Published online Dec 6, 2022. doi: 10.12998/wjcc.v10.i34.12750
Peer-review started: September 11, 2022
First decision: September 26, 2022
Revised: October 8, 2022
Accepted: November 8, 2022
Article in press: November 8, 2022
Published online: December 6, 2022
Processing time: 81 Days and 22.7 Hours
Cyclosporine is an immunosuppressive agent used effectively for treatment of a rare haematological disorder known as medullary aplasia. This drug prevents several side effects, including gingival enlargement (GE) which compromises aesthetics, phonetics and chewing, and also predisposes patients to periodontitis.
This clinical case reports a 41-year-old woman who presented with cyclosporine-induced GE with underlying periodontitis and medullary aplasia. The man
Multidisciplinary management of cyclosporine-induced GE and medullary aplasia allows for correct diagnosis and effective treatment of this pathological expression through a phased therapeutic approach.
Core Tip: Medullary aplasia is a marrow failure characterized by a total or partial disappearance of hematopoietic precursors in bone marrow, resulting in pancytopenia which is usually accompanied by hemorrhagic syndrome. The treatment involves immunosuppressive therapy with cyclosporine. This drug suppresses the activity of immune cells that damage the bone marrow, although it may produce an inflammatory reaction, with increased fibrotic response in the extracellular matrix, and gingival enlargement (GE). We report a case of GE induced by cyclosporine in a patient with rare medullary aplasia, which was managed with a phased periodontal treatment plan.
- Citation: Victory Rodríguez G, Ruiz Gutiérrez ADC, Gómez Sandoval JR, Lomelí Martínez SM. Gingival enlargement induced by cyclosporine in Medullary aplasia: A case report. World J Clin Cases 2022; 10(34): 12750-12760
- URL: https://www.wjgnet.com/2307-8960/full/v10/i34/12750.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i34.12750
Medullary aplasia is a disease characterized by failure of hematopoietic function and consequent peripheral cytopenias. Indeed, medullary aplasia is generally considered a medical emergency. It is characterized by bone marrow failure and total or partial disappearance of hematopoietic precursors which cause pancytopenia in the peripheral blood, as well as hemorrhagic syndrome[1]. Cyclosporine is one of the most widely used immunosuppressive drugs for this disease[2]. However, widespread clinical use of cyclosporine has been questioned due to its various inherent side effects such as nephrotoxicity, hepatotoxicity, hypertension, neurotoxicity, hirsutism, gastrointestinal toxicity, and gingival enlargement (GE)[3].
GE is a pathological condition characterized by excessive growth in mass and volume of gingival tissue, dense collagen stroma, and epithelial hyperplasia[4-6]. Cyclosporine interferes with the modulation of fibrotic response in gingival fibroblasts, and inhibits the secretion of proteases from the matrix which contributes to the accumulation of components of the extracellular matrix in the gingival connective tissue, resulting in gingival growth[7-10]. In addition, this immunosuppressant generates oral microbial dysbiosis in dental biofilm formed by bacteria, fungi and viruses[10].
The therapeutic procedure used in the management of GE with underlying periodontitis in a patient with medullary aplasia is not fixed because it can be modified according to the conditions, behavior, and severity of the oral pathological changes, as well as the patient's systemic condition. This article presents the therapeutic management and maintenance of a clinical case of severe cyclosporine-induced GE with underlying periodontitis in a patient with medullary aplasia.
A female patient aged 41 years was referred to the Periodontics Clinic of the University of Guadalajara by the Hematology Department of the Fray Antonio Alcalde Civil Hospital of Guadalajara, Jalisco. The reason for consultation was evaluation of enlarged gums, the patient perceived an increase in the sizes of the gums in both arches.
The patient had enlargement of gums without associated symptoms 1 year before coming to the Periodontics Clinic.
Her medical history showed that, in 2019, she was hospitalized for gingival and vaginal bleeding, and she received blood transfusion. She was diagnosed with hypothyroidism and medullary aplasia in 2019. The pharmacological treatment consisted of 100 mg Cyclosporine given in 3 daily doses, Tribedoce (1 daily, comprising 100 mg of thiamine, 5 mg of pyridoxine and 50 mg of cyanocobalamin); Bedoyecta Tri (1 daily, comprising 100 mg of hydroxycobalamin, 50 mg of thiamine, and 2 mL of pyridoxine); Cilocid (5 mg folic acid, 1 daily), and 600 Autrin (1 daily, consisting of iron and vitamins), as well as 100 mg of levothyroxine (1 daily) for hypothyroidism.
Her father was diagnosed with type 2 diabetes mellitus in 2020.
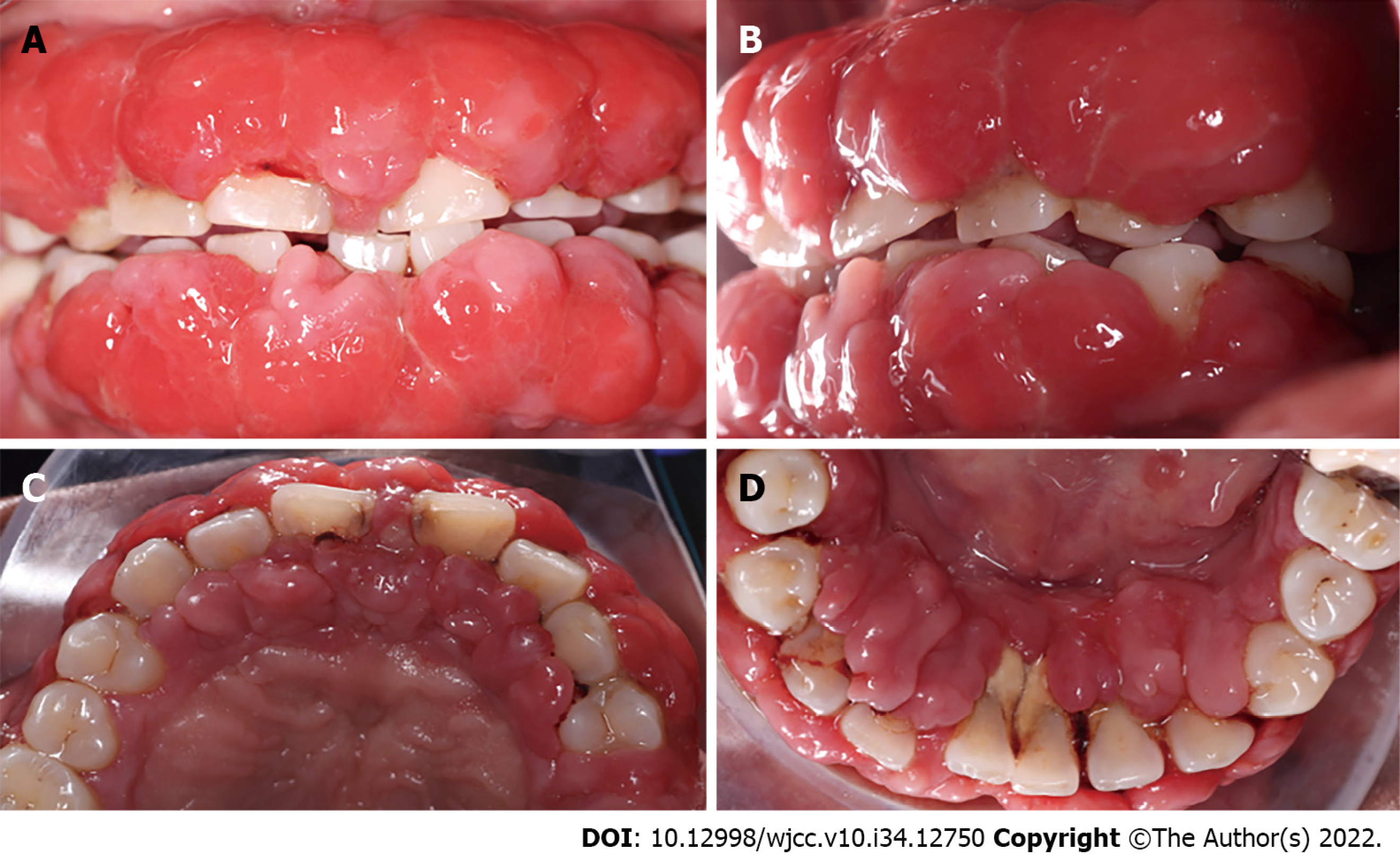
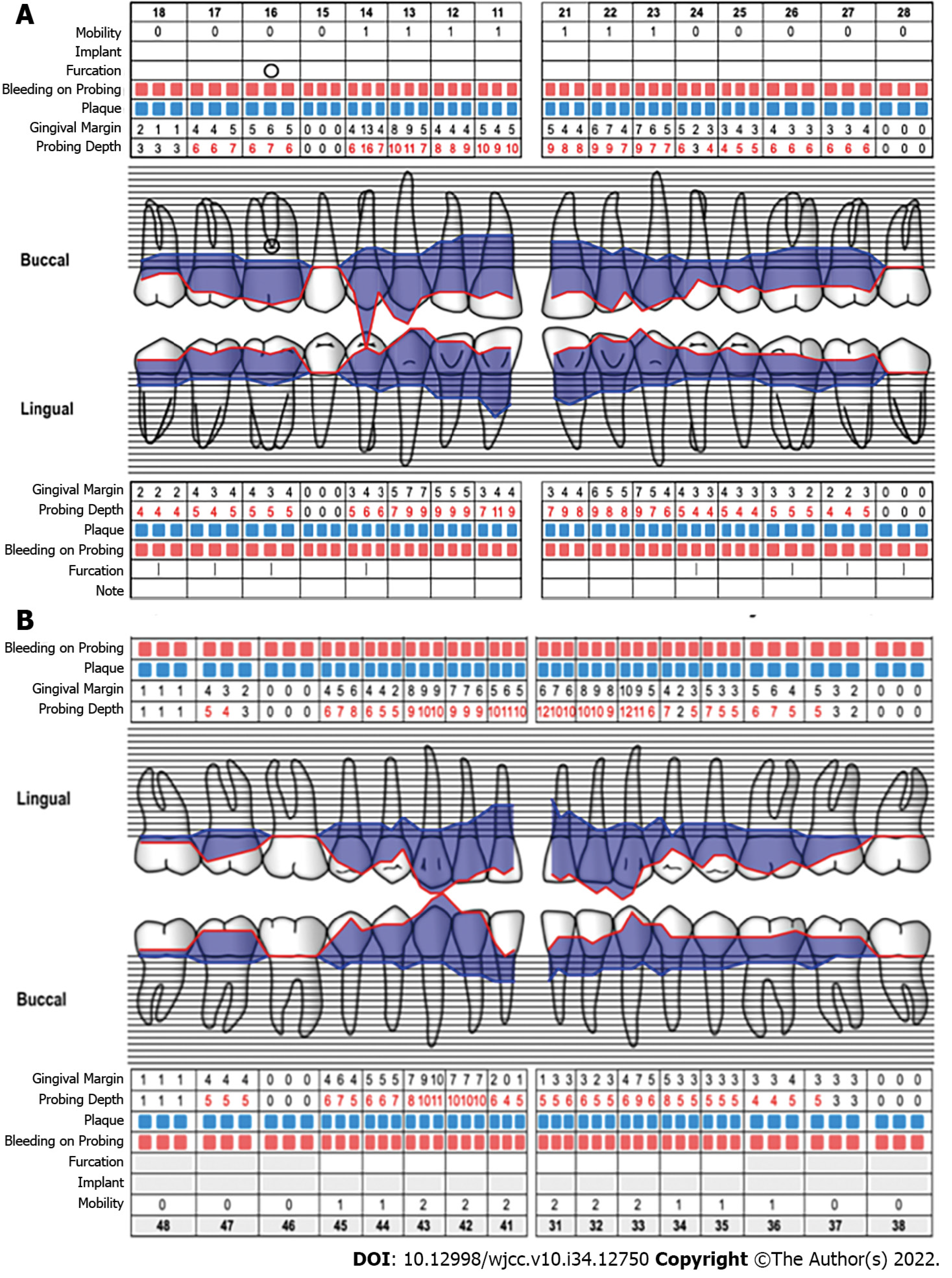
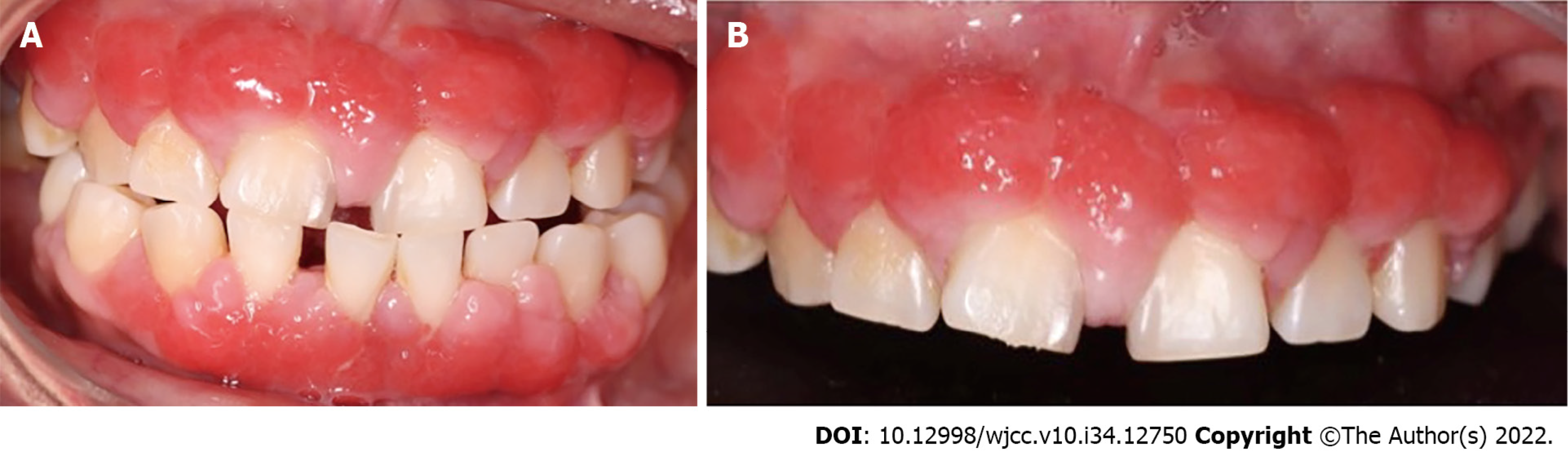
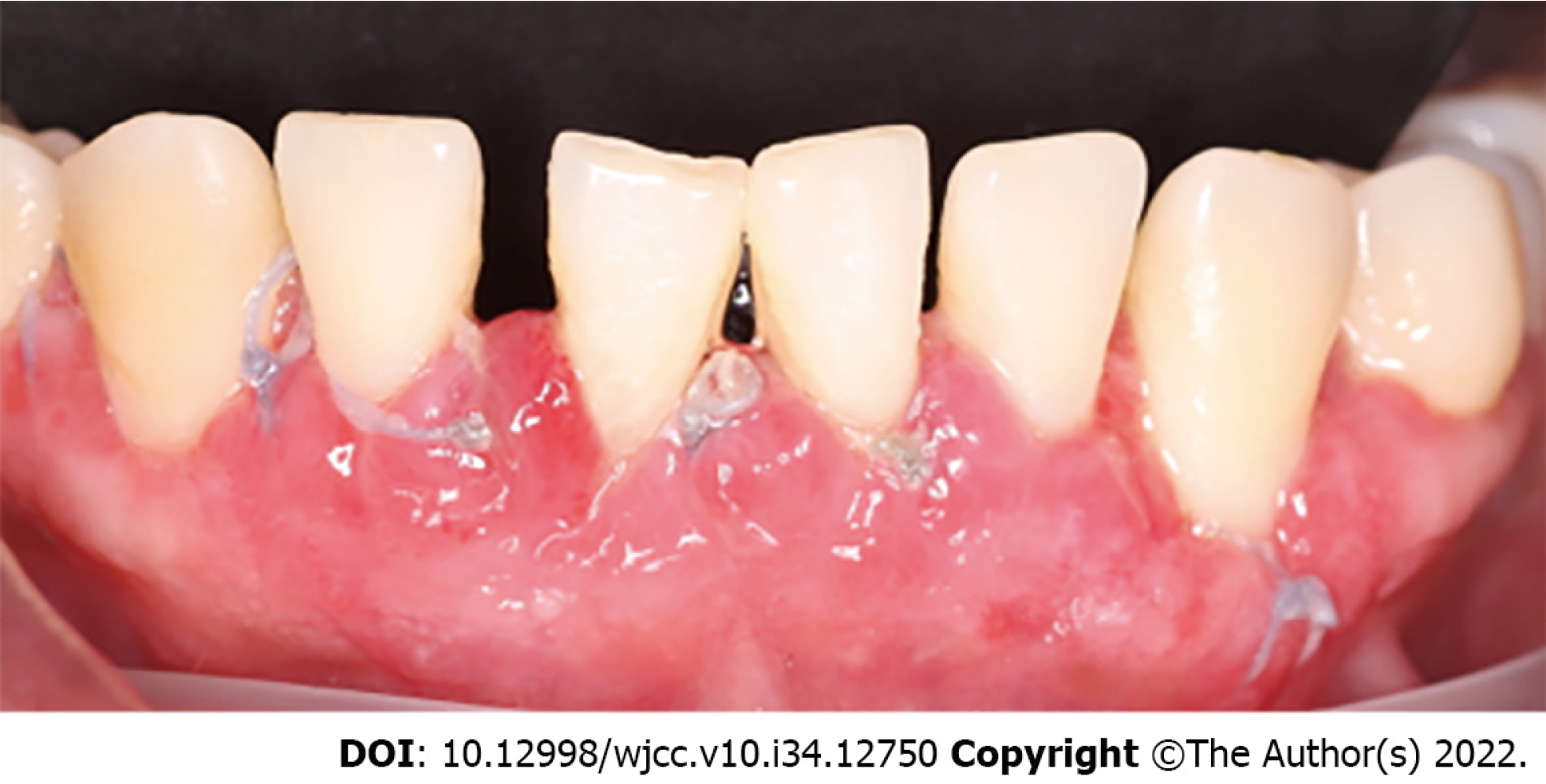
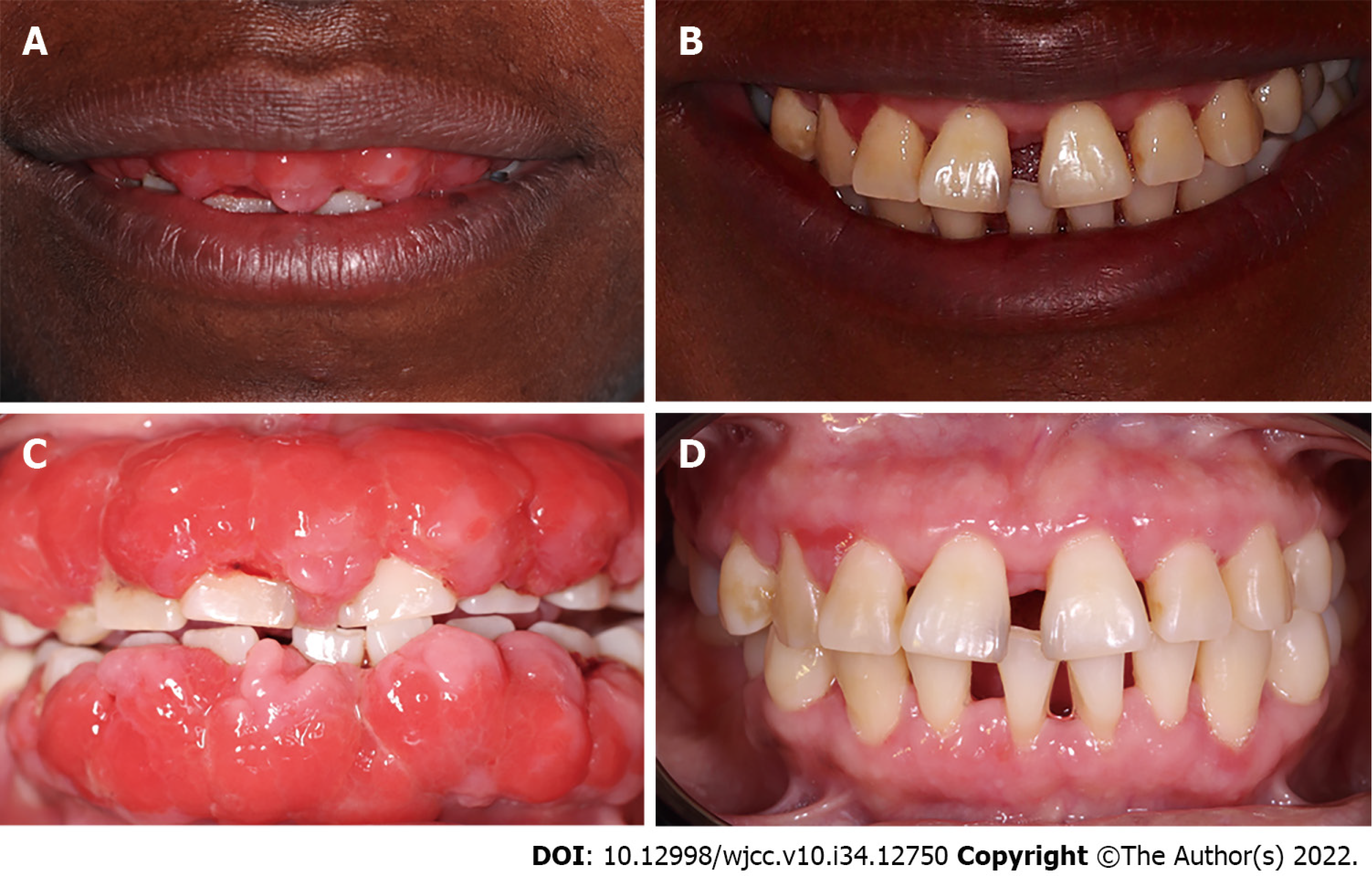
During the intraoral clinical examination, increased nodal gingival volume was observed in the upper and lower arcades located mainly in the anterior area through both vestibular and palatine/lingual access. There was erythema in all areas, with increased volume, biofilm, and generalized calculus; the root remained intact, and carious lesions were evident (Figure 1). The periodontal evaluation presented depths to the probe of up to 12 mm, mainly in anterior teeth; the generalized probing caused bleeding, and there was mobility in some of the dentition (Figure 2).
There were normal levels of platelets (275 × 103/µL) and white cells (5.68 × 103/µL), while hemoglobin level was slightly low (11.6 g/dL), and blood glucose level was 93 mg/dL. No abnormality was found in urine analysis.
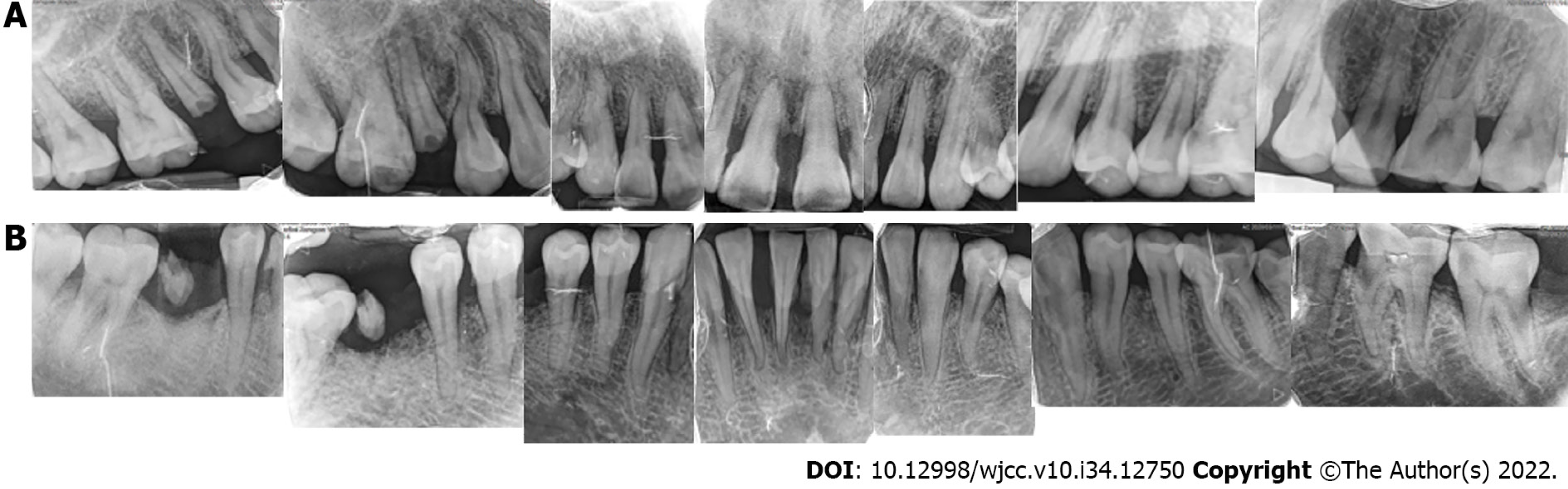
At the radiographic level, generalized bone loss was observed in a horizontal pattern, being more accentuated in teeth 11, 21, 31, and 41 where the loss extended to the middle third (Figure 3).
Based on all the information obtained from the clinical and radiographic examinations, the diagnosis was localized periodontitis stage III, grade C in teeth 11, 21, 31 and 41, and generalized periodontitis stage I, grade C. These were associated with a presumptive diagnosis of drug-induced GE. The periodontitis was classified according to the new classification system of periodontal diseases and conditions of the American Academy of Periodontology and the European Federation of Periodontology (2018).
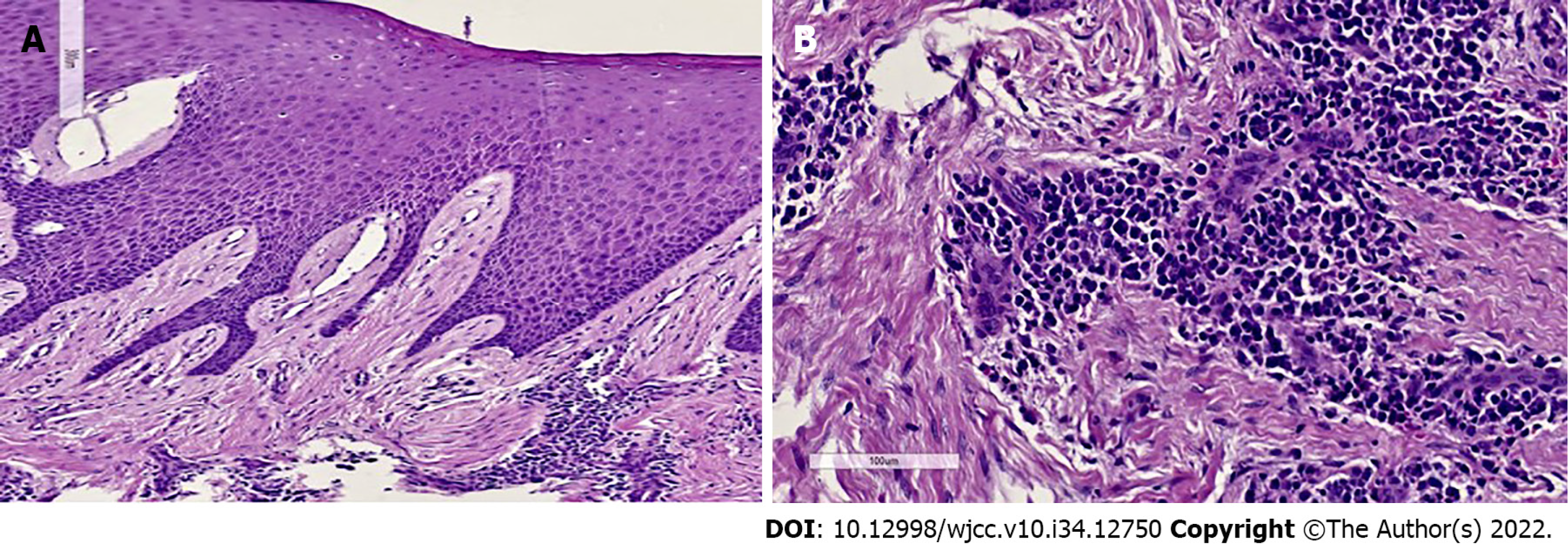
The histopathology study showed that the specimen presented fibroconnective tissue growth, epithelial nails exhibited elongation and pseudo-epithelionomatous hyperplasia. The connective tissue presented large focal areas of chronic inflammatory infiltrations and lymphoplasmacytic dispersion in a stroma of collagen fibers and active fibroblasts (Figure 4). This analysis corroborated the presumptive diagnosis of drug-associated GE.
The first periodontal phase began with generalized supragingival debridement and hygiene instructions which consisted of advising the patient on dental brushing, use of interproximal brushes, and rinsing with 0.12% chlorhexidine. In the following months, subgingival debridement was performed in the four quadrants, and due to the coronavirus disease 2019 pandemic, the patient was summoned after 3 mo. Upon the patient's return, control photographs were taken in which reductions in the color, size, and shape of the GE were clearly observed (Figure 5).
Consultation with the hematologist was carried out. The hematologist approved the surgical procedure and adjusted daily dose of cyclosporine from 3 capsules daily to 1 capsule every other day. Clindamycin (600 mg) was indicated 1 h before the intervention.
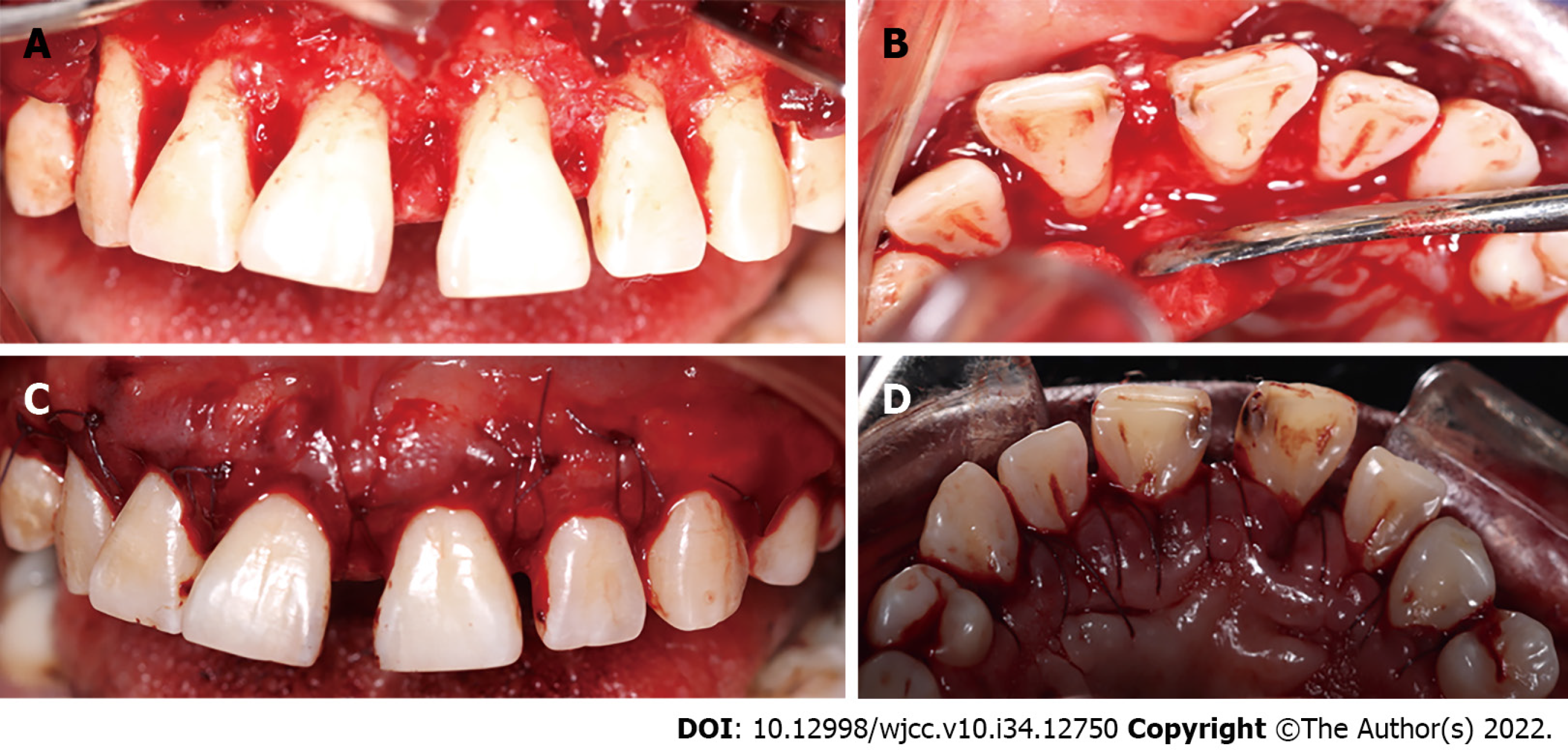
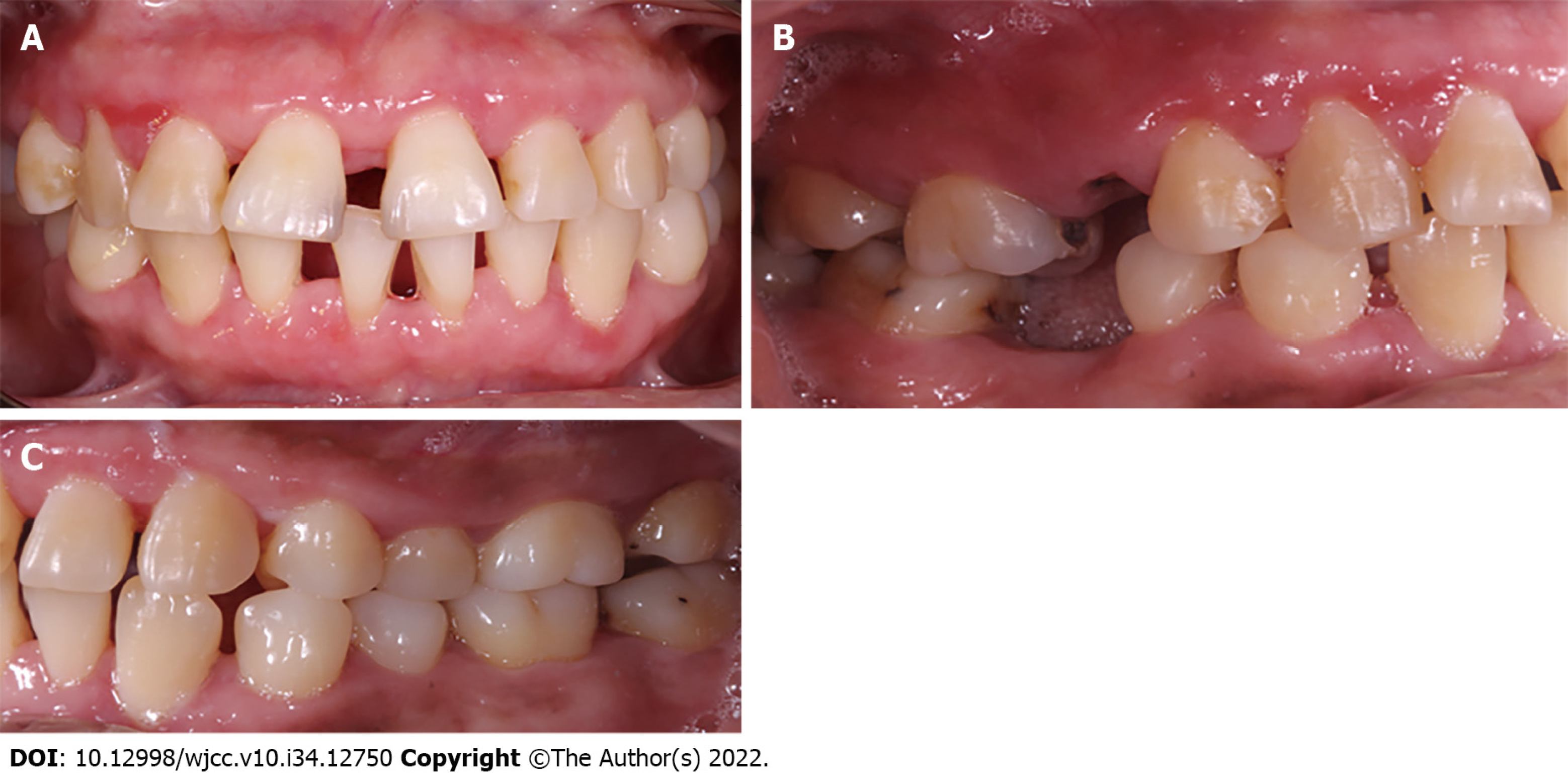
The surgical approach in the upper arch comprised premolar to premolar. Measurements were made to calculate the amount of gingival tissue above the clinical crown, and 3 mm coronal to the mucogingival line was considered as internal level for submarginal incisions to generate new papillae and thin the tissues, with a 15 C scalpel blade. Then, intrasulcular incisions were made to reflect full-thickness flaps in the vestibular and palatine (Figure 6).
Granulomatous tissue was removed with McCall 13-14 and 17-18 curettes, and the roots were scraped and smoothed. Osteoplasties were performed with carbide ball burs. Gingival nodules were thinned with LaGrange scissors, the flaps were adapted, and a tissue sample was taken for histopathological analysis. The incision site was sutured using polyglycolic acid 4-0 with modified horizontal mattress stitches, and periodontal dressing was applied. Paracetamol (500 mg) was administered for 3 d, and the prescription indicated that it was to be continued along with 300 mg clindamycin for 7 d. Moreover, 0.12% chlorhexidine gel was placed in the treated area 3 times daily for 15 d.
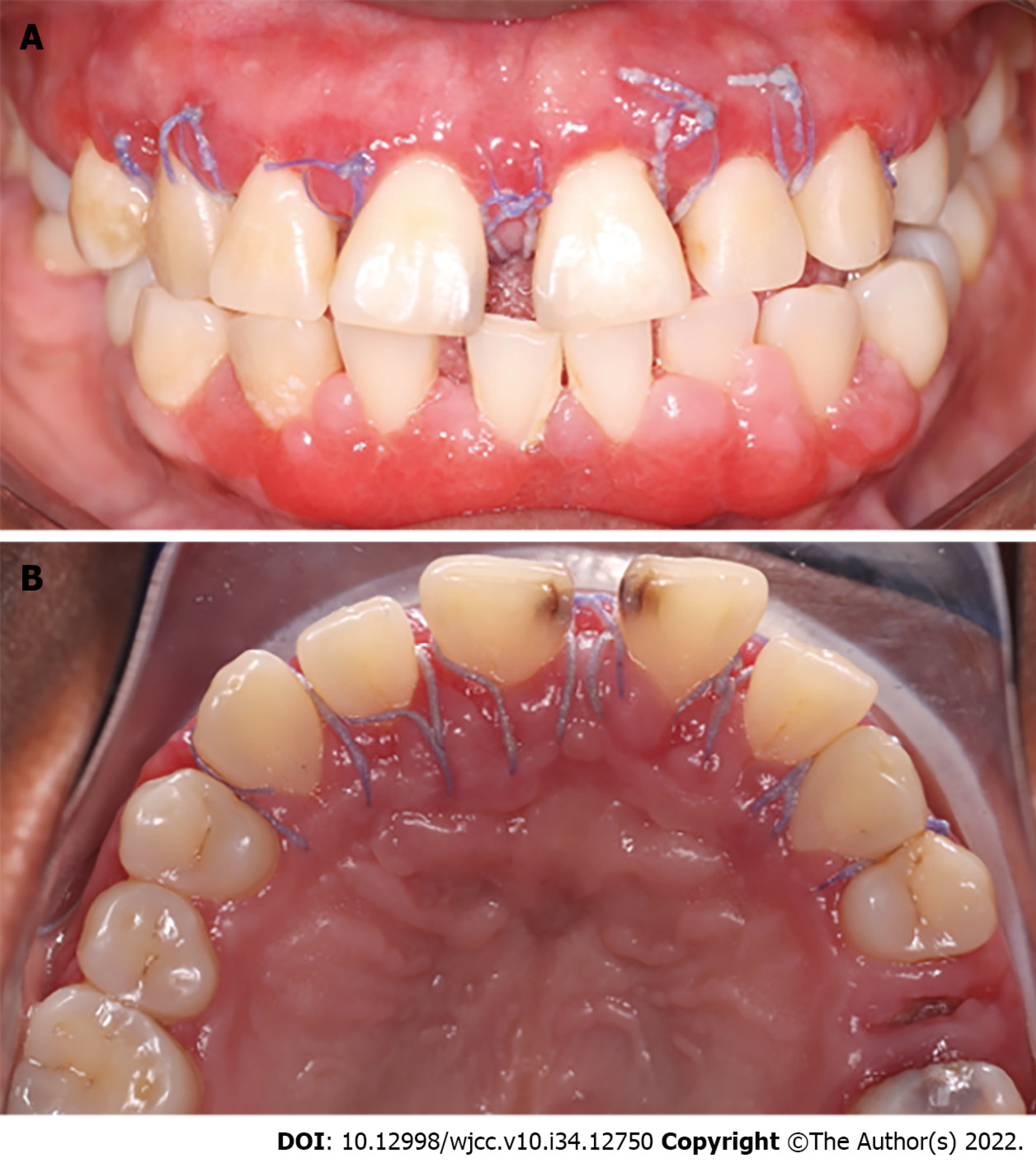
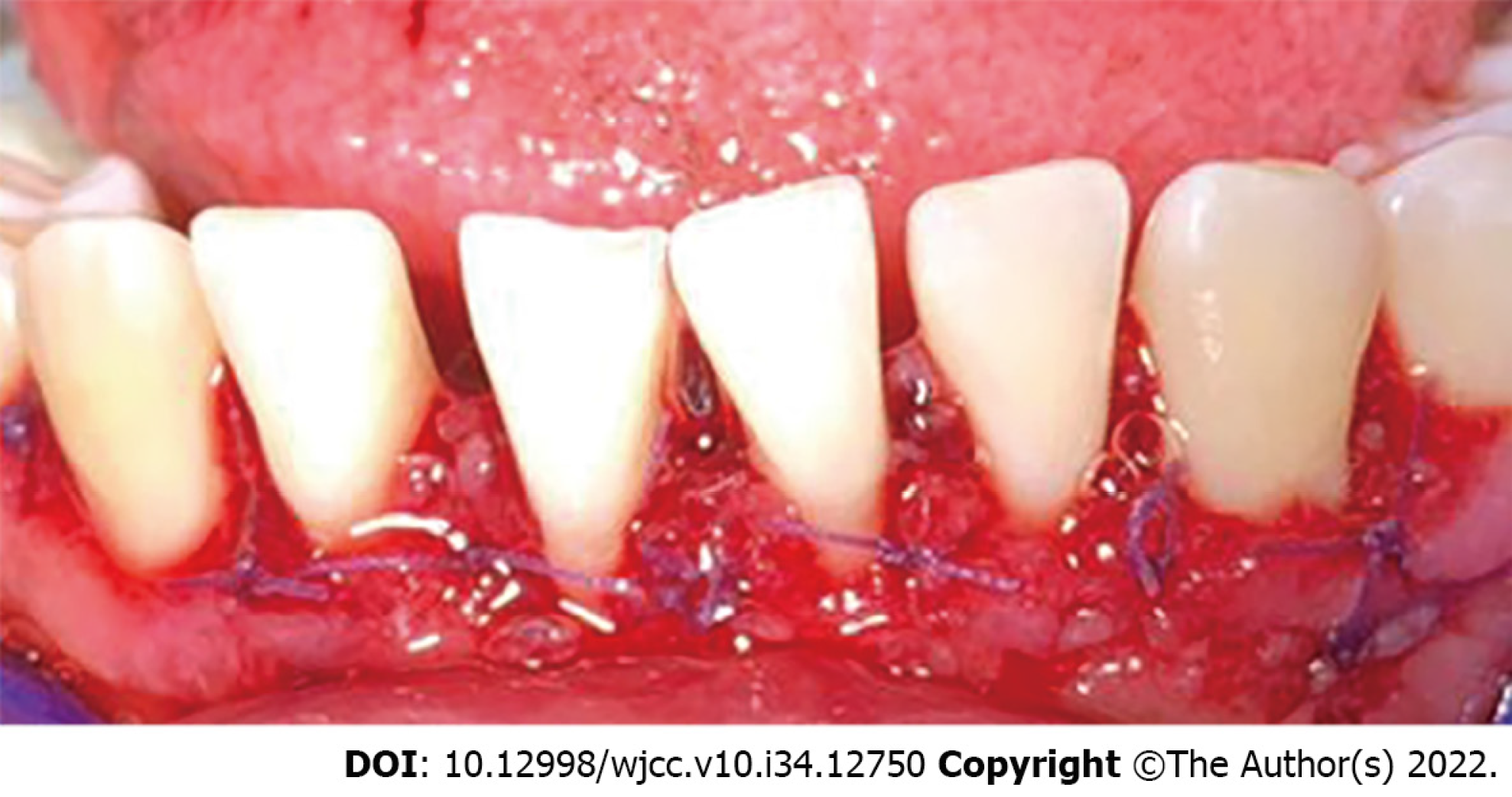
The patient's healing was reviewed at 2 postoperative weeks. When the sutures were removed, the tissue was in good condition, with slight inflammation corresponding to the healing process (Figure 7). At one month, surgery was performed on the lower arch from canine to canine, with the technique previously described, and in the same way, the sutures were removed at 2 wk (Figures 8 and 9). Histopathological analysis was performed at the Center for Research and Diagnosis of Pathology of the University of Guadalajara. The specimen presented fibroconnective tissue growth and pseudo-epithelionomatous hyperplasia. The connective tissue showed large focal areas of chronic inflammatory infiltration and lymphoplasmacytic dispersion in a stroma of collagen fibers and active fibroblasts (Figure 4). These lesions corroborated the presumptive diagnosis of drug-associated GE.
Three months after conclusion of the corrective phase, the periodontal status was evaluated. Inflammation was observed in marginal and papillary areas in teeth 12, 13, 22, 23, 41, 31, 42 and 33, as well as redness and moderate calculus. The maintenance stage was focused on maintaining control of dental biofilm. Therefore, supragingival debridement with ultrasound was performed, and indications of oral hygiene were reinforced. The next maintenance visit was at 6 mo post-surgery, at which period it was seen that the patient's hygiene was in constant control (Figure 10). A comparison of her initial photographs with the most recent photographs at 6 mo of the maintenance phase, revealed the changes obtained through periodontal therapy (Figure 11).
GE is currently defined as "gingival overgrowth" manifested as increase in size (hypertrophy) and increase in number (hyperplasia) of tissue cells. More than 20 prescription drugs are known to be used for GE. These drugs comprise dilanthine group medications, calcium channel blockers, and cyclosporine[11]. Enlargement of gingiva triggers inflammatory reactions in the periodontium, resulting in generation of periodontal disease, or aggravation of existing periodontal disease[6].
Recent studies revealed that the accumulation of gingival fibroblasts observed in drug-associated gingival hyperplasia resulted from inhibition of apoptosis[12]. It has been reported that the pathogenesis of cyclosporine-induced gingival hyperplasia is associated with up-regulated expression levels of proinflammatory cytokines in saliva i.e., interleukin (IL)-1α, IL-8, and IL-6[13].
Various risk factors for GE have been identified. These are plaque control, level of gingival inflammation, extent of periodontal destruction, dose and duration of cyclosporine therapy, plasma and tissue concentrations of cyclosporine and its metabolites, age of the patient, and perhaps the underlying comorbidities[14,15]. Although suspending the use of medication in most cyclosporine-induced GE cases is not a viable option, such was the case with the patient in this study. In previous studies, it was reported that maintenance of adequate oral hygiene in humans markedly reduced the severity of cyclosporine-induced gingival hyperplasia[16,17]. In this study, although a significant reduction in GE was observed after the initial phase, it did not prevent the development of gingival hyperplasia.
Periodontitis is a bacterial infection that, if not treated, may cause systemic complications such as bacteremia, systemic lesions by toxins from oral pathogens, and systemic inflammation due to soluble antigens of oral pathogens[18]. In addition, dental biofilm plays a role as a reservoir of multi-resistant pathogens such as S. aureus, P. aeruginosa, A. baumannii and C. albicans which are associated with severe infections such as meningitis, endocarditis, sepsis and necrotizing fasciitis, especially in immuno-compromised patients[19]. This is relevant because patients with medullary aplasia have altered immune responses due to immunosuppressive therapy. Therefore, the presence of periodontitis may trigger serious opportunistic infections. Therefore, the main objective of the periodontal treatment carried out in the present case was to reduce the pathogenic bacterial load in the oral cavity, in addition to use of initial therapy with rigorous hygiene which made it possible to reduce inflammatory response in gingival tissues[20].
Pharmacological treatments cannot be modified in most cases of severe GE, as this would lead to non-control of the systemic disease. In the case of the patient in this study, treatment modification aggravated the medullary aplasia, resulting in reappearance of the lesions after surgical removal, thereby requiring repeated interventions. Evidence suggests that 34% of cases show recurrence during 18 mo following periodontal surgery, regardless of medication[21]. In our patient’s case, consultation with a hematologist was carried out in a bid to modify the dose of cyclosporine administered, from the initial schedule of one 100-mg capsule three times a day, to one 100-mg capsule every other day. When compared with the initial phase, an important reduction in enlargement was observed. This reveals the importance of adequate multidisciplinary management for GE.
Periodontal therapeutic strategies for GE are generally categorized into initial, systemic, corrective and maintenance phases, depending on the severity and conditions of the disease[21]. The initial phase consists of oral hygiene instructions, dental biofilm control, and supra- and sub-gingival debridement. The aim of the systemic phase was to reduce the influence of cyclosporine on the results of periodontal therapy. This was the reason for which the hematologist reduced the drug dose. The corrective phase allows the return of periodontal tissues to states compatible with health. This phase is necessary when GE is severe or persistent, notwithstanding strict control of the dental biofilm. In this case, surgical correction is suggested. In the present case, the patient was subjected to the two periodontal phases, and at the end of the initial phase, a considerable decrease in GE was observed, the patient’s hygiene control was improved, but the enlargement persisted. The periodontal corrective phase was performed with the flap surgical technique which allowed for reduction of these defects while eliminating excess tissue, thereby resulting in gingival harmony that facilitated oral hygiene to the patient[5,6].
On the other hand, the maintenance phase began as soon as the periodontitis and GE were treated via the initial and corrective phase where inflammation was controlled, destruction of supporting tissues was stopped, and the enlargements in gingival tissue was removed[22]. The goal of this phase was to keep the teeth functional, without pain, excessive mobility, or long-term persistent infection. For this purpose, periodic visits were scheduled for maintenance of excellent oral hygiene, in addition to frequent monitoring of healing process, or recurrence of the disease. In this particular case, the added risk due to the use of immunosuppressive drugs was taken into consideration, since it makes GE patients highly susceptible to recurrence if optimal bacterial oral control is not maintained[23].
Microbial dysbiosis plays a role in GE. However, the accumulation of dental biofilm is another key factor in the severity of this pathology[5,7,8]. This case has revealed that at the end of the periodontal initial phase involving strict control of dental biofilm, administering the same initial dose of cyclosporine allowed the patient to control levels of dental biofilm and inflammation, resulting in a large decrease in GE. Moreover, at the level of soft tissue management, the reductions in inflammation and biofilm positively impacted the next phase of treatment.
Adequate multidisciplinary management of cyclosporine-induced GE patients with medullary aplasia allows for accurate diagnosis and treatment of the disease. The choice of therapeutic strategies depends on the tissue conditions and severity of the systemic disease. In the case studied, the patient´s response to medullary aplasia treatment was favorable. The patient was stable, and she kept appointments regularly. The patient had difficulty in cleaning the interproximal areas of the lower teeth, but in each maintenance appointment, physiotherapy was reinforced.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Yang J, China; Kakarmath SV, India; Tanaka H, Japan S-Editor: Wei ZH L-Editor: A P-Editor: Wei ZH
| 1. | Vallejo Llamas JC. Management of aplastic anemia during the phase of defervescence of the COVID-19 pandemic. Med Clin (Engl Ed). 2021;157:89-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Lorand IG, Souza CA, Caliani JA, Fabron Júnior A. [Medullary aplasia: diagnostic criteria, clinical course and prognostic factors]. AMB Rev Assoc Med Bras. 1983;29:215-218. [PubMed] |
| 3. | Khoori AH, Einollahi B, Ansari G, Moozeh MB. The effect of cyclosporine with and without nifedipine on gingival overgrowth in renal transplant patients. J Can Dent Assoc. 2003;69:236-241. [PubMed] |
| 4. | Hallmon WW, Rossmann JA. The role of drugs in the pathogenesis of gingival overgrowth. A collective review of current concepts. Periodontol 2000. 1999;21:176-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Morales D, González M. E., Rangel L. Agrandamiento Gingival Generalizado en un Paciente con Trasplante Renal. Revista Cubana de Medicina General Integral 2019; 35:658. |
| 6. | Ayanoglou CM, Lesty C. Cyclosporin A-induced gingival overgrowth in the rat: a histological, ultrastructural and histomorphometric evaluation. J Periodontal Res. 1999;34:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Moffitt ML, Bencivenni D, Cohen RE. Drug-induced gingival enlargement: an overview. Compend Contin Educ Dent. 2013;34:330-336. [PubMed] |
| 8. | Nakib N, Ashrafi SS. Drug-induced gingival overgrowth. Dis Mon. 2011;57:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Lauritano D, Palmieri A, Lucchese A, Di Stasio D, Moreo G, Carinci F. Role of Cyclosporine in Gingival Hyperplasia: An In Vitro Study on Gingival Fibroblasts. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Triveni MG, Rudrakshi C, Mehta DS. Amlodipine-induced gingival overgrowth. J Indian Soc Periodontol. 2009;13:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Agnihotri R, Bhat KM, Bhat GS, Pandurang P. Periodontal management of a patient with severe aplastic anemia: a case report. Spec Care Dentist. 2009;29:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Jung JY, Jeong YJ, Jeong TS, Chung HJ, Kim WJ. Inhibition of apoptotic signals in overgrowth of human gingival fibroblasts by cyclosporin A treatment. Arch Oral Biol. 2008;53:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Ruhl S, Hamberger S, Betz R, Sukkar T, Schmalz G, Seymour RA, Hiller KA, Thomason JM. Salivary proteins and cytokines in drug-induced gingival overgrowth. J Dent Res. 2004;83:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Parkar MH, Hussain F, Wickramaratna A, Olsen I. The immunosuppressant and hyperplasia-inducing drug cyclosporin A regulates the cell cycle and cyclin B1 gene expression in gingival fibroblasts in vitro. Cell Tissue Res. 2004;317:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Drozdzik M, Kurzawski M, Drozdzik A, Kotrych K, Banach J, Pawlik A. Interleukin-6 gene polymorphism in renal transplant patients with and without gingival overgrowth. J Clin Periodontol. 2005;32:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Seymour RA, Jacobs DJ. Cyclosporin and the gingival tissues. J Clin Periodontol. 1992;19:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Teshome A, Girma B, Aniley Z. The efficacy of azithromycin on cyclosporine-induced gingival enlargement: Systematic review and meta-analysis. J Oral Biol Craniofac Res. 2020;10:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 19. | Morton RS, Dongari-Bagtzoglou AI. Regulation of gingival fibroblast interleukin-6 secretion by cyclosporine A. J Periodontol. 1999;70:1464-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 21. | Lindhe J, Lang N, Berglundh T, Giannobile W, Sanz M. Lindhe's Clinical Periodontology and Implant Dentistry. 7th ed. 2 Volume Set. Wiley-Blackwell, 2021: 663-761. |
| 22. | Ilgenli T, Atilla G, Baylas H. Effectiveness of periodontal therapy in patients with drug-induced gingival overgrowth. Long-term results. J Periodontol. 1999;70:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Manresa C, Sanz-Miralles EC, Twigg J, Bravo M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst Rev. 2018;1:CD009376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |