Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12295
Peer-review started: June 20, 2022
First decision: August 4, 2022
Revised: August 16, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 26, 2022
Processing time: 155 Days and 12.2 Hours
Polycystic ovary syndrome (PCOS) is an endocrine disease that combines me
After two failed IVF cycles, a metabolic intervention, consisting of a ketogenic diet with daily consumption of 50 g of carbohydrates (CH), was indicated until pregnancy. Metabolic Syndrome was assessed using the Harmonizing Definition (3 of 5 pathologies: Central obesity, hypertension, hyperglycemia, hypertriglyceridemia, and dyslipidemia), and the Homeostatic Model Assessment of IR (HOMA-IR) was used to measure the level of IR. Once IR improved, endometrial quality improved. However, two day 5-thawed embryos (euploid, donated oocyte–partner's sperm) failed to implant, suggesting endometrial quality improvement was insufficient. Therefore, transmyometrial implantation of mesenchymal stem cells from the stromal vascular fraction of adipose tissue was performed to enrich the endometrial stem cell niche. Minimal endometrial mean thickness for embryo transfer (6.9 mm) was achieved three months after stem cell treatment and continuous dietary control of IR. Two euploid-day 5-thawed embryos (donated oocyte–partner's sperm) were transferred, and embryo implantation was confirmed on day 14 by β-hCG serum levels. Currently, a 37 wk baby girl is born.
In PCOS, endometrial quality can be improved by combining nutrient-based metabolic correction with endometrial stem cell niche enrichment.
Core Tip: Polycystic ovary syndrome (PCOS) is an endocrine disease that causes infertility due to ovulation disorders and impaired endometrial receptivity related to a pathological state of insulin resistance (IR). To date, endometrial dysfunctions are the rate-limiting factor for pregnancy in PCOS patients using in vitro fertilization. Here, an overweight PCOS patient with euploid embryos available for transfer achieved pregnancy only after a continuous nutritional intervention to correct IR and metabolic parameters and the enrichment of endometrial stem cell niche with mesenchymal stem cells from adipose tissue. In this case, endometrial thickness and receptivity were improved with a combination of nutritional and surgical interventions to achieve pregnancy.
- Citation: Hernández-Melchor D, Palafox-Gómez C, Madrazo I, Ortiz G, Padilla-Viveros A, López-Bayghen E. Surgical and nutritional interventions for endometrial receptivity: A case report and review of literature. World J Clin Cases 2022; 10(33): 12295-12304
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12295.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12295
Polycystic ovary syndrome (PCOS) is a complicated endocrine disease that combines metabolic, reproductive, and psychological dysfunctions. Ovulation disorders are causes of PCOS-related infertility[1]. In addition, alterations in the ability of the endometrium to accept an implanting embryo and a blastocyst's entry (endometrial receptivity) are critical factors of PCOS-related infertility[2,3]. Numerous studies have proved that hormonal disturbances and metabolic changes in PCOS patients could influence endometrial receptivity[4,5]. Insulin resistance (IR) refers to the complicated path
Stem cells are a powerful tool to respond to the needs of modern medicine, given their high potential in therapeutic applications[10]. Adipose tissue has become a convenient source for stem cell extraction after lipo-aspiration under local anesthesia, yielding significant quantities with a minimum invasive technique, low risk of morbidity, minimal discomfort, and almost zero chance for other possible complications[11]. Adipose-derived mesenchymal stem cells (ADMSC) have prolonged self-renewal ability and the capability to differentiate into various mature somatic lineages[12]. In addition, ADMSC possesses neovascularization, immune-modulating, and anti-inflammatory properties[13]. The Stromal Vascular Fraction (SVF) is the minimum manipulated heterogeneous cell population isolated from the adipose tissue with comparable regenerative potential as cultured ADMSC. SVF contains ADMSC, endothelial precursors, T-regulatory and smooth muscle cells, macrophages, pericytes, and preadi
A 37-year-old woman attended the Ingenes institute in México city for secondary infertility with one previous abortion in 2008 at 11 wk of gestation.
The patient and her current partner had been trying to conceive a pregnancy for two years, with 1 previous attempt in which the embryo implanted but resulted in an early abortion. Her periods are normal, and she does not remember any problems with her cycle. The patient was diagnosed with PCOS according to Rotterdam criteria, presenting signs of clinical hyperandrogenism (acne and hirsutism) and the polycystic ovary phenotype, which was confirmed with ultrasound (30-40 follicles in each ovary). She was counseled for IVF.
There is no relevant history of past illness.
The patient has had a previously failed pregnancy, which resulted in an early abortion, in 2008. Moreover, the patient had uterine synechiae in 2019, which was resolved by hysteroscopic adhesiolysis. She has no other medical history associated with reproduction and no history of alcohol or drug abuse. There was no significant history reported by the partner. Lastly, there was no relevant family history of reproductive complications.
Upon physical examination, the patient did not present any concerning symptoms. The patient's initial body-mass index (BMI) was 29.24 kg/m2. The anthropometric parameters are shown in Table 1. Due to the patient's weight and BMI, it was postulated that the patient has Metabolic Syndrome (MetS). Her waist circumference was elevated (> 80 cm for female Latinas) [18]. She was overweight and had at least one criteria of Metabolic Syndrome, according to the harmonizing definition.
| Categories | First cycle | Second cycle | Third cycle | Fourth cycle |
| Nutritional intervention | None | Caloric restrictiona | Ketogenic dietb | Ketogenic dietc |
| Stem cell treatment | None | None | None | Live cells trans-myometrial injection |
| Anthropometric parameters (at the cycle starting point) | ||||
| Age (years) | 37 (2019) | 38 (2020) | 39 (2021) | 40 (2021) |
| Weight (kg) | 73 | 69 | 59 | 59 |
| Waist circumference (cm) | 81 | 73 | 65 | 65 |
| BMI (kg/m2) | 29.24 | 27.64 | 23.63 | 23.63 |
| Blood pressure (mmHg) | 120/80 | 120/78 | 119/80 | 111/74 |
| Biochemical parameters | ||||
| Fasting glucose (mg/dL) | 104 | 102 | 86 | 78 |
| Urine ketones (mg/dL) | - | Negative | 40-80 | 50 |
| Insulin (U/mL) | - | 13.89 | 3.20 | 2.4 |
| HOMA-IR | - | 2.95 | 0.67 | 0.46 |
| Insulin resistance | - | Yes | Corrected | Corrected |
| Triglycerides (mg/dL) | - | 89 | 92 | 82 |
| HDL-Cholesterol (mg/dL) | - | 58 | 62 | 71 |
| IVF parameters | ||||
| Ova source | Patient | Patient | Donor | Donor |
| Ova collected | 26 | 15 | Not applicable | Not applicable |
| Own embryos frozen (day), quality | 7 (day 5), BC | 1 (day 5), BC | --- | --- |
| 1 (day 6), BC | ||||
| Endometrial intervention/treatment | None | Hysteroscopic evaluation and mild reactivation | Pentoxifylline | Pentoxifylline |
| E-Vitamin | E-Vitamin | |||
| L-Arginine | L-Arginine | |||
| Stem cells | ||||
| Endometrial thickness final size (mm) | 3.6 | 4.3 | 5.9 | 6.9 |
| Transferred thawed embryos and PGTA result | n = 2 | n = 2 | n = 2 | n = 2 |
| Quality: BC | Quality: BC | Quality: AC and BC | Quality: AC and BC | |
| Day 5: Euploid | Day 5 and 6: Euploid | Day 5: Euploid | Day 5: Euploid | |
| Transfer result | Failed | Failed | Failed | Success |
| β-hCG serum levels on day 14 | Negative | Negative | Negative | Positive, 90.89 mUI/mL |
| Clinical pregnancy | - | - | - | One sac, 158 bpm week 18 |
| Pregnancy outcome | - | - | - | Healthy baby girl, 37 wk, 2690 g; 47 cm; Apgar 9 |
Laboratory examinations were performed to assess the patient's metabolic status with respect to the MetS harmonized definition[18]. On initial evaluation, the patient’s fasting plasma glucose suggests that she is at risk for prediabetes. Two of five criteria were abnormal for MetS (waist circumference and fasting plasma glucose); systolic and diastolic blood pressure, tryglycerides, and high-density lipoprotein cholesterol (HDL-C) levels were normal. Values are presented as Biochemical parameters in Table 1.
Due to the patient's previous abortion and uterine synechiae, a sonohysterography was performed. There were no uterine synechiae or other issues associated with the uterus. However, more than 30 follicles on each ovary were detected by ultrasound (PCOS criteria).
There is no multidisciplinary expert consultation.
At the initial consultation, infertility associated with PCOS was diagnosed with IR.
In 2019, the patient underwent standard controlled ovarian stimulation (Gonal 150 UI and Merapur 150 UI). The stimulus was prolonged until the diameter of the leading follicles was > 18 mm. Afterward, recombinant human chorionic gonadotropin was administered. After 36 h, the oocytes were retrieved with ultrasound guidance. All 14-18 mm follicles were aspirated. Ova was collected, fertilized by intracytoplasmatic sperm injection with the partner’s sperm, and then cultured. The yields and quality of embryos are depicted in Table 1. Embryos were cryopreserved using the vitrification technique, thawed, and transferred after standard endometrial preparation. Two embryos were transferred. The endometrial mean thickness (EMT) was sub-optimal, and a thin endometrium was diagnosed (EMT = 3.6 mm). On day 14, there was no detectable amount of β-hCG.
As the patient was overweight and following an unbalanced standard American 2000 calories diet with 45% CHO, 47% fat, and 7% protein content (direct food intake questionnaire), a caloric restriction diet (averaging a total of approximately 1800 calories) was advised with macronutrients adjustment to 55% CHO, 25% protein and 25% fat. This resulted in a 4-kg weight loss but not a BMI reduction to normal weight status. The standard controlled ovarian stimulation protocol was repeated as before. Hysteroscopy was performed because of the thin endometrium diagnosis (EMT=3.6 mm). Again, we ruled out synechiae and found a normal cervical channel, normal uterine cavity, and a visible and permeable ostium; no other abnormalities were found. Gentle endometrial reactivation was performed. Endometrial preparation was carried out using primogyn (estradiol valerate) with incremental dosing for 12 d (2 mg/d for four days, 4 mg/d for four days, and 6 mg/d for four days)[19]. Luteal phase support was carried out with utrogestan (300 mg/vaginal, three times a day). Two cryopreserved embryos were thawed and transferred. On day 14, there was no detectable amount of β-hCG.
Because the patient's initial BMI was 29.24 kg/m2 and PCOS diagnosis, metabolic parameters were assessed (Table 1). Triglycerides and HDL-C were within the normal range. Fasting glucose was above the normal range, suggesting the patient was prediabetic. In addition, IR was calculated using HOMA-IR (Table 1). The patient had significant IR. A nutritional intervention was started to correct for IR, with a secondary goal to increase endometrium and ova quality. The patient was instructed to follow a ketogenic diet, which typical consists of 1800 calories/day, daily consumption of ≤ 50 g of total CHO (15% or less), 1.5 g of protein per kilogram of body weight (25% maximum), with the remainder being fat (approximately 60%). The patient auto-registered all food consumption in the MyFitnessPal app, and the dietician confirmed the macros every week, correcting them when needed. In addition, the patient received nutritional education (video) that focused on controlling the glucose load. The purpose was to teach food choices and avoid ultra-processed food, starches, juices, bread, sweets, sugared beverages, and CHO-rich food (rice, beans, tortilla, legumes). The patient was followed via electronic messaging every two to three days, inquiring about any doubts, symptoms, changes in weight, fasting glucose, ketone detection in urine, and counseling about the general guidelines. Before the start and during all nutritional interventions, the patient auto-monitored their urinary ketone levels using the Ketone Test Strips (acetoacetate), measuring at least twice a week with MUNDO-Keto reactive strips. Once, laboratory tests confirmation of improve IR and with the patient's approval, the physician and the specialist in Reproductive Medicine moved to ova donation. Donated oocytes were fertilized by ICSI with the partner's sperm, which produced four embryos (two day 5 and two day 6 embryos). Before all embryos were vitrified, Preimplantation Genetic Testing for Aneuploidies (PGT-A) was performed following a standardized protocol[20]. Endometrial preparation consisted of Primogyn (same as described before) while adding 400 mg of Pentoxifylline twice a day, 1 g of L-Arginine (vasodilator), and 1 g of Vitamin E daily (vascular and antioxidant effects), which improved the EMT (Table 1). Embryo transfer was performed with one embryo AC and one embryo BC. Both embryos failed to implant.
Autologous mesenchymal stem cells in the SVF of adipose tissue were placed in the patient's uterine cavity to improve endometrial quality. Micro liposuction was performed to obtain 20 mL of abdominal fat. Adipose tissue was washed, mechanically disaggregated, and treated with collagenase type I to isolate SVF. A total of 6.27 × 107 live cells were isolated and transmyometrial injected. Changes in the EMT were monitored for three months until an EMT of 6.9 mm was reached. Two Day 5, euploid-thawed embryos (from oocyte donor and partner's sperm) were transferred. The uterine transfers occurred during a controlled endometrial development cycle for frozen embryos, free of gonadotropin stimulation, but with the addition of Pentoxifylline, L-Arginine, and E-Vitamin (same as described before). The ketogenic intervention was sustained until pregnancy was achieved. Embryo implantation was determined on day 14 by serum β-hCG concentrations (>10 mUI/mL) and by the presence of a fetal heartbeat using ultrasound at six weeks (Table 1).
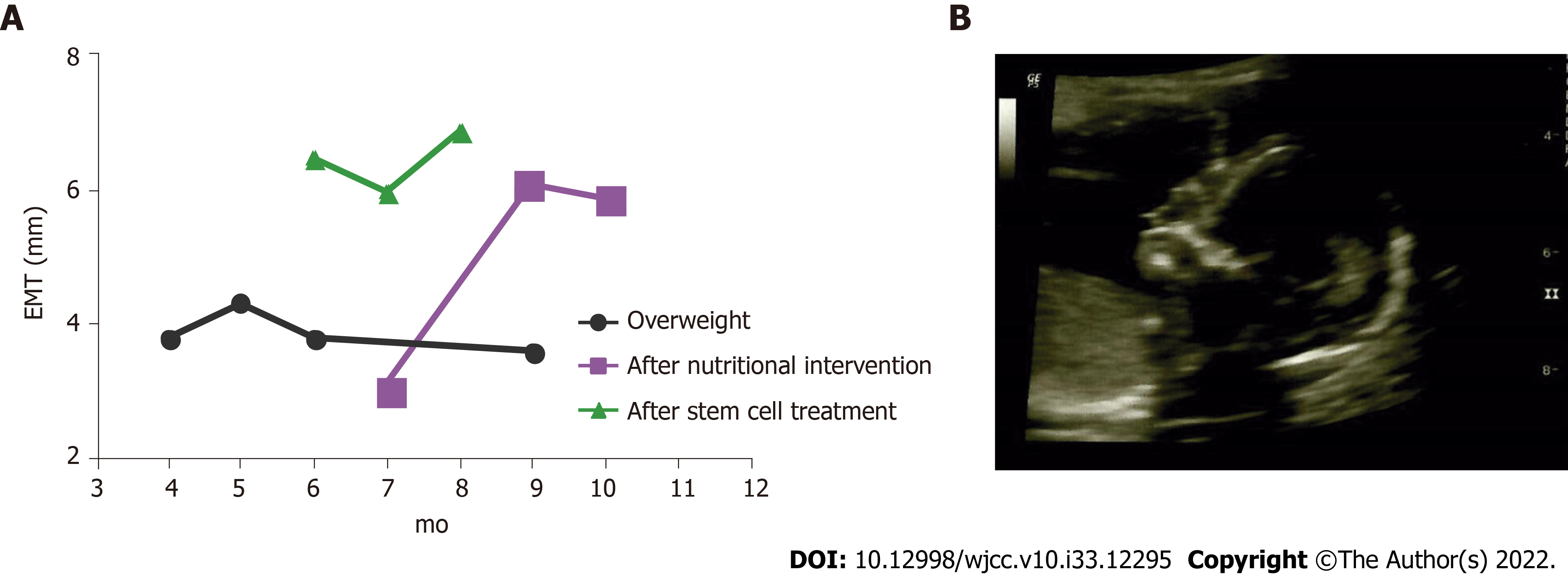
Table 1 depicts all results and details of the interventions during the four IVF cycles. After induction and maintenance of nutritional ketosis, IR was corrected. As a result, BMI decreased to 23.63 kg/m2 and the final fasting glucose was 78 mg/dL. Figure 1A shows the changes in the EMT after the two major interventions: nutritional ketosis and endometrial regeneration. Pregnancy was finally achieved after a continuous dietary intervention, which resulted in metabolic normalization when complemented with endometrial reparation using mesenchymal stem cells and the transfer of euploid embryos from donated ova. One gestational sac with a fetal heartbeat was detected via the ultrasound at week 6. The gestational sac presented with 158 beats per minute, normal amniotic liquid, and the absence of ultrasound markers for chromosomopathies. No apparent structural alterations were detected at week 18 (Figure 1B). Metabolic control continued to avoid the risk of gestational diabetes with the proper caloric increase by trimester and 100 g - 150 g of CHO for daily consumption. Pregnancy resulted in the born of a healthy baby girl at 37 wk (2690 g; 47 cm; Apgar 9).
In a PCOS-related infertility case, a patient achieved pregnancy after combining interventions, specifically implementing a ketogenic diet, and improving the endometrium receptivity with mesenchymal stem cells. The most concerning problem with PCOS is infertility at the reproductive age, as this degenerative disease predisposes women to reproductive complications and possible infertility. Lower pregnancy rates are observed in obese PCOS compared to non-obese PCOS patients[21,22], demonstrating a crucial metabolic component for PCOS women concerning fertility. IR-PCOS patients develop sub-optimal oocytes, with fewer MII oocytes[23]. Moreover, IR-PCOS patients have lower pregnancy rates after IVF, even if oocyte development, embryo quality[7], or risk for embryonic aneuploidy was not affected[24], suggesting that the effects of IR on endometrial function and embryo implantation underlie the decreased pregnancy rates[7].
Evidence in adult women indicated that treatment of IR, either by lifestyle changes or pharmacological support, improves reproductive and metabolic abnormalities[25]. Thus, it was expected that the nutritional intervention would improve the reproduction potential of our PCOS patients. IR is mainly caused due to a constant glucose overload, leading to continuous hyperinsulinemia; therefore, restricting glucose exposure via dietary modifications should improve IR and reproductive outcomes for PCOS women[26]. Therefore, it is crucial to determine a subject’s IR status before considering any intervention containing a diet. However, not all diet modifications are optimal for PCOS. Here, a low caloric diet with standard macro nutrients distribution improved weight but did not correct IR (Table 1). Diets with lower carbohydrate content are more likely to improve IR in PCOS women with severe IR[19,27,28]. In this case, a diet modification with CHO limitation to a maximal daily consumption of 50 g successfully corrected IR and improved pregnancy chances. Even when Metformin is widely used for IR as an important insulin sensitizer, a recent meta-analysis shows that Metformin does not improve insulin sensitivity over hypocaloric diets in women with polycystic ovary syndrome[19]. When pre- and post-intervention values for fasting plasma glucose, fasting plasma insulin, and IR indices (HOMA-IR, ISI, and QUICKI) were compared, any benefit of using Metformin was already achieved when a diet intervention was implemented. This means that adding Metformin to hypocaloric diets did not improve serum glucose or insulin concentrations, or IR in PCOS women, but controlling the CHO intake does.
In PCOS patients, endometrial tissue function is altered due to abnormal glucose homeostasis and insulin action[29], presumably due to GLUT4 alterations. GLUT4 is the main glucose transporter in charge of glucose uptake at the cellular level, regulated by insulin through protein synthesis and translocation. Hyperinsulinemia and PCOS are conditions associated with decreased GLUT4 expression at the endometrial level[30]. Lifestyle modification (physical exercise and low CHO/high protein diet) improves glucose homeostasis in PCOS patients. Consequently, the endometrial function is restored due to GLUT4 down-regulation, resulting from the up-regulation of endometrial IRS1 and GLUT1[31]. In support of this, we show that a continuous and closely supervised nutritional intervention resulted in total correction in IR, complete normalization of metabolic parameters, and improved endometrial growth. Improving endometrial function may be a necessary approach for PCOS-related infertility[9], as for improving metabolic health was not enough to resolve the patient’s reproductive issues. The uterine lining in our patient improved after the nutritional intervention (Figure 1), with the EMT consistently increasing above 6 mm. Nevertheless, considering past failed embryo transfers and lack of abnor
Three mechanisms of action have been proposed for stem cell therapy to improve endometrial quality in the injured uterus of murine models: (1) Stem cell engraftment followed by trans-differentiation; (2) Environment modulation through trophic factors; and (3) Angiogenesis promotion. First, mesenchymal stem cells are highly proliferative cells that can transdifferentiate into various non-hematopoietic cell types. This differentiation potential in transplanted bone marrow-derived stem cells allows stem cell engraftment in the uterus, then differentiation into an endometrial phenotype expressing vimentin and lacking CD45 expression[36]. Second, LIF and integrins are regulators of endometrial function, markers for endometrial receptivity, and essential proteins for embryo implantation. Expression of cytokeratin, vimentin, integrin αγβ3, and LIF significantly increased after bone marrow-derived stem cells were transplanted, mimicking levels found in normal conditions, suggesting that stem cell treatments improve endometrial thickness but also contribute to endometrial receptivity[37]. Last, human endometrial mesenchymal stem cells derived from menstrual blood (MenSCs) have been demonstrated to promote angiogenesis in treating an endometrial injury. Newly formed blood vessels were observed after MenSCs were transplanted in vivo under the control of the AKT and ERK signaling pathways, suggesting that revascularization and angiogenesis can improve the injured endometrium. Furthermore, this revascularization process allows paracrine signaling (cytokines and growth factors) to repair injured tissues[38]. SVF is a minimum manipulated heterogeneous cell pool containing ADMSC and is efficiently obtained via minimal liposuction. Its use has been extended and represents a convenient source for stem cells[39]. Considering its therapeutic potential, comparable to cultured ADSC[14], we propose a surgical intervention to implant SVF-containing stem cells into the patient's uterus to achieve minimal EMT for embryo transfer. Three months after stem cell treatment, endometrial thickness improved up to 6.9 mm, allowing successful embryo implantation and pregnancy. We assume that more than one of the proposed mechanisms for stem cell therapy helped in our case. The endometrium thickness was improved, and receptivity and paracrine signaling were boosted, allowing successful embryo implantation.
Our study has two significant limitations. First, the patient’s advanced maternal age. The effect age has on the result presented here still needs to be investigated. Nevertheless, we demonstrate that for certain circumstances, the use of a ketogenic diet and stem cell treatment maybe required for even the transfer euploid embryos. Lastly, the quality of the SVF was not specifically measured, with respect to the other components of the fluid. It is possible other components, such as endothelial precursors, macrophages, pericytes, and preadipocytes as well as the concentration of cytokine and adipokine, could improve or inhibit the effect present here.
We show that a continuous nutritional intervention resulted in correction of IR, normalization of metabolic parameters, and improved endometrial growth. Furthermore, stem cell treatment improved endometrial quality concerning endometrial thickness and receptivity. We conclude that correcting IR in PCOS patients may improve reproductive outcomes and stem cell treatment, using SVF, could become a handy intervention to improve endometrial receptivity.
We want to express our gratitude to this study's participant and the IVF and medical staff at Ingenes SC and Regenera SC. In addition, we would like to thank Dr. Leonardo M P for his critical reading of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Reproductive biology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elfayoumy KN, Egypt; Shuang W, China S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Niño OMS, da Costa CS, Torres KM, Zanol JF, Freitas-Lima LC, Miranda-Alves L, Graceli JB. High-refined carbohydrate diet leads to polycystic ovary syndrome-like features and reduced ovarian reserve in female rats. Toxicol Lett. 2020;332:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Bai X, Zheng L, Li D, Xu Y. Research progress of endometrial receptivity in patients with polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol. 2021;19:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Lopes IM, Baracat MC, Simões Mde J, Simões RS, Baracat EC, Soares JM Jr. Endometrium in women with polycystic ovary syndrome during the window of implantation. Rev Assoc Med Bras (1992). 2011;57:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ejzenberg D, Gomes TJO, Monteleone PAA, Serafini PC, Soares-Jr JM, Baracat EC. Prognostic factors for pregnancy after intrauterine insemination. Int J Gynaecol Obstet. 2019;147:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Giordano MV, Giordano LA, Gomes RC, Simões RS, Nader HB, Giordano MG, Baracat EC, Soares Júnior JM. The evaluation of endometrial sulfate glycosaminoglycans in women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Qi J, Wang W, Zhu Q, He Y, Lu Y, Wang Y, Li X, Chen ZJ, Sun Y. Local Cortisol Elevation Contributes to Endometrial Insulin Resistance in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2018;103:2457-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Chang EM, Han JE, Seok HH, Lee DR, Yoon TK, Lee WS. Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation-in vitro fertilization-embryo transfer cycle. Clin Endocrinol (Oxf). 2013;79:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Liu Y, Li J, Yan Z, Liu D, Ma J, Tong N. Improvement of Insulin Sensitivity Increases Pregnancy Rate in Infertile PCOS Women: A Systemic Review. Front Endocrinol (Lausanne). 2021;12:657889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Jiang NX, Li XL. The Disorders of Endometrial Receptivity in PCOS and Its Mechanisms. Reprod Sci. 2022;29:2465-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 11. | Sudoma I, Pylyp L, Kremenska Y, Goncharova Y. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J Stem Cell Ther Transplant. 2019;3:1-8. [DOI] [Full Text] |
| 12. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12636] [Article Influence: 702.0] [Reference Citation Analysis (2)] |
| 13. | Feisst V, Meidinger S, Locke MB. From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning. 2015;8:149-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: A regenerative reality? J Plast Reconstr Aesthet Surg. 2016;69:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Ogawa R. The importance of adipose-derived stem cells and vascularized tissue regeneration in the field of tissue transplantation. Curr Stem Cell Res Ther. 2006;1:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: A novel cell based therapy. J Hum Reprod Sci. 2014;7:93-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, Palmero J, Remohí J, Pellicer A, Simón C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman's syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 18. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10517] [Article Influence: 657.3] [Reference Citation Analysis (0)] |
| 19. | Garcia-Hernandez SC, Porchia LM, Pacheco-Soto BT, López-Bayghen E, Gonzalez-Mejia ME. Metformin does not improve insulin sensitivity over hypocaloric diets in women with polycystic ovary syndrome: a systematic review of 12 studies. Gynecol Endocrinol. 2021;37:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 20. | Schaeffer E, López-Bayghen B, Neumann A, Porchia LM, Camacho R, Garrido E, Gómez R, Camargo F, López-Bayghen E. Whole Genome Amplification of Day 3 or Day 5 Human Embryos Biopsies Provides a Suitable DNA Template for PCR-Based Techniques for Genotyping, a Complement of Preimplantation Genetic Testing. Biomed Res Int. 2017;2017:1209158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Tziomalos K, Dinas K. Obesity and Outcome of Assisted Reproduction in Patients With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2018;9:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Bailey AP, Hawkins LK, Missmer SA, Correia KF, Yanushpolsky EH. Effect of body mass index on in vitro fertilization outcomes in women with polycystic ovary syndrome. Am J Obstet Gynecol. 2014;211:163.e1-163.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Hassani F, Oryan S, Eftekhari-Yazdi P, Bazrgar M, Moini A, Nasiri N, Ghaheri A. Association between The Number of Retrieved Mature Oocytes and Insulin Resistance or Sensitivity in Infertile Women with Polycystic Ovary Syndrome. Int J Fertil Steril. 2019;12:310-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 24. | Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Marshall JC, Dunaif A. Should all women with PCOS be treated for insulin resistance? Fertil Steril. 2012;97:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 26. | Hajishafiee N, Ghiasvand R, Feizi A, Askari G. Dietary patterns and ovulatory infertility: a case-control study. Journal of Nutritional Sciences and Dietetics. 2017;3. [DOI] [Full Text] |
| 27. | Alwahab UA, Pantalone KM, Burguera B. A ketogenic diet may restore fertility in women with polycystic ovary syndrome: a case series. AACE Clin Case Rep. 2018;4:e427-e431. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Mavropoulos JC, Yancy WS, Hepburn J, Westman EC. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutr Metab (Lond). 2005;2:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Oróstica L, Astorga I, Plaza-Parrochia F, Vera C, García V, Carvajal R, Gabler F, Romero C, Vega M. Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int J Obes (Lond). 2016;40:1715-1722. [PubMed] [DOI] [Full Text] |
| 30. | Mioni R, Chiarelli S, Xamin N, Zuliani L, Granzotto M, Mozzanega B, Maffei P, Martini C, Blandamura S, Sicolo N, Vettor R. Evidence for the presence of glucose transporter 4 in the endometrium and its regulation in polycystic ovary syndrome patients. J Clin Endocrinol Metab. 2004;89:4089-4096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Ujvari D, Hulchiy M, Calaby A, Nybacka Å, Byström B, Hirschberg AL. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33:1883-1888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 218] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 33. | Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 34. | Cousins FL, Pandoy R, Jin S, Gargett CE. The Elusive Endometrial Epithelial Stem/Progenitor Cells. Front Cell Dev Biol. 2021;9:640319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani AH, Gargett CE. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front Cell Dev Biol. 2020;8:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 36. | Lee YJ, Yi KW. Bone marrow-derived stem cells contribute to regeneration of the endometrium. Clin Exp Reprod Med. 2018;45:149-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, Zhang S. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152:389-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5752] [Article Influence: 239.7] [Reference Citation Analysis (0)] |