Published online Nov 26, 2022. doi: 10.12998/wjcc.v10.i33.12175
Peer-review started: July 24, 2022
First decision: August 22, 2022
Revised: September 13, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 26, 2022
Processing time: 121 Days and 22.2 Hours
Optic nerve sheath diameter (ONSD) measurement is one of the non-invasive methods recommended for increased intracranial pressure (ICP) monitoring.
This study aimed to evaluate the roles of optic nerve sheath diameter (ONSD) and ONSD/eyeball transverse diameter (ETD) ratio in predicting prognosis of death in comatose patients with acute stroke during their hospitalization.
A total of 67 comatose patients with acute stroke were retrospectively recruited. The ONSD and ETD were measured by cranial computed tomography (CT) scan. All patients underwent cranial CT scan within 24 h after coma onset. Patients were divided into death group and survival group according to their survival status at discharge. The differences of the ONSD and ONSD/ETD ratio between the two groups and their prognostic values were compared.
The ONSD and ONSD/ETD ratio were 6.07 ± 0.72 mm and 0.27 ± 0.03 in the comatose patients, respectively. The ONSD was significantly greater in the death group than that in the survival group (6.32 ± 0.67 mm vs 5.65 ± 0.62 mm, t = 4.078,
The mortality increased in comatose patients with acute stroke when the ONSD was > 5.7 mm or the ONSD/ETD ratio was > 0.25. Both indexes could be used as prognostic tools for comatose patients with acute stroke. The ONSD/ETD ratio was more stable than the ONSD alone, which would be preferred in clinical practice.
Core Tip: Optic nerve sheath diameter (ONSD) and ONSD/eyeball transverse diameter (ETD) were correlated with intracranial pressure and prognosis. This study aimed to evaluate the roles of ONSD and ONSD/ETD ratio in predicting prognosis of death in comatose patients with acute stroke during hospitalization. Total 67 comatose patients were retrospectively recruited. ONSD and ETD were measured by cranial computed tomography scans within 24 h after coma onset. It was found that the mortality increased when ONSD > 5.7 mm or ONSD/ETD ratio > 0.25. The ONSD/ETD ratio was more stable than ONSD alone, which be preferred in clinical practice.
- Citation: Zhu S, Cheng C, Wang LL, Zhao DJ, Zhao YL, Liu XZ. Prognostic values of optic nerve sheath diameter for comatose patients with acute stroke: An observational study. World J Clin Cases 2022; 10(33): 12175-12183
- URL: https://www.wjgnet.com/2307-8960/full/v10/i33/12175.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i33.12175
Stroke is a leading cause of death and disability worldwide[1]. As the world’s largest developing country, China had the largest number of incident cases and deaths related to stroke[2]. Stroke is mainly accompanied by an increased intracranial pressure (ICP), leading to more severe brain damage via secondary cerebral ischemia and hernia[3]. Coma is the most severe disturbance of consciousness. A sudden rise of ICP is typically observed in comatose patients with acute stroke. Therefore, ICP monitoring is of particular importance for comatose patients.
In recent years, non-invasive ICP monitoring methods have been developed, such as transcranial doppler ultrasound, cerebral blood flow velocity measurement, venous intraocular pressure measure
Optic nerve sheath is a continuation of the dura mater and contains a subarachnoid space. It is continuous with the intracranial subarachnoid space, and an increased ICP can be directly transmitted to the optic nerve sheath[4,5]. Previous studies have shown that computed tomography (CT) can be used to accurately measure ONSD. The strong correlation between ICP and ONSD has been generally identified[6-8]. Therefore, ONSD measurement is regarded as a reliable and noninvasive tool for ICP monitoring. However, the ONSD of normal and sick individuals mainly varies noticeably, indicating a large SD range. In subsequent studies, it was found that the ONSD was strongly correlated with eyeball transverse diameter (ETD) in healthy individuals[9]. The ONSD/ETD ratio slightly varies, indicating a small SD range. Therefore, the ONSD/ETD ratio has a smaller variability and a higher stability, and it may be more appropriate for ICP monitoring[7,10]. To date, several studies have shown that the ONSD/ETD ratio is more related to ICP than the ONSD[6,7,10]. Therefore, it is reasonable to speculate that the ONSD/ETD ratio has a higher value in predicting the prognosis of neurological function. Previous studies have largely concentrated on the correlation between the ONSD and prognosis. It has been reported that the ONSD can help predict the prognosis of patients with cerebral hemorrhage (CH)[11]. However, there are few studies on the prognostic role of the ONSD/ETD ratio in stroke.
Unenhanced cranial CT scan is the most common examination for acute stroke, especially for comatose patients, which can rapidly identify the type of stroke and determine the scope of associated structural changes. In the current study, unenhanced cranial CT scan was used to measure the ONSD and ETD, and the prognostic values of the ONSD and ONSD/ETD ratio in comatose patients with acute stroke were compared.
Comatose patients with acute stroke who were admitted to the neurological intensive care unit of Peking University International Hospital (Beijing, China) from August 2015 to September 2021 were retrospectively enrolled. The inclusion criteria were as follows: (1) Patients who were aged ≥ 18 and ≤ 80 years old; (2) Meeting the diagnostic criteria of acute cerebral infarction (ACI), CH, and spontaneous subarachnoid hemorrhage (SAH); and (3) Comatose patients with glasgow coma scale (GCS) scores ≤ 8 upon admission. The exclusion criteria were as follows: (1) Patients with a history of glaucoma, thyroid-associated ophthalmopathy, or optic neuropathy; (2) Patients with acute brain stem and cerebellar strokes, and a history of SAH; and (3) Severe complications (e.g., hematological diseases and tumors) that might affect life expectancy. This retrospective study was approved by the Ethics Committee of Peking University International Hospital [Approval No. 2021-001 (BMR)].
Patients' baseline clinical data were collected, including age, gender, body weight, body mass index (BMI), mean arterial pressure (MAP), stroke type, GCS scores, surgery during hospitalization (e.g., clearance of hematoma and decompressive craniectomy), and the survival status at discharge (alive or dead).
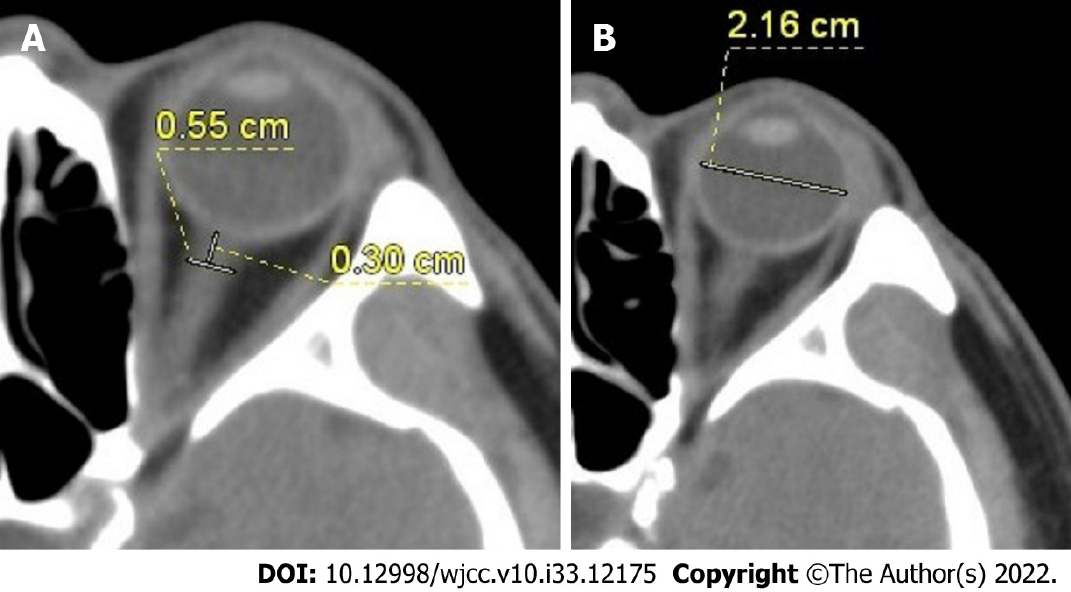
CT scan was performed using a 64-row spiral CT scanner (Siemens, Munich, Germany), and the scanning parameters were as follows: Tube voltage of 120 kV, tube current of 200-300 mA, slice thickness of 2 mm, slice interval of 3 mm, and pitch of 1. All patients received cranial CT scan within 24 h upon coma onset. The ONSD and ETD were measured by two experienced radiologists, who were blinded to patients' clinical data. The ONSD and ETD were measured at fixed mediastinal window setting (width, 300; level, 35). The direction of the optic nerve was determined by three-dimensional reconstruction of CT data. The ONSD was measured vertically at 3 mm behind the eyeball (Figure 1A). ETD was measured from one side of the retina behind the lens to the other side for the maximum diameter (Figure 1B). The values measured by two radiologists were averaged. The ONSD and ETD were measured bilaterally, and their average was taken for each case to calculate the ONSD/ETD ratio.
The statistical analysis was performed using MedCalc 19.6 software (MedCalc Software Ltd., Ostend, Belgium). Continuous variables were expressed as mean ± SD. Categorical variables were expressed as count (percentage). Death was set to 0 and survival to 1 depending on the patient's survival status at discharge. The χ2 test was used for the statistical analysis of categorical variables, such as gender, GCS, and stroke type. The independent-samples t-test was applied for the statistical analysis of continuous variables, including age, body weight, BMI, ONSD, and ONSD/ETD ratio. The Mann-Whitney U test was utilized for the statistical analysis of height and MAP. The ONSD and ONSD/ETD ratio were included in the logistic regression model for multivariate analysis. The diagnostic performance of the ONSD and ONSD/ETD ratio was assessed by the receiver operating characteristic (ROC) curve analysis. All tests were two-sided, and P < 0.05 was considered statistically significant.
A total of 67 comatose patients with acute stroke were included. The baseline characteristics of the comatose patients and healthy controls at baseline are summarized in Table 1. In the ACI group, 3 patients (23%) underwent decompressive craniectomy. In the CH group, 15 patients (38%) received hematoma evacuation. In the SAH group, 1 patient (7%) underwent ventricular puncture and drainage.
| Characteristics | Value |
| Age (yr) | 59.72 ± 16.72 |
| Gender, male, n (%) | 32 (47.76) |
| Height (cm) | 164.88 ± 7.93 |
| Body weight (kg) | 65.86 ± 13.59 |
| BMI | 23.62 ± 4.43 |
| MAP | 89.03 ± 23.25 |
| Stroke type | |
| ACI | 13 (19.4) |
| CH | 39 (58.2) |
| SAH | 15 (22.4) |
| Stroke causes | |
| ACI | |
| Atherosclerosis | 6 (46.2) |
| Cardiogenic cerebral embolism | 4 (30.8) |
| Moyamoya disease | 3 (23.1) |
| CH | |
| Hypertension | 24 (61.5) |
| Vascular malformation | 13 (33.3) |
| Amyloidosis | 2 (5.1) |
| SAH | |
| Aneurysm | 11 (73.3) |
| Vascular malformation | 4 (26.7) |
| Mortality | 42 (62.69) |
| ACI | 10 (76.92) |
| CH | 18 (46.2) |
| SAH | 14 (93.3) |
| Surgery | 19 (28.36) |
| ACI | 3 (23) |
| CH | 15 (38) |
| SAH | 1 (7) |
In this study, 42 (62.7%) patients died, while 25 (37.3%) patients survived at discharge. As shown in Table 2, there were significant differences in the GCS score (χ2 = 49.809, P < 0.0001), stroke type (χ2 = 11.981, P = 0.003), ONSD (t = 4.078, P < 0.0001), ONSD/ETD ratio (t = 4.625, P < 0.0001), and surgery (χ2 = 4.803, P = 0.048) between the two groups.
| Parameter | Death group (n = 42) | Survival group (n = 25) | χ2/t/Z value | P value |
| Age (yr) | 62.3 ± 14.7 | 56.1 ± 18.9 | 1.405 | 0.165 |
| Gender, male, n (%) | 21 (50.0) | 11 (44.0) | 0.226 | 0.801 |
| Height (cm) | 164.1 ± 8.1 | 166.6 ± 8.0 | -1.296 | 0.195 |
| Body weight (kg) | 64.7 ± 14.3 | 67.8 ± 13.6 | -0.823 | 0.414 |
| BMI | 23.6 ± 3.5 | 23.6 ± 5.9 | 0.006 | 0.995 |
| MAP | 80.3 ± 18.8 | 103.5 ± 26.0 | -3.585 | < 0.0001 |
| GCS score | 49.809 | < 0.0001 | ||
| 3 | 33 | 0 | ||
| 4 | 3 | 2 | ||
| 5 | 3 | 4 | ||
| 6 | 0 | 4 | ||
| 7 | 1 | 5 | ||
| 8 | 2 | 10 | ||
| Stroke | 11.981 | 0.003 | ||
| ACI | 10 | 3 | ||
| CH | 18 | 21 | ||
| SAH | 14 | 1 | ||
| ONSD (mm) | 6.32 ± 0.67 | 5.65 ± 0.62 | 4.078 | < 0.0001 |
| ONSD/ETD | 0.28 ± 0.03 | 0.25 ± 0.02 | 4.625 | < 0.0001 |
| Surgery, n (%) | 8 (19.0) | 11 (44.0) | 4.803 | 0.048 |
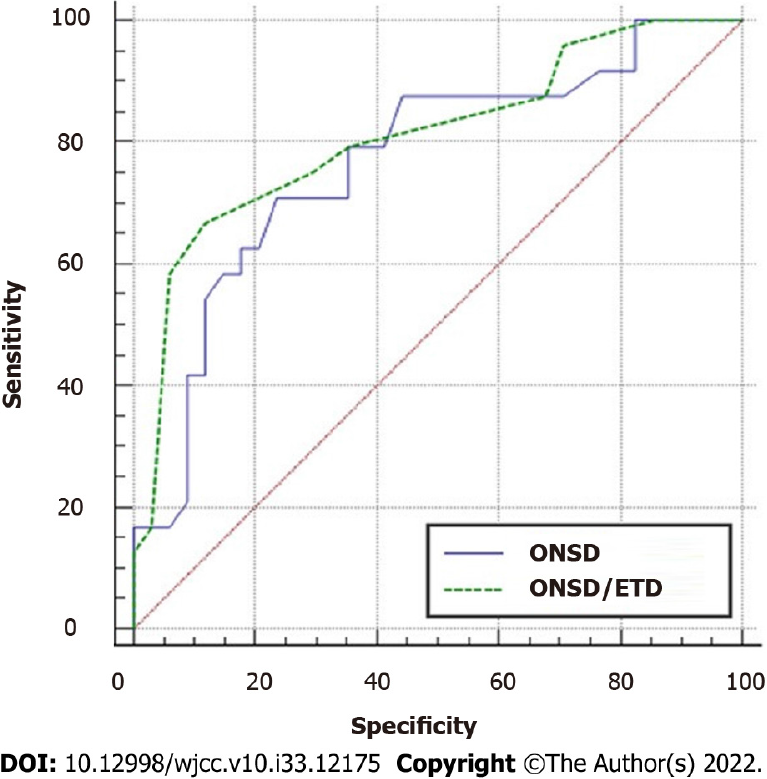
The ONSD and ONSD/ETD ratio were included in the logistic regression model for multivariate analysis, respectively. After adjusting for age, MAP, GCS score, stroke type, and surgery, the associations among the ONSD (P = 0.04), ONSD/ETD ratio (P = 0.036), and mortality were still significant. The performance of the ONSD and ONSD/ETD ratio in predicting prognosis of comatose patients with acute stroke is shown in Figure 2. The area under the curve (AUC) of the ONSD was 0.760 (95%CI: 0.637-0.882, P < 0.0001), with a sensitivity of 81.0% and a specificity of 64.0% at a cut-off value of 5.7 mm. The AUC of the ONSD/ETD ratio was 0.808 (95%CI: 0.696-0.920, P < 0.0001), with a sensitivity of 92.9% and a specificity of 68.0% at a cut-off value of 0.25. The AUC values of the ROC curves of the ONSD and ONSD/ETD ratio were compared. There was no significant difference in the AUC values of the two indices (Z = 1.333, P = 0.1826).
In the present study, the ONSD measured in patients who died was 6.32 ± 0.67 mm, while that in patients who were alive was 5.65 ± 0.62 mm. The ONSD/ETD ratio was 0.28 ± 0.03 in the death group compared with 0.25 ± 0.02 in the survival group. These two parameters had almost the same predictive performance for prognosis of comatose patients with acute stroke.
A large number of studies on the ONSD for ICP assessment have been published since 1996. The direct relationship between the increased ICP and ONSD in patients with traumatic and nontraumatic brain injuries was reported[7,8,12]. However, the normal ranges and critical values of ICP and the ONSD for predicting the prognosis of such patients have still remained elusive. Jenjitranan et al[13] showed that the cut-off value of the ONSD for predicting the increased ICP on cranial CT was 4.8 mm. Vaiman et al[6] found that ONSD > 5.5 mm on CT could predict an increased ICP in patients with hemorrhagic stroke. A study on CH showed that the ONSD was 6.40 ± 0.70 mm in patients with an increased ICP compared with 4.70 ± 0.40 mm in those without an increased ICP, indicating a significant difference[14]. In another study, the ONSD was measured by ultrasound to predict early prognosis of patients with stroke. It was found that the ONSD in the death group and survival group was 5.5 ± 0.4 and 4.4 ± 0.5 mm, respectively[15]. The results of our study revealed that the ONSD in the death group was significantly greater than that in the survival group, demonstrating that the ONSD was an indirect monitoring marker for ICP. A higher mortality was predicted when the ONSD was > 5.7 mm.
The clinical application of the ONSD has some limitations. First, factors affecting the ONSD are not clear. Second, the normal range or threshold of the ONSD for the diagnosis of ICP is uncertain, and the two ranges may overlap. Third, the reliability of the ONSD needs to be improved. Therefore, researchers have attempted to apply different indices to various imaging techniques, such as ONSD/ETD ratio.
The ONSD/ETD ratio was first described in 2014 and has recently attracted scholars' attention. To date, it has not been determined whether the ONSD could be related to age, sex, height, body weight, BMI, MAP, and head circumference. However, the ONSD/ETD ratio is irrelevant to the abovementioned factors based on previous studies[16,17]. In addition, the ONSD/ETD ratio has a lower variability than the ONSD alone. Overall, the ONSD/ETD ratio is more[16], while that was 0.19 ± 0.02 on CT[18]. The ONSD/ETD ratio is a better predictor of increased ICP compared with the ONSD in brain-injured patients[19]. The ONSD/ETD ratio of 0.21 can be used as a simple reference tool for the diagnosis of papilledema and elevated ICP in pediatric patients[20]. The mortality noticeably increased in comatose patients with acute stroke when the ONSD was > 5.0 mm or the ONSD/ETD ratio was > 0.25[15]. Our previous study indicated that ONSD > 6.4 mm or ONSD/ETD ratio > 0.25 predicted the poor prognosis of comatose patients with supratentorial lesions[21]. The ONSD/ETD ratio was negatively correlated with glasgow outcome scale score (r = -0.64)[22].
In the present study, the mortality increased in comatose patients with acute stroke when the ONSD was > 5.7 mm or the ONSD/ETD ratio was > 0.25. The abovementioned studies indicated a higher consistency of the ONSD/ETD ratio in the prognostic prediction. In addition, the AUC values of the ONSD and ONSD/ETD ratio for predicting the prognosis of comatose patients with acute stroke were 0.760 (95%CI: 0.637-0.882) and 0.808 (95%CI: 0.696-0.920), respectively. Although there was no significant difference in the performance of the two parameters in predicting the prognosis, the AUC of the ONSD/ETD ratio seemed to be higher than that of the ONSD. Therefore, additional large-scale studies are required to confirm the cut-off values of the two parameters and their predictive values.
Moreover, we found a significant difference in MAP between the death group and the survival group, which could be attributed to a higher hemodynamic instability in the death group. Although ischemic stroke is prevalent, hemorrhagic stroke can lead to a higher mortality[23]. In the present study, we reported the highest mortality in the SAH group, followed by ACI and CH groups. During hospitalization, surgeries were performed on 23% of the patients with ACI, 38% of the patients with CH, and 7% of the patients with SAH. The highest mortality of SAH might be explained by a higher ICP and a greater incidence of seizures. The lowest mortality observed in CH might be related to active decompressive surgery during hospitalization. These results confirmed the necessity of decompressive surgery for comatose patients with acute stroke to prevent cerebral hernia and to improve the prognosis.
However, the present study had some limitations. Firstly, the study was retrospective and had a small sample size, with unavoidable biases. Secondly, we only classified patients based on their survival status upon discharge, and a long-term follow-up was not conducted. In the future, the prognostic values of the ONSD and ONSD/ETD ratio in comatose patients with acute stroke will be further verified by large-scale multi-center studies.
According to our study, the ONSD and ONSD/ETD ratio were significantly higher in comatose patients with acute stroke in the death group than those in the survival group. The mortality increased in comatose patients with acute stroke when the ONSD was > 5.7 mm or the ONSD/ETD ratio was > 0.25. Both indexes could be used as prognostic indicators for comatose patients with acute stroke. However, the ONSD/ETD ratio was more stable than the ONSD and would be preferred in clinical practice.
Stroke is mainly accompanied by an increased intracranial pressure (ICP), leading to more severe brain damage via secondary cerebral ischemia and hernia. A sudden rise of ICP is typically observed in comatose patients with acute stroke. Measuring optic nerve sheath diameter (ONSD) is noninvasive and convenient in comatose patients. Previous studies have shown that computed tomography (CT) can be used to accurately measure ONSD. The strong correlation between ICP and ONSD has been generally identified.
However, the ONSD of normal and sick individuals mainly varies noticeably. The ONSD/ETD ratio slightly varies. Therefore, the ONSD/ETD ratio has a smaller variability and a higher stability, and it may be more appropriate for ICP monitoring. To date, several studies have shown that the ONSD/ETD ratio is more related to ICP than the ONSD. Therefore, it is reasonable to speculate that the ONSD/ETD ratio has a higher value in predicting the prognosis of neurological function.
In the current study, unenhanced cranial CT scan was used to measure the ONSD and ETD, and the prognostic values of the ONSD and ONSD/ETD ratio in comatose patients with acute stroke were compared. the area under the curve (AUC) values of the ONSD and ONSD/ETD ratio for predicting the prognosis of comatose patients with acute stroke were 0.760 (95%CI: 0.637-0.882) and 0.808 (95%CI: 0.696-0.920), respectively. Although there was no significant difference in the performance of the two parameters in predicting the prognosis, the AUC of the ONSD/ETD ratio seemed to be higher than that of the ONSD. This study confirmed that ONSD/ETD can better predict neurological outcomes, and the variation is small, which provides a reference for non-invasive ICP monitoring and prediction of neurological outcomes in the future.
A total of 67 comatose patients with acute stroke were retrospectively recruited. The ONSD and ETD were measured by cranial computed tomography (CT) scan. The ONSD was measured vertically at 3 mm behind the eyeball. ETD was measured from one side of the retina behind the lens to the other side for the maximum diameter. All patients underwent cranial CT scan within 24 h after coma onset. Patients' baseline clinical data were collected, including age, gender, body weight, body mass index, mean arterial pressure (MAP), stroke type, glasgow coma scale (GCS) scores, surgery during hospitalization (e.g., clearance of hematoma and decompressive craniectomy). Patients were divided into death group and survival group according to their survival status at discharge. The differences of the ONSD and ONSD/ETD ratio between the two groups and their prognostic values were compared by MedCalc software.
In this study, 42 (62.7%) patients died, while 25 (37.3%) patients survived at discharge. there were significant differences in the GCS score (χ2 = 49.809, P < 0.0001), stroke type (χ2 = 11.981, P = 0.003), ONSD (t = 4.078, P < 0.0001), ONSD/ETD ratio (t = 4.625, P < 0.0001), and surgery (χ2 = 4.803, P = 0.048) between the two groups. The ONSD and ONSD/ETD ratio were included in the logistic regression model for multivariate analysis, respectively. After adjusting for age, MAP, GCS score, stroke type, and surgery, the associations among the ONSD (P = 0.04), ONSD/ETD ratio (P = 0.036), and mortality were still significant.
The AUC of the ONSD was 0.760 (95%CI: 0.637-0.882, P < 0.0001), with a sensitivity of 81.0% and a specificity of 64.0% at a cut-off value of 5.7 mm. The AUC of the ONSD/ETD ratio was 0.808 (95%CI: 0.696-0.920, P < 0.0001), with a sensitivity of 92.9% and a specificity of 68.0% at a cut-off value of 0.25. The AUC values of the receiver operating characteristic (ROC) curves of the ONSD and ONSD/ETD ratio were compared. There was no significant difference in the AUC values of the two indices (Z = 1.333, P = 0.1826).
The results of this study provide a reference for noninvasive ICP monitoring and prediction of neurological outcomes in the future.
In the future, we need to expand the sample size to determine the value of ONSD/ETD ratio in predicting increased ICP and poor prognosis.
In this study, the area under the ROC curve of ONSD and ONSD/ETD ratio was compared by MedCalc for the first time. Although there was no significant statistical difference in P value, the area under the ROC curve of ONSD/ETD ratio tended to increase compared with ONSD, and the degree of variation was smaller. Therefore, ONSD/ETD ratio is more recommended for ICP monitoring and predicting prognosis.
This study compared and evaluated the prognostic value of ONSD and ONSD/ETD ratio in comatose patients with stroke. The mortality increased in comatose patients with acute stroke when the ONSD was > 5.7 mm or the ONSD/ETD ratio was > 0.25. In addition, ONSD/ETD ratio was more reliable than ONSD. However, it is necessary to expand the sample size to study single etiology.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; Pitton Rissardo J, Brazil S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, Sandercock P, Wang Y, Huang Y, Cui L, Pu C, Jia J, Zhang T, Liu X, Zhang S, Xie P, Fan D, Ji X, Wong KL, Wang L; China Stroke Study Collaboration. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 1018] [Article Influence: 203.6] [Reference Citation Analysis (0)] |
| 2. | Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Feigin VL; NESS-China Investigators. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation. 2017;135:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1461] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 3. | Harary M, Dolmans RGF, Gormley WB. Intracranial Pressure Monitoring-Review and Avenues for Development. Sensors (Basel). 2018;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 4. | Killer HE, Laeng HR, Flammer J, Groscurth P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 2003;87:777-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Hassen GW, Bruck I, Donahue J, Mason B, Sweeney B, Saab W, Weedon J, Patel N, Perry K, Matari H, Jaiswal R, Kalantari H. Accuracy of optic nerve sheath diameter measurement by emergency physicians using bedside ultrasound. J Emerg Med. 2015;48:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Vaiman M, Sigal T, Kimiagar I, Bekerman I. Noninvasive assessment of the intracranial pressure in non-traumatic intracranial hemorrhage. J Clin Neurosci. 2016;34:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Bekerman I, Sigal T, Kimiagar I, Ben Ely A, Vaiman M. The quantitative evaluation of intracranial pressure by optic nerve sheath diameter/eye diameter CT measurement. Am J Emerg Med. 2016;34:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Sekhon MS, Griesdale DE, Robba C, McGlashan N, Needham E, Walland K, Shook AC, Smielewski P, Czosnyka M, Gupta AK, Menon DK. Optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;40:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Vaiman M, Gottlieb P, Bekerman I. Quantitative relations between the eyeball, the optic nerve, and the optic canal important for intracranial pressure monitoring. Head Face Med. 2014;10:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Du J, Deng Y, Li H, Qiao S, Yu M, Xu Q, Wang C. Ratio of Optic Nerve Sheath Diameter to Eyeball Transverse Diameter by Ultrasound Can Predict Intracranial Hypertension in Traumatic Brain Injury Patients: A Prospective Study. Neurocrit Care. 2020;32:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Bender M, Lakicevic S, Pravdic N, Schreiber S, Malojcic B. Optic nerve sheath diameter sonography during the acute stage of intracerebral hemorrhage: a potential role in monitoring neurocritical patients. Ultrasound J. 2020;12:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Raffiz M, Abdullah JM. Optic nerve sheath diameter measurement: a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med. 2017;35:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Jenjitranant P, Tunlayadechanont P, Prachanukool T, Kaewlai R. Correlation between optic nerve sheath diameter measured on imaging with acute pathologies found on computed tomography of trauma patients. Eur J Radiol. 2020;125:108875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Naldi A, Pivetta E, Coppo L, Cantello R, Comi C, Stecco A, Cerrato P, Lesmeister M, Lochner P. Ultrasonography Monitoring of Optic Nerve Sheath Diameter and Retinal Vessels in Patients with Cerebral Hemorrhage. J Neuroimaging. 2019;29:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhao L, Huang Q, Huang P, Zhao Q, Xie H, Wang R. [Optic nerve sheath diameter and eyeball transverse diameter as a useful tool for the clinical prognosis in patients with stroke during hospitalization]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:1242-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Kim DH, Jun JS, Kim R. Measurement of the Optic Nerve Sheath Diameter with Magnetic Resonance Imaging and Its Association with Eyeball Diameter in Healthy Adults. J Clin Neurol. 2018;14:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Kim DH, Jun JS, Kim R. Ultrasonographic measurement of the optic nerve sheath diameter and its association with eyeball transverse diameter in 585 healthy volunteers. Sci Rep. 2017;7:15906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Vaiman M, Sigal T, Kimiagar I, Bekerman I. Intracranial Pressure Assessment in Traumatic Head Injury with Hemorrhage Via Optic Nerve Sheath Diameter. J Neurotrauma. 2016;33:2147-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Youm JY, Lee JH, Park HS. Comparison of transorbital ultrasound measurements to predict intracranial pressure in brain-injured patients requiring external ventricular drainage. J Neurosurg. 2022;136:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Bartsikhovsky T, Klar MM, Bekerman I, Nagieva S, Tal S. Diagnostic tool for initial evaluation of the intracranial pressure on computed tomography in pediatric patients with headache. PLoS One. 2019;14:e0216812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Zhu S, Cheng C, Zhao D, Zhao Y, Liu X, Zhang J. The clinical and prognostic values of optic nerve sheath diameter and optic nerve sheath diameter/eyeball transverse diameter ratio in comatose patients with supratentorial lesions. BMC Neurol. 2021;21:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Bekerman I, Sigal T, Kimiagar I, Vaiman M. Initial evaluation of the intracranial pressure in cases of traumatic brain injury without hemorrhage. J Neurol Sci. 2016;368:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Feigin VL, Norrving B, Mensah GA. Global Burden of Stroke. Circ Res. 2017;120:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1348] [Article Influence: 168.5] [Reference Citation Analysis (0)] |