinal malformation, the neural tissue protrudes through the spina bifida opening with the surrounding enlarged subarachnoid space. Myelomeningocele refers to the presence of a sac containing neural tissue and subarachnoid fluid. Meningocele refers to the presence of only subarachnoid space in the sac, and myelocele refers to the presence of only neural tissue in the sac. Chiari 2 malformation is characterized by a small posterior fossa and inferior heniation of the brain stem and cerebellar tonsils from the foramen magnum, ventricular dilatation and callosal dysgenesis in accompanying all cases of open spinal dysraphism[41]. The banana sign appears when the inferiorly herniated cerebellum is tightly wrapped around the brain stem and the cisterna magna disappears. In addition, the lemon sign describing the appearance of an indentation in the bone can be seen in the frontoparietal suture. In the presence of isolated lemon sign, craniosynostosis should also be included in the differential diagnosis[41,42] (Figures 16 and 17).
Figure 16 Myelomeningocele.
A: Ultrasonography showed an anechoic sac (diameters) extending outward from the posterior defect at the cervical level, reaching 3 cm in diameter; B: There is seen neural roots (arrows) within the sac.
Figure 17 Spinal dysraphism.
A-C: On ultrasonography, spina bifida was seen in the vertebral column at the lumbar-sacral level (arrows); D: There were lemon sign characterized by angulation in the bilateral frontoparietal suture (arrows); E: Additionaly, there were bilateral mild ventriculomegaly (diameters); F: There were banana sign (arrows) in the posterior fossa.
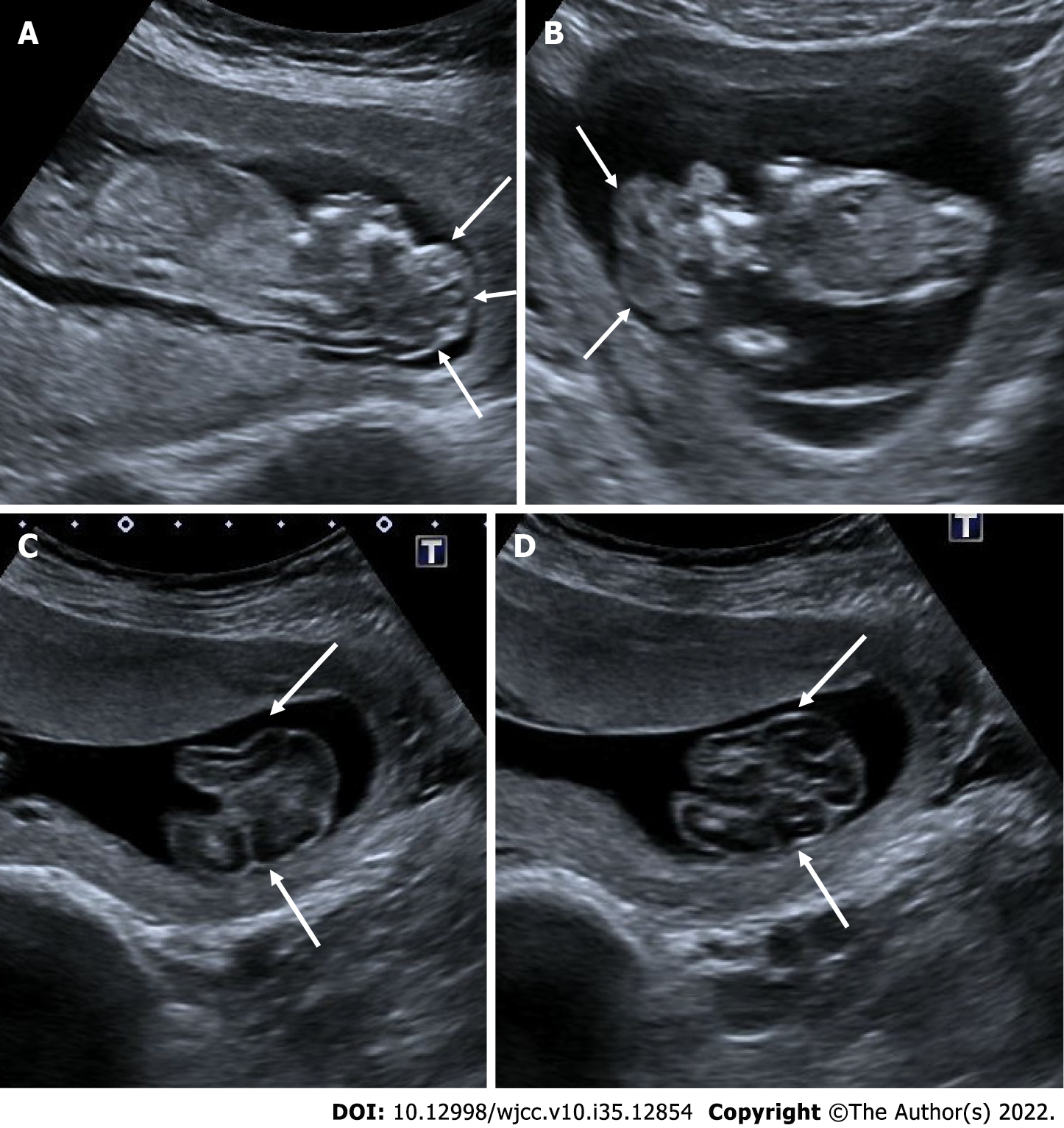
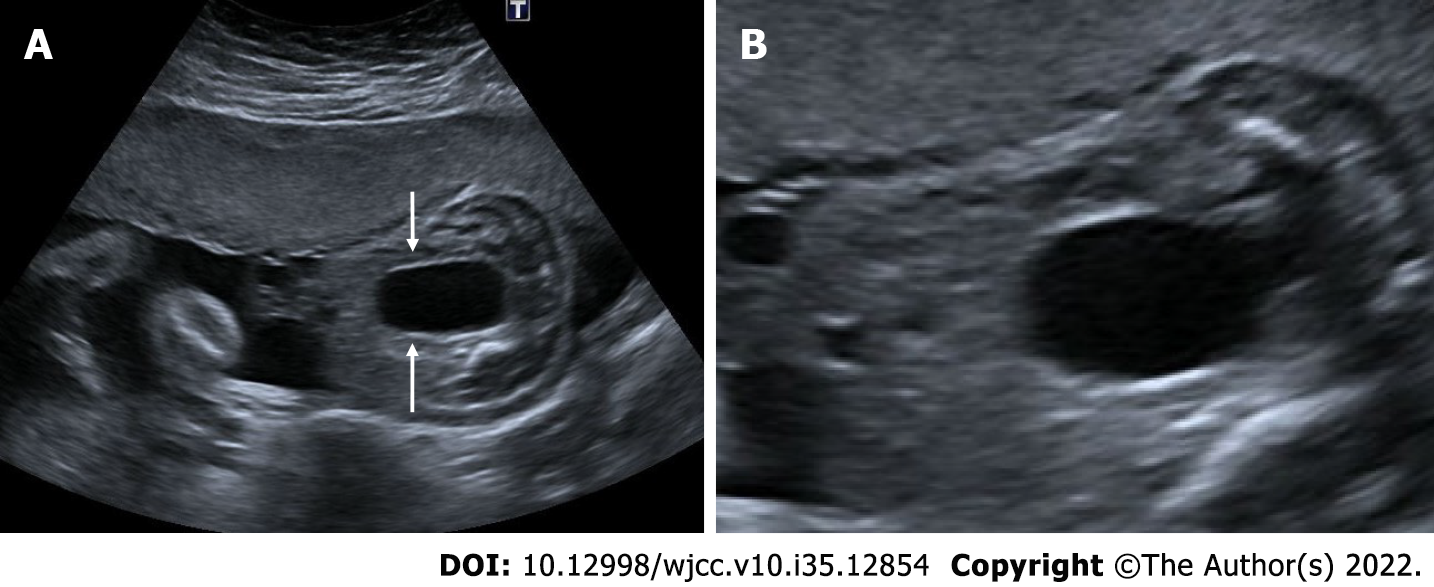
Occipital encephalocele
Encephalocele is a neural tube defect characterized by the herniation of brain tissue and meninges through a cranial defect. The occipital encephalocele is the most common type of encephalocele. Frontoethmoidal, basal, temporal and parietal encephaloceles can also be seen. Occipital encephalocele may be associated with Chiari malformations, Dandy Walker malformations and Meckel Gruber syndrome[43,44] (Figure 18).
Figure 18 Occipital encephalocele.
30-year-old, second pregnancy, 20 wk of gestation. A-C: On ultrasonography, there was a herniated sac from the bone defect in the occipital region (thin arrows); C: A portion of the occipital lobe was present in the sac (thick arrow).
Bladder outlet obstruction, fetal megacystis
Fetal bladder outlet obstruction or lower urinary tract obstruction is most commonly caused by posterior urethral valve or second most frequently by urethral atresia. Kidney development is impaired and may lead to pulmonary hypoplasia. It is associated with a high rate of perinatal morbidity and mortality. It occurs due to an obstruction in the lower urinary tract (bladder outlet), leading to megacystis, a thickened bladder wall, and bilateral hydronephrosis. It may be accompanied by renal cystic dysplasia. Oligohydramnios and secondary clubfoot and pulmonary hypoplasia may be present. If bladder or kidney rupture occurs, there may be urinary acid and perinephric urinomas. For the diagnosis of megacystis in the first trimester, the maximum sagittal length of the bladder is considered to be 7 mm. In the 2nd and 3rd trimesters, there is no clearly defined value. There are studies describing the removal of 5 mm from the gestational week as the limit in the second and third trimesters. The keyhole appearance is observed in the posterior urethral valve and indicates an enlarged posterior urethra. Lower urinary tract obstruction is usually seen in isolated. Differential diagnosis includes Prune-Belly syndrome, aneuploidy, megacystis-megaureter syndrome and megacystis-microcolon syndrome[45-47] (Figure 19).
Figure 19 Megacystis.
A: 25 wk of gestation. On ultrasonography, there was a distension reaching 4.5 cm in the bladder that did not regress in the follow-ups (arrows); B: Keyhole sign indicating an enlarged urethra is seen in the inferior of the bladder.
Urinary tract dilation
There are numerous terms for urinary tract dilatation, including pyelectasis, pelviectasis, and hydronephrosis. In urinary tract dilatation, the anterior-posterior diameter of the renal pelvis is measured in the axial plane. Less than 4 mm in the 2nd trimester and less than 7 mm in the 3rd trimester are normal. Pyelectasis and pelviectasis are generally used instead of mild hydronephrosis, and 4-7 mm in the 2nd trimester and 7-10 mm in the 3rd trimester are mild hydronephrosis. More than 10 mm in the 2nd trimester and more than 15 mm in the 3rd trimester is severe hydronephrosis. Urinary tract dilatation is often transient and a common variation. Urinary tract dilatation can be a marker of trisomy 21 as well as kidney and urinary tract pathologies. It is considered a soft marker for trisomy 21. 80% of patients with mild hydronephrosis measuring 4-7 mm in the 2nd trimester recover spontaneously in the 3rd trimester. It is recommended to follow up the child after birth. The most common pathological causes of urinary tract dilatation include vesicoureteral reflux, ureteropelvic junction obstruction, and posterior urethral valve[29,48-50] (Figure 20).
Figure 20 Bilateral mild hydronephrosis.
A and B: 23 wk of gestation. On ultrasonography, bilateral renal pelvis was wider than normal (arrows). Renal pelvis diameters were 6 mm on the right and 5 mm on the left. There was no dilatation of the bilateral ureters.
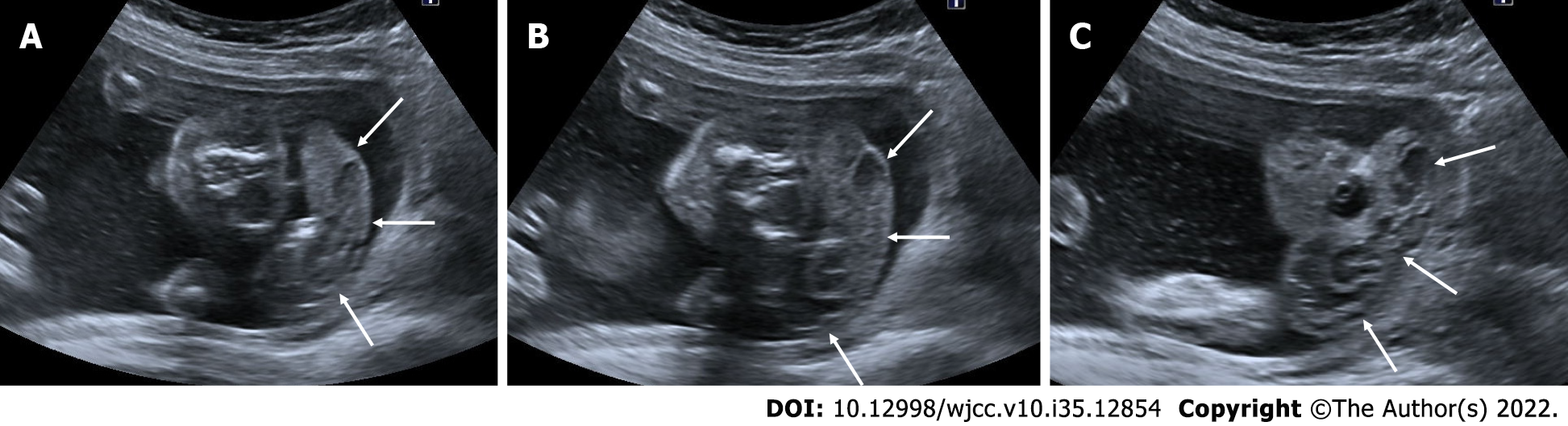
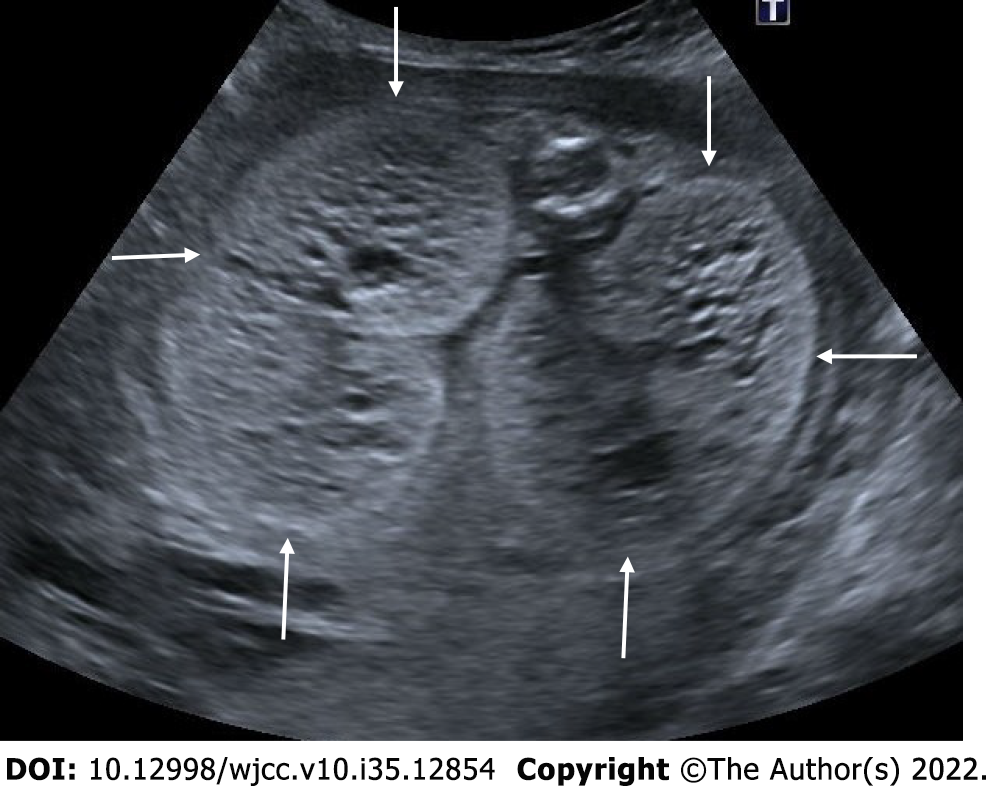
Multicystic dysplastic kidney
Most renal anomalies are isolated and the risk of association with aneuploidy or genetic syndromes is low. The exception is autosomal dominant or autosomal recessive polycystic kidney disease. Therefore, in the presence of cystic kidney disease, a careful evaluation should be made in terms of other anomalies and hereditary diseases[50].
The multicystic dysplastic kidney is characterized by unrelated and varied size multiple cysts, increased echogenicity and increased diameters of kidney without hydronephrosis. It is often unilateral and generally has a good prognosis. Rarely, the prognosis may be poor if the disease is bilateral or accompanied by additional anomalies. Absence of a fluid-filled bladder and oligohydramnios indicate an anomaly in the contralateral kidney. Compensatory hypertrophy may accompany if the contralateral kidney is normal[51,52] (Figure 21).
Figure 21 Multicystic dysplastic kidney.
In two different pregnant women who underwent 2nd trimester obstetric sonography. A and B: Case 1, there was a multicystic appearance in different sizes with an increase in size in one kidney (arrows); C and D: Case 2; there was another case with a multicystic appearance in different sizes in one kidney (arrows).
Autosomal recessive polycystic kidney disease
Autosomal recessive polycystic kidney disease is caused by a rare genetic disorder. It has a poor prognosis, especially when diagnosed prenatally, and is associated with a high rate of morbidity and mortality. On ultrasonography, both kidneys are enlarged and hyperechoic, and there is loss of corticomedullary differentiation. Millimetric cystic areas can be seen. In addition, oligohydramnios or anhydramnios is common. It may be accompanied by pulmonary hypoplasia. In the third trimester, the size of millimeter-sized cysts may exceed 3 mm. Rarely, no abnormality is detected in antenatal ultrasonography. Although hepatic fibrosis and biliary dysgenesis are common in affected patients, prenatal detection of these conditions is frequently not possible. Differential diagnosis includes Bardet-Biedl syndrome and Meckel Gruber syndrome. Postaxial polydactyly is common in Bardet-Biedl syndrome and the renal parenchyma appears more homogeneous. Cysts usually appear earlier in Meckel-Gruber syndrome, and central nervous system malformations and postaxial polydactyly are common[53,54] (Figure 22).
Figure 22 Autosomal recessive polycystic kidney disease.
27 wk of gestation. On ultrasonography, there was a significant increase in bilateral kidney sizes, multiple millimetric cystic heterogeneous echogenic kidneys (arrows). The patient also had oligohydramnios, cardiac and cranial anomalies (not shown in the figure).
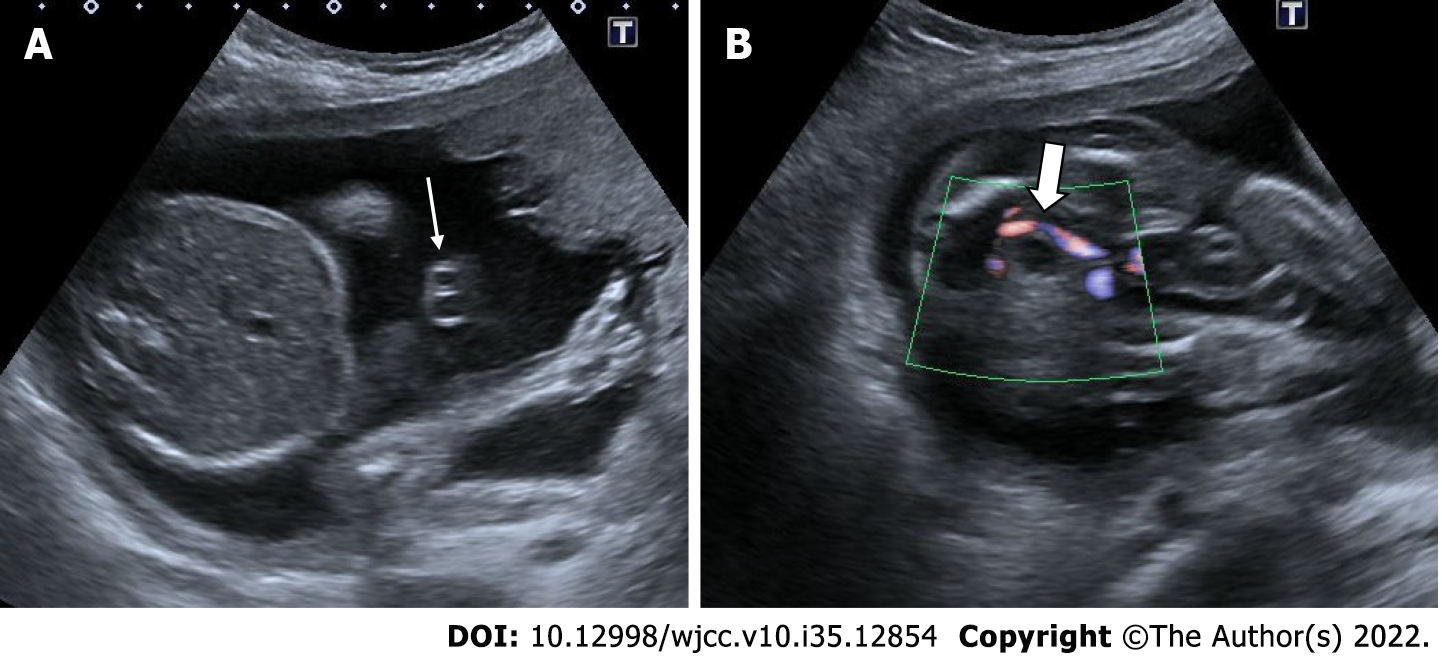
Persistent right umbilical vein
Early in fetal development, both umbilical veins are connected to the sinus venosus. Between the 4-7th weeks of pregnancy, the right umbilical vein obliterates and the left umbilical vein carries all of the blood to the portal vein. Persistent right umbilical vein is an embryological development characterized by the regression of the left umbilical vein and the persistence of the right umbilical vein. Persistent right umbilical vein has a generally good prognosis. However, it may also be associated with congenital absence of the ductus venosus and other serious fetal malformations. Absence of the ductus venosus may result in hemodynamic decompensation. On ultrasonography, the umbilical vein has an abnormal path towards the stomach. The gallbladder medializes towards the umbilical vein. Normally, the arrangement in the form of stomach-umbilical vein-gallbladder is seen as the stomach-gallbladder-umbilical vein, respectively. It may be isolated or associated with chromosomal abnormalities. It can also be seen together with a single umbilical artery[55-57] (Figure 23).
Figure 23 Persistent right umbilical vein.
22 wk of gestation. A: On ultrasonography, while the stomach was in its normal position (asterisk); B and C: The umbilical vein (thin arrow) was seen to the right of the gallbladder (thick arrow).
Orofacial cleft
Orofacial clefts are a common facial anomaly, most frequently manifesting as a cleft lip and a cleft palate. Isolated cleft lip or isolated cleft palate are also common. Most clefts are paramedian. It can be unilateral or bilateral. The cleft palate may extend to include the premaxillary region-hard palate or more dorsally the soft palate. Isolated cleft palate often evades prenatal diagnosis. Cleft palate and cleft lip can be seen isolated or as an additional finding in many genetic anomalies. If there is a cleft palate or cleft lip, a detailed neurosonogram and fetal echocardiogram and genetic analysis should be performed to identify any additional anomalies[58,59] (Figure 24).
Figure 24 Isolated cleft lip.
23 wk of gestation. A: Isolated cleft lip view in coronal section (arrow); B: Isolated cleft lip view in axial section (arrows).
Cystic lymphangioma
Fetal lymphangioma is a rare congenital lymphatic malformation affecting the skin and subcutaneous tissue. It is usually seen in the neck and is called cystic hygroma. There is an increased risk of chromosomal anomalies, especially trisomy 21. It can be seen in localizations such as the chest wall, abdomen, pelvis, extremities and axilla. It looks like a mass lesion with multiple cystic areas on ultrasonography. On color Doppler examination, no vascularity is detected[60,61] (Figure 25).
Figure 25 Cystic lymphangioma.
24 wk of gestation. A and B: On ultrasonography, a complex cystic heterogeneous lesion with multicystic areas on the right side of the fetal thoracic wall, under the skin (arrows); C: There was seen no vascularity on Doppler sonography; D: There is a view of the cystic lesion (arrows) on the chest wall from a different section.
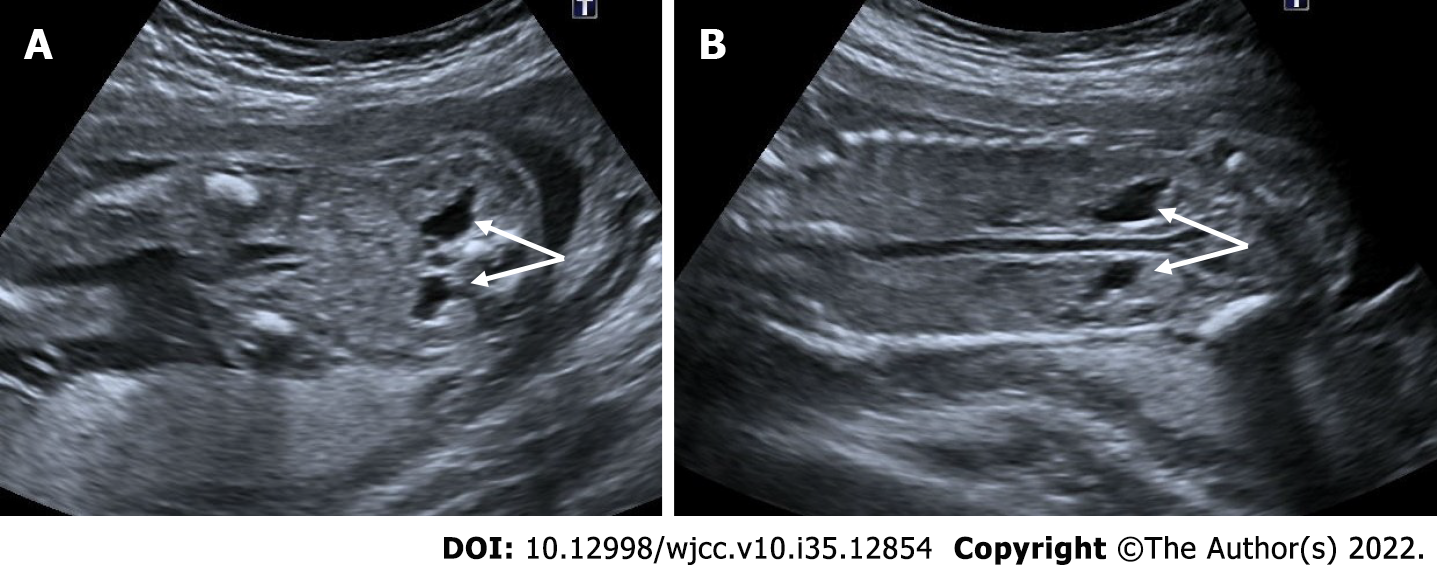
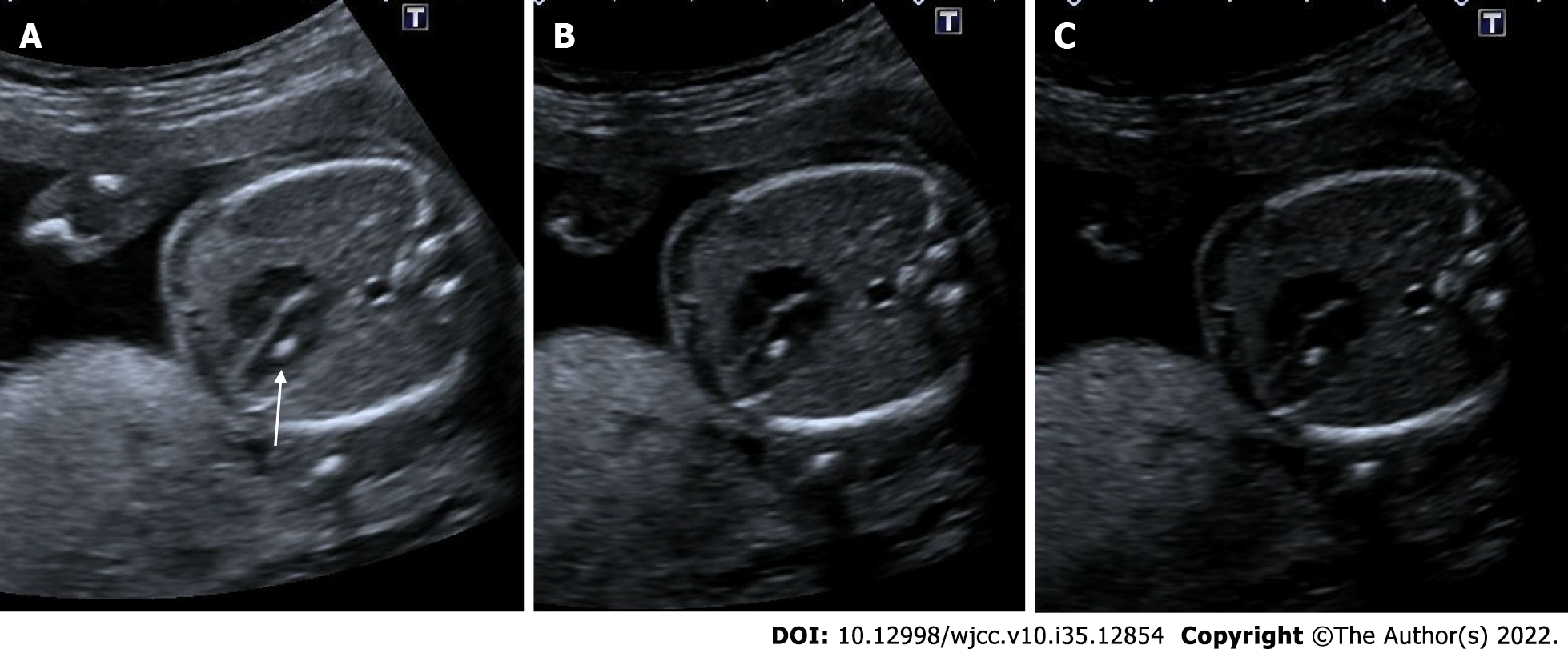
Echogenic intracardiac focus
Intracardiac echogenic focus is defined as an echogenic focus that is less than 6 millimeters in diameter and as bright as a bone, and is often visible in a four-chamber image within the heart, viewed in at least two planes. Although it is more common on the left ventricle, it can seen in both ventricles. It is believed to represent microcalcification within the papillary muscles. Generally, it disappears in the 3rd trimester. It is not a structural or functional cardiac anomaly. It is not associated with cardiac malformations. It is considered a soft marker for trisomy 21. No additional diagnostic procedure is required if it is detected isolated, but amniocentesis is recommended if it is seen together with other anomalies[29,62,63] (Figure 26).
Figure 26 Echogenic intracardiac focus.
21 wk of gestation. A: On ultrasonography, there was one echogenic focus (arrow) in the left ventricle of the fetal heart; B and C: Although the gain settings were decreased, the hyperechoic appearance persisted.
Amniotic band sequence
The amniotic band sequence is a series of malformations that the result of fetal parts being compressed by intrauterine fibrous amniotic bands. Depending on the affected organ, the severity of amniotic band syndrome can be so severe as to be fatal to the fetus. It most frequently results in limb deformities. Typically, constriction ring deformities are seen in the extremities. These include edematous and non-edematous fingers and toes, usually in the distal extremity. These constriction rings can lead to amputation of limbs or fingers and the appearance of syndactyly on ultrasound (pseudosyndactyly). Craniofacial deformities may include single orbital involvement, severe clefts that do not conform to the normal pattern, severe nasal deformity, or asymmetric encephaloceles[64,65] (Figure 27).
Figure 27 Amniotic band sequence.
16 wk of gestation. A: On ultrasonography, there was amniotic band formation (thin arrows) in the right half of the amniotic cavity; B-D: Fetal cranium (thick arrows) was fixed in the secondary cavity formed at this level, and a deformed appearance was noted in the fetal cranium.
Echogenic fetal bowel
An echogenic fetal bowel finding is the appearance of fetal bowels that are echogenic or more echogenic than the neighboring iliac bone. It is often detected isolated in second trimester ultrasonography. If echogenic fetal bowel is present, there is an increased incidence of renal and cardiac anomalies. Although transient or idiopathic, it may also be associated with various pathological conditions such as aneuploidy, cystic fibrosis, congenital viral infection, primary gastrointestinal pathology, intraamniotic hemorrhage and fetal growth restriction. The most common associated aneuploidy is trisomy 21. In addition to having an echogenic bowel, having dilated bowel loops increases the risk of cystic fibrosis. In this situation, the parents' cystic fibrosis carriage can be explored. The most common congenital infection is cytomegalovirus, but toxoplasma, rubella, herpes, chickenpox, and parvovirus infections are also seen. Additionally, it is possible to observe primary gastrointestinal diseases such as intestinal obstruction, atresia, and perforation[29,66] (Figure 28).
Figure 28 Echogenic fetal bowel.
22 wk of gestation. A and B: On ultrasonography, the intestines showed a diffuse echogenicity increase (arrows). Although intestinal echogenicity was not as echogenic as the bone structures in the same section, this case was later diagnosed as prenatal trisomy 21.
Omphalocele
Omphalocele is the herniation of abdominal contents through the anterior abdominal wall near the umbilical cord's base. It occurs when the physiological herniation of the intestine does not end up to 10-12 wk and does not return to the abdomen. Omphalocele is usually associated with chromosomal anomalies. An omphalocele is detected during routine ultrasonographic examination of the fetus or during investigation of an elevated level of alpha-fetoprotein[67] (Figure 29).
Figure 29 Omphalocele.
24 wk of gestation. A: On ultrasonography, abdominal wall defect and herniation at the level of umbilical cord insertion (thin arrows) are observed and the presence of intra-abdominal tissues and gall bladder (thick arrow) was noted within the herniation; B: The level of herniation is from the side of the umbilical cord, and the umbilical cord is seen in color Doppler; C: There is seen sagittal section view of the herniation.