Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12837
Peer-review started: August 2, 2022
First decision: October 5, 2022
Revised: October 13, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 16, 2022
Processing time: 133 Days and 16.5 Hours
Two years after the coronavirus disease 2019 (COVID-19) pandemic, acute hepatitis of unknown etiology in children (AHUCD) began to be reported world
Core Tip: In this review, we summarize the existing evidence related to the outbreak of acute hepatitis of unknown etiology in children (AHUCD). Some scholars believe that the outbreak of AHUCD caused by the coronavirus disease 2019 (COVID-19) pandemic is caused by immune dysfunction and liver injury after COVID-19, and children with reduced exposure to pathogens and increased physical susceptibility due to isolation and mask wearing. Secondly, human adenovirus or other pathogens can interact with each other, leading to the outbreak of AHUCD. However, the relationship between COVID-19, adenovirus hepatitis and AHUCD is still unclear. Future studies should focus on the etiology and mechanism of AHUCD.
- Citation: Zhong R, Yi F, Xiang F, Qiu YF, Zhu L, Zou YH, Wang W, Zhang Q. Hepatitis of unknown etiology in children: Current evidence and association . World J Clin Cases 2022; 10(35): 12837-12843
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12837.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12837
Infectious disease outbreaks have long been a major global health concern. Coronavirus disease (COVID-19) has continued to proliferate worldwide since 2019, and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shows no signs of eradication. Approximately two years after the onset of the COVID-19 pandemic, on October 1, 2021, five pediatric patients with severe hepatitis were reported at a children's hospital in Alabama, USA, and four similar cases were reported in the following three months[1,2]. Since April 2022, acute hepatitis of unknown etiology in children (AHUCD) has been reported in many areas worldwide. Clinical manifestations of severe AHUCD include acute onset, nausea, vomiting, diarrhea, abdominal pain, and other gastrointestinal symptoms, resulting in clinical conditions such as jaundice and abnormal liver function. A small number of patients may progress to acute liver failure within a short period, presenting with hepatic encephalopathy and progressive exacerbation of jaundice[3]. Recent studies have not determined the etiology of this recent AHUCD outbreak. However, it has been speculated that the outbreak was related to the sequelae of COVID-19 or adenovirus infection. Therefore, this brief review aimed to synthesize existing clinical evidence related to AHUCD to elucidate its origins and determine best practices for diagnosis and treatment.
We searched databases such as Reference Citation Analysis, PubMed/Medline, Cochrane Library, Embase, and Web of Science, and websites such as UK Health Security Agency, European Centre for Disease Prevention and Control, World Health Organization (WHO), and others for articles with the keyword AHUCD.
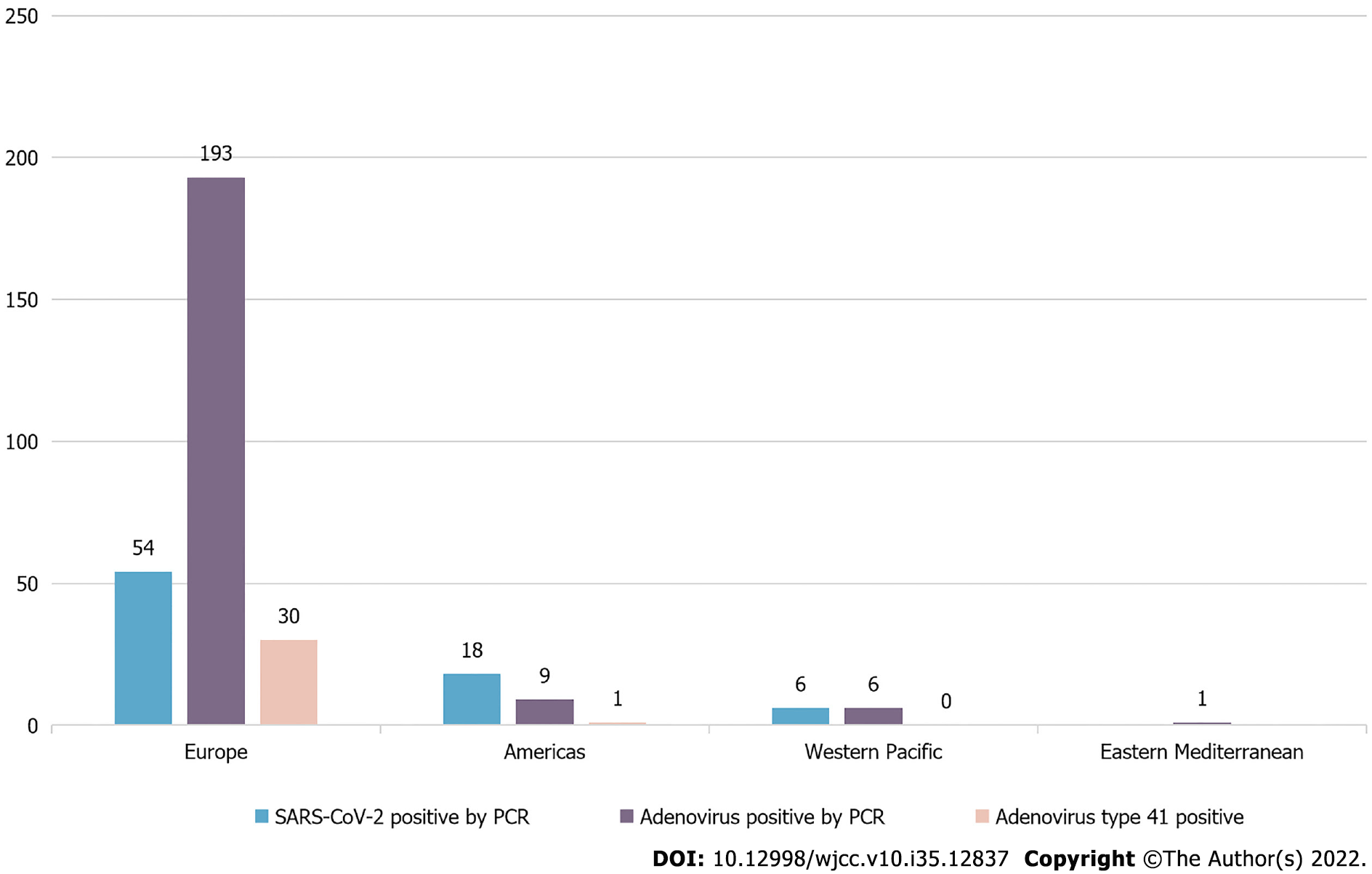
According to the WHO, as of July 8, 2022, five WHO regions (Europe, the Americas, and Asia) comprising 35 countries have reported a total of 1,010 cases of AHUCD. Of these, liver transplantation was required in 46 cases (4.6%) and death occurred in 22 cases (2.2%). Polymerase chain reaction (PCR) testing confirmed that 78 cases (7.7%) were positive for SARS-CoV-2 and 209 cases (20.7%) were positive for adenovirus. Among the adenovirus positive cases, 31 (3.1%) were of adenovirus type 41F[4], as presented in Figure 1. As of June 13, 2022, the United Kingdom reported[5] 260 cases of AHUCD in children under 16 years of age, with serum transaminase levels > 500 IU/L, 12 of which required liver transplantation. Adenovirus and novel coronavirus were the primary potential pathogens detected in these cases; 64.7% of the cases were positive for adenovirus, primarily adenovirus 41F, and 17.3% of the cases tested positive for SARS-CoV-2 upon admission. The 41F adenovirus subtype primarily affects young children and individuals with immunodeficiency. However, there are no previous reports of AHUCD caused by adenovirus 41F in patients who did not undergo liver transplantation.
Most patients presented with jaundice, significantly elevated hepatic transaminase levels [alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels > 500 IU/L][6,7], and severe acute hepatitis that required hospitalization. In most cases, the onset of jaundice was preceded by abdominal pain, vomiting, diarrhea, and fever[2,8]. Additionally, there was no history of the common hepatitis viruses that cause acute viral hepatitis (hepatitis A, B, C, D, and E viruses) in these cases, and their tests at presentation were negative[9]. The cases had no clear epidemiological link, occurred sporadically, had no history of being in affected areas, and had no established exposure (such as exposure to specific poisons, drugs, food, or water). Moreover, most children had no history of COVID-19 vaccination because they were too young to receive them at the time[10].
During the COVID-19 pandemic, the association between liver injury and SARS-CoV-2 infection should not be ignored. Some researchers have suggested that temporary liver damage caused by certain drugs[11] or antigen-specific immune activation triggered by SARS-CoV-2 may lead to AHUCD[12]. Currently, the etiology of AHUCD is under investigation and research, and the association between AHUCD and COVID-19 remains unclear and continues to attract the attention of researchers. This review explores the underlying mechanisms of AHUCD, which are believed to have several possible causes, as shown in Figure 2.
Adenovirus is a 70–90 nm particle with no envelope[13]. It is composed of 252 shells in a 20-hedral arrangement, each with a diameter of 7–9 nm. Currently, adenoviruses are best known for their potential use as therapeutic vectors, such as those processed as vectors into one of the novel coronavirus vaccines[14,15].
Adenoviruses have rarely been reported to cause severe hepatitis in children who have not undergone liver transplantation[7,16]. As of July 8, 2022, the WHO reported 209 cases (20.7%) of children with AHUCD that were positive for adenovirus by PCR. In June 2022, the Lancet Infectious Diseases[17] also reported several cases of AHUCD who tested positive for adenovirus. Therefore, adenovirus infection is considered a potential cause of AHUCD.
Adenovirus-associated hepatitis occurs primarily in immunosuppressed patients with defective adaptive cellular immune responses, including T cell-mediated immunity[18]. Therefore, the frequency of adenovirus hepatitis infection in immunocompromised patients is high[19]. This finding links the immunodeficiency status of COVID-19 patients with gastrointestinal manifestations of adenovirus infections. Co-infection may also be a possible cause[9]. The United Kingdom Health Security Agency[16] has also suggested that AHUCD may result from abnormal susceptibility or host response to co-infection caused by adenovirus, SARS-CoV-2, other pathogens, toxins, drugs, or environmental exposure. According to Brodin et al[20], the "superantigen hypothesis" suggests that a novel coronavirus infection can lead to the formation of viral reservoirs. AHUCD may be due to viral reservoirs in the intestines after infection with the novel coronavirus, followed by adenovirus infection and then abnormal host immune-mediated hepatitis.
The incidence of multisystem inflammatory syndrome in children (MIS-C) combined with liver injury in COVID-19 patients was 29.4% (5/17)[21]. This evidence supports the hypothesis of an increased adeno-associated virus incidence of adenoviral hepatitis in children with COVID-19.
Another hypothesis is that adenoviruses evolve through molecular homologous recombination, different tissue orientation and enhanced virulence of new viruses are generally caused by mutation, and their virulence and clinical characteristics are obviously different from those of other adenoviruses, which may be a causative factor of AHUCD[22].
Some researchers have proposed a connection between the novel coronavirus infection and MIS-C[23,24]. Hepatitis is a common condition among children with MIS-C. According to Cantor et al[25], most MIS-C patients were negative for novel coronavirus by reverse transcription PCR (56.8%) but positive for novel coronavirus antibodies (92.3%). Yasuhara et al[21] conducted a meta-analysis of clinical characteristics and liver injury in children with COVID-19, including 114 samples from 46 studies. The results showed that the levels of D-dimer (52%) and C-reactive protein (40%) were elevated, and the incidence of lymphocytopenia was 33%. Additionally, AST (22.2%) and ALT (16.4%) levels were elevated and the incidence of MIS-C was 15%. These results suggest the presence of acute and long-term hepatic sequelae of COVID-19 in pediatric patients. In addition, many hepatotropic viruses can cause hepatocyte injury through immune-mediated inflammation[10]. Therefore, it cannot be ruled out that pathological changes caused by novel coronavirus-related inflammation are cofactors or that the virus is an independent factor in children with AHUCD.
In patients with uncertain pediatric acute liver failure, when liver failure occurs, tissue resident memory CD8+T cells infiltrate mainly abnormally immunoactivated leukocytes. SARS-CoV-2-specific CD4+ and CD8+ memory T cells were positive in close contacts and recovered patients with COVID-19[26]. Therefore, some researchers have speculated that AHUCD may have memory T-cell responses due to close contact with COVID-19 patients. During reinfection with the novel coronavirus, abnormal immune responses can lead to acute liver injury and even liver failure[27].
In Israel, hepatologists have suggested that 5 of 12 cases of AHUCD were classified as sequelae of COVID-19. The interval between infection with novel coronavirus and hepatitis onset was approximately 3.5 mo. These cases resembled autoimmune hepatitis; however, there were no positive results. Recently, researchers speculated that the VVVNASN mutation in a novel coronavirus strain infected with ORF1ab may lead to autoimmune T-cell responses with "molecular mimicry" and that there is a connection to AHUCD[10].
In addition, owing to COVID-19 restriction measures, for example maintaining social distance, children may not be exposed to pathogens. Samarasekera believed that these children were not exposed to these childhood viruses at a normal age; therefore, when they were first exposed, it had an unexpected and serious impact[28], suggesting that COVID-19 may have altered their immunity to these viruses.
Some researchers[29] believe that vaccination with mRNA vaccines and prolonged exposure to pathogenic bacteria cannot be excluded to reduce resistance in some children. Researchers have also estimated that in 84% of reported cases of AHUCD, children were too young to be vaccinated[30]. On July 23, 2022, the COVID-19 immunization rate in China was 92.1% for the first dose, 89.7% for the full course, and 71.7% for the booster[31]. The age range of AHUCD is generally 3–5 years[6], and the reported rate of AHUCD in China was 0. Therefore, it can be inferred that vaccination is not associated with AHUCD (Table 1).
| Etiological speculation | Possible causes | Ref. |
| Association with adenovirus infection | ||
| Adenovirus infection | Adenovirus was detected by PCR in 209 (20.7%) patients with AHUCD. | World Health Organization[4] |
| Adenovirus mutation | Adenoviruses generate new viruses exhibiting various tissue tropism and increased virulence through molecular mutations of homologous recombination. | Tian X et al[22] |
| Secondary adenovirus infection "superantigen hypothesis" | Viral reservoirs emerge in the intestine after COVID-19 infection, followed by adenovirus infection, which triggers aberrant host immune-mediated hepatitis. | Brodin P et al[20] |
| Association with COVID-19 infection | ||
| COVID-19 infection | SARS-CoV-2-specific CD4+ and CD8+ memory T cells can be detected not only in patients recovering from COVID-19, but also in close contacts. Memory T-cell response after close contact with SARS-CoV-2 infection. During reinfection with SARS-CoV-2, a dysregulated immune response leads to rapid liver damage and acute liver failure. | Wang Z et al[26]; Zhu M et al[27] |
| Secondary multisystem inflammatory syndrome in children | Hepatitis is common in children with multisystem inflammatory syndrome, and MIS-C appears after COVID-19 infection, resulting in liver sequelae. | Cantor A et al[25]; Yasuhara J et al[21] |
| Pathogen exposure reduction | Children are not exposed to the viruses that children are generally exposed to; therefore, when they are first exposed to these viruses, it has an unexpected and serious impact. | Samarasekera U[28] |
| Association with vaccination against COVID-19 | Vaccination against COVID-19 induces antigen-specific immune responses in children. | Sergi CM[29] |
| Adenovirus and SARS-CoV-2 co-infection | ||
| Co-infection ofadenovirus and SARS-CoV-2 or other pathogens | Adenovirus-associated hepatitis mainly occurs in immunosuppressed patients. Co-infection with adenovirus and SARS-CoV-2 or other pathogens leads to immune dysfunction, increasing the likelihood of adenovirus-associated hepatitis infection. | Lion T[18]; Li L et al[19]; Mendez-Sanchez N et al[9] |
The association between COVID-19, adenovirus hepatitis, and AHUCD remains unclear, and further research is required to understand the etiology, mechanism, pathophysiology, and other aspects related to this evolving outbreak. Currently, there is an urgent need to improve AHUCD control and prevention. Healthcare workers, especially pediatric providers, should pay close attention to WHO reports and be on high alert for clinical signs of hepatitis in children.
Overall, no evidence of an association between medical activity and human-to-human transmission of AHUCD has been found; however, a possible association cannot be ruled out. Existing evidence for the cause of AHUCD outbreaks tend to point to the sequelae of COVID-19, immunocompromised patients, patients with liver damage, reduced exposure to pathogens, and increased susceptibility in children due to isolation and mask wearing. Therefore, it is possible that adenoviruses or other pathogens interact with each other, leading to an outbreak of AHUCD. However, there is no direct evidence of an association between COVID-19 vaccination and AHUCD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Silva LD, Brazil S-Editor: Liu GL L-Editor: Webster JR P-Editor: Liu GL
| 1. | Binnicker M. Adenovirus or Covid-19: What is behind the outbreak in child hepatitis cases? [Internet] [accessed 21 July 2022]. Available from: https://www.forbes.com/sites/coronavirusfrontlines/2022/05/19/adenovirus-or-covid-19-what-is-behind-the-outbreak-in-child-hepatitis-cases. |
| 2. | Baker JM, Buchfellner M, Britt W, Sanchez V, Potter JL, Ingram LA, Shiau H, Gutierrez Sanchez LH, Saaybi S, Kelly D, Lu X, Vega EM, Ayers-Millsap S, Willeford WG, Rassaei N, Bullock H, Reagan-Steiner S, Martin A, Moulton EA, Lamson DM, St George K, Parashar UD, Hall AJ, MacNeil A, Tate JE, Kirking HL. Acute Hepatitis and Adenovirus Infection Among Children - Alabama, October 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:638-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 98] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Acute Hepatitis of Unknown Aetiology-the United Kingdom of Great Britain and Northern Ireland. [Internet] [accessed 15 April 2022]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/acute-hepatitis-of-unknown-aetiology---the-united-kingdom-ofgreat-britain-and-northern-ireland. |
| 4. | World Health Organization. Severe acute hepatitis of unknown aetiology in children - Multi-country. [Internet] [accessed 12 July 2022]. Availabe from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON400. |
| 5. | UK Health Security Agency. Investigation into acute hepatitis of unknown aetiology in children in England: case update.[Internet] [accessed 28 July 2022]. Availabe from: https://www.gov.uk/government/publications/acute-hepatitis-technical-briefing/investigation-into-acute-hepatitis-of-unknown-aetiology-in-children-in-england-case-update. |
| 6. | Marsh K, Tayler R, Pollock L, Roy K, Lakha F, Ho A, Henderson D, Divala T, Currie S, Yirrell D, Lockhart M, Rossi MK, Phin N. Investigation into cases of hepatitis of unknown aetiology among young children, Scotland, 1 January 2022 to 12 April 2022. Euro Surveill. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 7. | World Health Organization. Multi-Country – Acute, severe hepatitis of unknown origin in children. [Internet] [accessed 23 April 2022]. Availabe from: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON376. |
| 8. | European Centre for Disease Prevention and Control. Increase in severe acute hepatitis cases of unknown aetiology in children.[Internet] [accessed 28 April 2022]. Availabe from: https://www.ecdc.europa.eu/en/publications-data/increase-severe-acute-hepatitis-cases-unknown-aetiology-children. |
| 9. | Mendez-Sanchez N, Pal SC. Editorial: Acute Hepatitis of Unknown Origin in Children. Is Autoimmunity at Play? Med Sci Monit. 2022;28:e937371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Zhang LY, Huang LS, Yue YH, Fawaz R, Lim JK, Fan JG. Acute Hepatitis of Unknown Origin in Children: Early Observations from the 2022 Outbreak. J Clin Transl Hepatol. 2022;10:522-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Sergi CM. Epigallocatechin-3-Gallate Toxicity in Children: A Potential and Current Toxicological Event in the Differential Diagnosis With Virus-Triggered Fulminant Hepatic Failure. Front Pharmacol. 2019;10:1563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 13. | Gallardo J, Pérez-Illana M, Martín-González N, San Martín C. Adenovirus Structure: What Is New? Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 14. | Kremer EJ. Pros and Cons of Adenovirus-Based SARS-CoV-2 Vaccines. Mol Ther. 2020;28:2303-2304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Hasanpourghadi M, Novikov M, Ertl HCJ. COVID-19 Vaccines Based on Adenovirus Vectors. Trends Biochem Sci. 2021;46:429-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | UK Health Security Agency. Investigation into acute hepatitis of unknown aetiology in children in England—technical briefing 2. [Internet] [accessed 6 May 2022]. Availabe from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1073704/acute-hepatitis-technical-briefing-2.pdf. |
| 17. | The Lancet Infectious Diseases. Explaining the unexplained hepatitis in children. Lancet Infect Dis. 2022;22:743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 19. | Li L, Hsu SH, Wang C, Li B, Sun L, Shi J, Ren Y, Wang J, Zhang X, Liu J. Characteristics of viral pneumonia in non-HIV immunocompromised and immunocompetent patients: a retrospective cohort study. BMC Infect Dis. 2021;21:767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Brodin P, Arditi M. Severe acute hepatitis in children: investigate SARS-CoV-2 superantigens. Lancet Gastroenterol Hepatol. 2022;7:594-595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 21. | Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020;55:2565-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 22. | Tian X, Wu H, Zhou R. Molecular evolution of human adenovirus type 16 through multiple recombination events. Virus Genes. 2019;55:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Noval Rivas M, Porritt RA, Cheng MH, Bahar I, Arditi M. COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome-the superantigen hypothesis. J Allergy Clin Immunol. 2021;147:57-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 24. | Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 334] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 25. | Cantor A, Miller J, Zachariah P, DaSilva B, Margolis K, Martinez M. Acute Hepatitis Is a Prominent Presentation of the Multisystem Inflammatory Syndrome in Children: A Single-Center Report. Hepatology. 2020;72:1522-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 26. | Wang Z, Yang X, Zhong J, Zhou Y, Tang Z, Zhou H, He J, Mei X, Tang Y, Lin B, Chen Z, McCluskey J, Yang J, Corbett AJ, Ran P. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat Commun. 2021;12:1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 27. | Zhu M, Chen L. Hepatitis of unknown etiology in children: What we know and what we can do? Front Microbiol. 2022;13:956887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Samarasekera U. Mystery outbreak of severe acute hepatitis in children. Lancet Gastroenterol Hepatol. 2022;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Sergi CM. Acute Hepatitis of Unknown Origin (AHUO)-The Puzzle Ahead. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Pourkarim M R. Acute Hepatitis of Unknown Origin in Children; Lessons Learned from the COVID-19 Pandemic. Hepat Mon. 22:e128796. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | National Health Commission of the People's Republic of China. A press conference under the Joint Prevention and control mechanism of The State Council on July 23, 2022 Introduce the safety and efficacy of novel coronavirus vaccine. [Internet] [accessed 27 July 2022]. Availabe from: http://www.nhc.gov.cn/xwzb/webcontroller.do?titleSeq=11464&gecstype=1. |