Published online Dec 16, 2022. doi: 10.12998/wjcc.v10.i35.12804
Peer-review started: August 1, 2022
First decision: September 5, 2022
Revised: September 19, 2022
Accepted: November 10, 2022
Article in press: November 10, 2022
Published online: December 16, 2022
Processing time: 135 Days and 5.7 Hours
Esophageal squamous cell carcinoma is one of the most common malignant tumors in the digestive system in China and the world. Most patients are diagnosed as locally advanced or advanced stage. Concurrent chemoradiotherapy is the standard treatment for locally advanced esophageal squamous cell carcinoma. This study intends to summarize the evidence-based medical evidence of the treatment principle of locally advanced esophageal squamous cell carcinoma, the selection of radiotherapy dose, the outline of radiotherapy target and the selection of chemotherapy scheme. As a result, the effect of radiotherapy and chemotherapy is equivalent to that of surgery for the radical treatment of esophageal squamous cell carcinoma. In the era of immunization, it is recom
Core Tip: For the radical treatment of esophageal squamous cell carcinoma, the effect of radiotherapy and chemotherapy is equivalent to that of surgery. In the era of immunization, it is recommended to use involved field irradiation. Fluorouracil plus cisplatin regimen is the standard chemotherapy regimen, FOLFOX regimen and paclitaxel plus fluorouracil regimen are optional concurrent chemotherapy regimens. The toxic and side effects of different chemotherapy regimens are very different, which can be selected according to the actual situation of patients.
- Citation: Zhang XF, Liu PY, Zhang SJ, Zhao KL, Zhao WX. Principle and progress of radical treatment for locally advanced esophageal squamous cell carcinoma. World J Clin Cases 2022; 10(35): 12804-12811
- URL: https://www.wjgnet.com/2307-8960/full/v10/i35/12804.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i35.12804
Esophageal squamous carcinoma is the most common tumor in China[1,2]. The diagnosis of esophageal squamous carcinoma needs to be made by gastroscopic biopsy, and there is no effective and rapid screening method, therefore, most patients are diagnosed at advanced stage. Concurrent chemoradiotherapy is the standard treatment for locally advanced esophageal squamous carcinoma[3,4]. In this study, we intend to summarize the evidence-based clinical principles for radical treatment of locally advanced esophageal squamous carcinoma, including the choice of treatment modality for radical treatment, the dose selection of radical radiotherapy, the target delineation and the choice of chemotherapy regimen combined with radical radiotherapy for esophageal squamous carcinoma.
Radical treatment for locally advanced esophageal squamous carcinoma includes radical surgery and radical radiotherapy. Patients with staging of T1b stage (AJCC 8th) or above must undergo radical treatment. The following conditions should be met to enable radical surgery: T0-4aN0-1M0 stage and the distance from the lesion to the esophageal inlet is greater than 5 cm[5-7]. Other than this, locally advanced squamous esophageal cancer requires radiotherapy treatment[8,9].
The FFCD9102 study showed that even if surgery could be performed, the efficacy of concurent chemoradiotherapy vs surgery was comparable[10]. The study enrolled 444 patients with squamous esophageal cancer in the thoracic segment of T3N0-1M0, with a 8:1 ratio of squamous to adenocarcinoma, with the data suggest that, in patients with locally advanced thoracic esophageal cancers, especially epidermoid, who respond to chemoradiation, there is no benefit for the addition of surgery after chemoradiation compared with the continuation of additional chemoradiation.
In other studies[11,12], Adding surgery to chemoradiotherapy improves local tumor control but does not increase survival of patients with locally advanced esophageal squamous cell carcinoma. A randomized controlled study of surgery and radiotherapy for operable esophageal cancer showed that the curative effect of late course accelerated hyperfractionation conformal radiotherapy for operable esophageal cancer was equivalent to that of surgery[13].
Another CURE study is a prospective randomized controlled study of concurrent radiotherapy and chemotherapy vs surgery for potentially resectable esophageal cancer. Eighty patients with potentially resectable squamous cell carcinoma of the middle and lower thoracic esophagus were randomly assigned to radiotherapy and chemotherapy group and surgical treatment group. The study concluded that progression-free survival (PFS) and overall survival (OS) are similar between standard resection and radiotherapy and chemotherapy for potentially resectable esophageal squamous cell carcinoma. The recurrence rate in the mediastinum was slightly higher in the operation group, and the recurrence rate in the neck or abdomen was higher in the radiotherapy and chemotherapy group[14].
Therefore, even for patients with locally advanced squamous esophageal cancer who can undergo radical surgery, the efficacy of treatment with radical radiotherapy is comparable to that of surgery, so radiotherapy is important for the treatment of locally advanced squamous esophageal cancer.
According to evidence-based medical evidence, 50-50.4 Gy is the standard dose for radical radiotherapy and chemotherapy of locally advanced esophageal squamous cell carcinoma. Sixty Gy to seventy Gy is mainly used in China, and 61.2 Gy is mostly used in our center. The main basis is as follows.
The study of RTOG8501 compared radiotherapy alone at 64 Gy/32 Fx/44 d and radiotherapy at 50 Gy/25 Fx/38 d with a chemotherapy regimen of cisplatin 75 mg/m2 /d, d1, and fluorouracil 1000 mg/m2/d, 96h, for four courses. The study included 106 (88%) squamous carcinomas and 15 (12%) adenocarcinomas. The included stages were T1-2 = 98 (81%), T3 = 23 (19%), N0 = 91 (75%), and N1 = 30 (25%), respectively. The results showed that the 3-, 5-year OS, local failure and distant metastasis rates were superior in the 50 Gy than in the 60 Gy radiotherapy alone group, but the side effect rate was higher in the 50 Gy radiotherapy group[3]. Based on the above results of RTOG8501 study, the efficacy of radiotherapy was not satisfying, therefore, RTOG9405 study was proposed with increased dose. The radiation doses of the two groups were 50.4 Gy and 64.8 Gy respectively, and with same chemotherapy regimen of fluorouracil plus cisplatin. The results showed that the 2-year OS and 3-year OS of the low-dose group compared with the high-dose group was 40% vs 31% and 33% vs 25%. The results suggest that the 50.4 Gy dose group has better efficacy[4].
However, the 2-year OS rates for esophageal cancer range from 36% to 56% and the 3-year OS rates range from 20% to 33% at the above dose conditions[4]. Since three-dimensional radiotherapy techni
ARTDECO study, radiation dose escalation up to 61.6 Gy to the primary tumor did not result in a significant increase in local control over 50.4 Gy[17].
Therefore, based on the above, for the dose of radical radiotherapy for esophageal squamous carcinoma, 50-50.4 Gy is recommend for concurrent chemoradiotherapy and 60-61.2 Gy (1.8-2.0 Gy per fraction a day) is mostly used for radiotherapy alone in China[15].
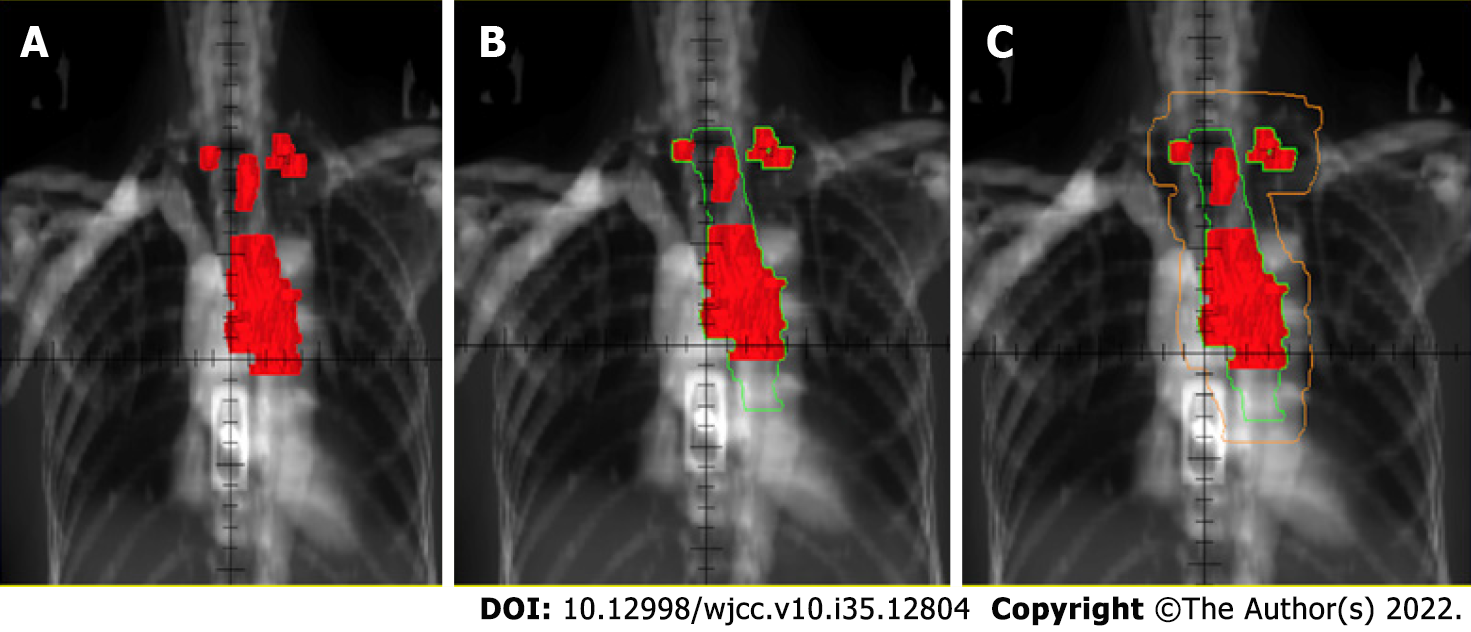
The target area of radical radiotherapy for locally advanced esophageal squamous cell carcinoma was treated with involved field irradiation in our center. The gross tumor volume (GTV) includes the visible lesions of the tumor including the primary esophageal foci plus metastatic lymph nodes. The clinical target volume (CTV) is to put the GTV up and down for 3 cm without putting it outside and the planning target volume (PTV) is 1cm outside the CTV (1.5 cm outside if the target area is located in the cardia), as in Figure 1.
For GTV outline, CT, esophagogram, gastroscopy, intraluminal ultrasound, and positron emission computed tomography (PET-CT) need to be consulted. The distance between the esophageal lesion and the surrounding anatomical landmarks is first assessed by gastroscopy and esophageal film to understand the general location of the lesion, and CT has advantages in the display of intramural invasive lesions. In addition, PET-CT can improve the accuracy of GTV outline[18,19]. It should be noted that if the above imaging can not see the primary focus, you can first place the silver clip mark under the esophagoscope, determine the position under the simulator, and then outline the target area. The criteria for confirming lymph node metastasis of esophageal squamous cell carcinoma on CT images are: the short diameter of cervical and supraclavicular lymph nodes ≥ 5 mm, and the short diameter of mediastinal and hilar lymph nodes ≥ 10 mm[20]. For tracheoesophageal groove lymph nodes, Gu et al's study on the clinical significance of CT observation of tracheoesophageal groove lymph node metastasis of thoracic esophageal squamous cell carcinoma shows that each lymph nodes in tracheoesophageal groove are considered to be positive[21]. In this study, each tracheoesophageal groove lymph node has postoperative pathology as the gold standard for diagnosis.
The determination of CTV is based on the following studies. A study published in 2006 analyzed the pathologic features of squamous esophageal cancer, defined as normal esophageal mucosal coating epithelium, but the presence of cancer cells or cancer nests can be seen under the mucosa or muscle. In this study, the incidence of intramural infiltration was found to be 78.8%, and for 95% of intramural infiltrates, a 5 cm proximal and distal resection was required, and for 90% of intramural infiltrates, a 4.7 cm and 3.9 cm proximal and distal resection were required[22]. Another study also showed that for squamous esophageal carcinoma, a 3-cm proximal and distal margin could cover 94% and 97% of the proximal and distal microinfiltrates[23]. Based on the above, the GTV is placed up and down for 3 cm and not placed around to form a CTV. Attention should be paid to the following matters. GTV is placed 3 cm up and down and drawn layer by layer manually along the esophageal wall, which is not a direct external placement. If GTV is in the cardia, it should be manually outlined layer by layer along the gastric wall. In addition, relevant studies show that different filling degrees of the stomach have little effect on the target dose. Therefore, we do not limit the filling degree of the stomach in patients with esophageal squamous cell carcinoma during radiotherapy[24].
Currently, both involved field and prophylactic field irradiation are used in l clinical practice. RTOG0436 study used the prophylactic irradiation[25], while RTOG0246 and SCOPE1[26,27] studies used involved field irradiation. Pooling RTOG8501, RTOG9405, and Prof. Zhao's studies[28,29], the 3-year OS of prophylactic field irradiation study was 25%-27%, compared with 33%-44% in involved field study. This proves the comparable efficacy of the irradiation range of involved field irradiation. Four studies on involved field irradiation compared to prophylactic field irradiation suggest comparable efficacy of the involved field vs the preventive field for the cervical and upper thoracic esophageal cancer and for people older than 70 years[30-33]. In conclusion, we suggest involved field irradiation be used to protect normal tissues.
We analyzed the pattern of failure after the involved field irradiation, which included 53 patients, 26% survived tumor-free and 74% failed treatment. Of the total failure population, 41% had distant metastases, 8% had outfield lymph node recurrence, 5% had distant metastases and local recurrence, and 44% had in-field recurrence. Therefore, the proportion of field recurrence in this study is low, indicating that the radiation range of the involved field is sufficient[28].
Based on the results of the RTOG8501 study, fluorouracil plus cisplatin is the standard chemotherapy regimen for concurrent chemoradiotherapy, but 42% of patients in this study experienced grade 3 acute toxicity, 25% experienced grade 3 distant toxicity, and the 5-year OS rate was 26%, so this regimen still needs to be optimized[3]. A randomized study in France comparing the efficacy of the FOLFOX (oxaliplatin combined with fluorouracil and calcium folinate) with the standard fluorouracil combined with cisplatin regimen for concurrent chemoradiotherapy showed comparable efficacy of both regimens, but the FOLFOX regimen was administered in a more convenient manner than the fluorouracil combined with cisplatin regimen[8]. Several studies have demonstrated the use of docetaxel in locally advanced esophageal cancer. Font et al[34] evaluated the efficacy and tolerability of docetaxel concurrent with radiotherapy in inoperable esophageal cancer patients showing a better toxicity profile compared to standard cisplatin/5-FU-based chemoradiotherapy. KDOG 0501 trial reported the optimal dose of definitive chemoradiotherapy with docetaxel in patients with advanced esophageal carcinoma.The main toxicities were myelotoxicity and esophagitis, which was tolerable[35]. Spigel et al[36] reported that chemoradiotherapy with docetaxel was safe, with a high pathological curative effect. Previous studies also have shown that the paclitaxel plus fluorouracil is better tolerated in radiotherapy for esophageal squamous cancer[15].
The Chinese ESO-Shanghai1 study was a phase III study evaluating patients receiving paclitaxel combined with fluorouracil vs the standard fluorouracil combined with cisplatin regimen in concurrent chemoradiotherapy for esophageal squamous carcinoma[15]. Patients in both groups received the same radiotherapy regimen with a total dose of 61.2 Gy/34 F (1.8 Gy/1 F, five times a week). The primary endpoint was the 3-year OS rate, and secondary study endpoints included PFS and safety. This study showed that fluorouracil combined with cisplatin remains the standard chemotherapy regimen for radical radiotherapy of locally advanced esophageal squamous carcinoma, and the OS of the radiotherapy regimen of paclitaxel combined with fluorouracil was comparable to that of standard fluorouracil combined with cisplatin. With regard to safety, the paclitaxel combined with fluorouracil regimen had a higher incidence of severe leukopenia, radiation dermatitis, and radiation pneumonitis, and a lower incidence of anemia, thrombocytopenia, gastrointestinal toxicity, and malaise. It should be noted that the intensity of radiation dose using fluorouracil combined with cisplatin in this study was lower than in the RTOG8501 study and the PRODIGE study[3,8], but the radiation dose was higher than in these two studies. In conclusion, the standard chemotherapy regimen for radical concurrent radiotherapy for esophageal squamous carcinoma remains fluorouracil combined with cisplatin. All in ALL, FOLFOX regimen and paclitaxel combined with fluorouracil regimen are optional chemotherapy regimens, and the toxic effects of different chemotherapy regimens are different and can be chosen clinically according to the actual situation of patients.
Immunotherapy has made rapid progress in the treatment of esophageal cancer. The field of esophageal cancer is no exception. At present, a number of prospective RCT studies of immunization combined with concurrent radiotherapy and chemotherapy are being carried out[37,38]. However, there is still no final conclusion on how to arrange troops for immunization and concurrent radiotherapy and chemotherapy. At present, both concurrent and sequential modes are optional.
For radical treatment of esophageal squamous carcinoma, concurrent chemoradiotherapy is comparable to surgery. 50-50.4 Gy is the standard dose, and 60 Gy is mainly used in China. the efficacy of involved field and prophylactic irradiation is comparable. With the advent of the immune era, we suggest that the involved field irradiation should be used to preserve the immune protective function of normal lymph nodes. Fluorouracil combined with cisplatin regimen is the standard chemotherapy regimen, and FOLFOX regimen and paclitaxel combined with fluorouracil regimen are optional chemotherapy regimens. The toxic effects of different chemotherapy regimens are very different and can be chosen clinically according to the patient's actual situation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chien CR, Taiwan; Xu J, China S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1261] [Cited by in RCA: 1373] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 4. | Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose vs standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 876] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 5. | Steyerberg EW, Neville BA, Koppert LB, Lemmens VE, Tilanus HW, Coebergh JW, Weeks JC, Earle CC. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006;24:4277-4284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Fujita H, Sueyoshi S, Yamana H, Shinozaki K, Toh U, Tanaka Y, Mine T, Kubota M, Shirouzu K, Toyonaga A, Harada H, Ban S, Watanabe M, Toda Y, Tabuchi E, Hayabuchi N, Inutsuka H. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection vs radical esophagectomy. World J Surg. 2001;25:424-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Hofstetter WL. Lymph Node Dissection in Esophageal Cancer. In: Yang SC, Cameron DE. Current Therapies in Thoracic and Cardiovascular Surgery. Philadelphia: Mosby, Inc., 2004: 360-363. |
| 8. | Conroy T, Galais MP, Raoul JL, Bouché O, Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay I, Michel P, Llacer-Moscardo C, François E, Créhange G, Abdelghani MB, Juzyna B, Bedenne L, Adenis A; Fédération Francophone de Cancérologie Digestive and UNICANCER-GI Group. Definitive chemoradiotherapy with FOLFOX vs fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 9. | Li QQ, Liu MZ, Hu YH, Liu H, He ZY, Lin HX. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus. 2010;23:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, Pezet D, Roullet B, Seitz JF, Herr JP, Paillot B, Arveux P, Bonnetain F, Binquet C. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 879] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 11. | Qian D, Chen X, Shang X, Wang Y, Tang P, Han D, Jiang H, Chen C, Zhao G, Zhou D, Cao F, Er P, Zhang W, Li X, Zhang T, Zhang B, Guan Y, Wang J, Yuan Z, Yu Z, Wang P, Pang Q. Definitive chemoradiotherapy vs neoadjuvant chemoradiotherapy followed by surgery in patients with locally advanced esophageal squamous cell carcinoma who achieved clinical complete response when induction chemoradiation finished: A phase II random. Radiother Oncol. 2022;174:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 12. | Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, Klump B, Budach W, Teichmann R, Schmitt M, Schmitt G, Franke C, Wilke H. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 928] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 13. | Sun XD, Yu JM, Fan XL, Ren RM, Li MH, Zhang GL. [Randomized clinical study of surgery vs radiotherapy alone in the treatment of resectable esophageal cancer in the chest]. Zhonghua Zhong Liu Za Zhi. 2006;28:784-787. [PubMed] |
| 14. | Chiu PW, Chan AC, Leung SF, Leong HT, Kwong KH, Li MK, Au-Yeung AC, Chung SC, Ng EK. Multicenter prospective randomized trial comparing standard esophagectomy with chemoradiotherapy for treatment of squamous esophageal cancer: early results from the Chinese University Research Group for Esophageal Cancer (CURE). J Gastrointest Surg. 2005;9:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, Tang H, Fan M, Li L, Lin Q, Xia Y, Li Y, Li J, Jia H, Lu S, Zhang Z, Zhao K. Comparing Paclitaxel Plus Fluorouracil Versus Cisplatin Plus Fluorouracil in Chemoradiotherapy for Locally Advanced Esophageal Squamous Cell Cancer: A Randomized, Multicenter, Phase III Clinical Trial. J Clin Oncol. 2019;37:1695-1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Xu Y, Dong B, Zhu W, Li J, Huang R, Sun Z, Yang X, Liu L, He H, Liao Z, Guan N, Kong Y, Wang W, Chen J, Qiu G, Zeng M, Pu J, Hu W, Bao Y, Liu Z, Ma J, Jiang H, Du X, Hu J, Zhuang T, Cai J, Huang J, Tao H, Liu Y, Liang X, Zhou J, Tao G, Zheng X, Chen M. A Phase III Multicenter Randomized Clinical Trial of 60 Gy vs 50 Gy Radiation Dose in Concurrent Chemoradiotherapy for Inoperable Esophageal Squamous Cell Carcinoma. Clin Cancer Res. 2022;28:1792-1799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | Hulshof MCCM, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, Nuyttens JJME, van der Sangen MJC, Jeene PM, Reinders JG, van Berge Henegouwen MI, Thano A, van Hooft JE, van Laarhoven HWM, van der Gaast A. Randomized Study on Dose Escalation in Definitive Chemoradiation for Patients With Locally Advanced Esophageal Cancer (ARTDECO Study). J Clin Oncol. 2021;39:2816-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 18. | Munden RF, Macapinlac HA, Erasmus JJ. Esophageal cancer: the role of integrated CT-PET in initial staging and response assessment after preoperative therapy. J Thorac Imaging. 2006;21:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Thomas L, Lapa C, Bundschuh RA, Polat B, Sonke JJ, Guckenberger M. Tumour delineation in oesophageal cancer - A prospective study of delineation in PET and CT with and without endoscopically placed clip markers. Radiother Oncol. 2015;116:269-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Mizowaki T, Nishimura Y, Shimada Y, Nakano Y, Imamura M, Konishi J, Hiraoka M. Optimal size criteria of malignant lymph nodes in the treatment planning of radiotherapy for esophageal cancer: evaluation by computed tomography and magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1996;36:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Gu JY, Wang JH, Xiang JQ, Ma LF. A study on clinical value of CT features of tracheoesophageal groove lymph node metastasis of thoracic esophageal carcinoma. Zhonghua Fangshe Xue Zazhi. 2002;36. |
| 22. | Shi HY, Zhu SC, Zhai FS, Su JW, Li R, Han C. Influence of pathological characteristics on radiotherapeutic target area of esophageal squamous cell carcinoma. Zhonghua Fangshe Zhongliu Xue Zazhi. 2006;4. |
| 23. | Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, Wang X, Gao G, Wu TT, Komaki R, Chang JY. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys. 2007;67:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Van De Voorde L, Larue R, Persoon L, Öllers M, Nijsten S, Bosmans G, Berbée M, Swinnen A, van Elmpt W, Vanneste B, Verhaegen F, Lambin P. The influence of gastric filling instructions on dose delivery in patients with oesophageal cancer: A prospective study. Radiother Oncol. 2015;117:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar H, Horiba N, Dubey A, Greenberger JS, Raben A, Giguere J, Roof K, Videtic G, Pollock J, Safran H, Crane CH. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2017;3:1520-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 26. | Swisher SG, Winter KA, Komaki RU, Ajani JA, Wu TT, Hofstetter WL, Konski AA, Willett CG. A Phase II study of a paclitaxel-based chemoradiation regimen with selective surgical salvage for resectable locoregionally advanced esophageal cancer: initial reporting of RTOG 0246. Int J Radiat Oncol Biol Phys. 2012;82:1967-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, Cunningham D, Blazeby J, Roy R, Maughan T, Griffiths G. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 290] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary? Int J Radiat Oncol Biol Phys. 2010;76:446-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Li M, Zhang X, Zhao F, Luo Y, Kong L, Yu J. Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice. Radiat Oncol. 2016;11:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, Nakagawa K. Involved-field radiotherapy (IFRT) vs elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol. 2015;10:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Liu M, Zhao K, Chen Y, Jiang GL. Evaluation of the value of ENI in radiotherapy for cervical and upper thoracic esophageal cancer: a retrospective analysis. Radiat Oncol. 2014;9:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ, Kong L. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie. 2011;34:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, Chen L, Lu B, Chen H, Yang J, Wang Q, Zhang J, Ma Y, Liu R, Hage A, Lang J. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med. 2020;9:7460-7468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Font A, Arellano A, Fernández-Llamazares J, Casas D, Boix J, Cardenal J, Margelí M, Manzano JL, Abad A, Rosell R. Weekly docetaxel with concomitant radiotherapy in patients with inoperable oesophageal cancer. Clin Transl Oncol. 2007;9:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Ishiyama H, Hayakawa K. A phase I trial of definitive chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) for advanced esophageal carcinoma: Kitasato digestive disease & oncology group trial (KDOG 0501). Radiother Oncol. 2008;87:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Spigel DR, Greco FA, Meluch AA, Lane CM, Farley C, Gray JR, Clark BL, Burris HA 3rd, Hainsworth JD. Phase I/II trial of preoperative oxaliplatin, docetaxel, and capecitabine with concurrent radiation therapy in localized carcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2010;28:2213-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, Bordia S, Bhagia P, Shih CS, Desai A, Enzinger P. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. 2021;17:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 38. | Yu R, Wang W, Li T, Li J, Zhao K, Liang L, Wu H, Ai T, Huang W, Li L, Yu W, Wei C, Wang Y, Shen W, Xiao Z. RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol. 2021;17:4081-4089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |