INTRODUCTION
Perioperative neonatal pneumothorax (NP) is a very rare incidental event that can rapidly develop life-threatening complications. With the exception of perioperative neonatal pneumothorax (PNP) risk factors, most incidents of PNP are related to surgery or anesthesia (Figure 1)[1]. The importance of early diagnosis of pneumothorax is very clear, but diagnosis is difficult for the following reasons: The operating space is limited, the newborn is relatively difficult to access under the sterile sheet, and there are many other factors other than pneumothorax that may affect the judgment of the anesthesiologist. Therefore, it is very important to quickly identify and accurately diagnose pneumothorax in the neonatal perioperative period and to conduct a timely and effective clinical intervention.
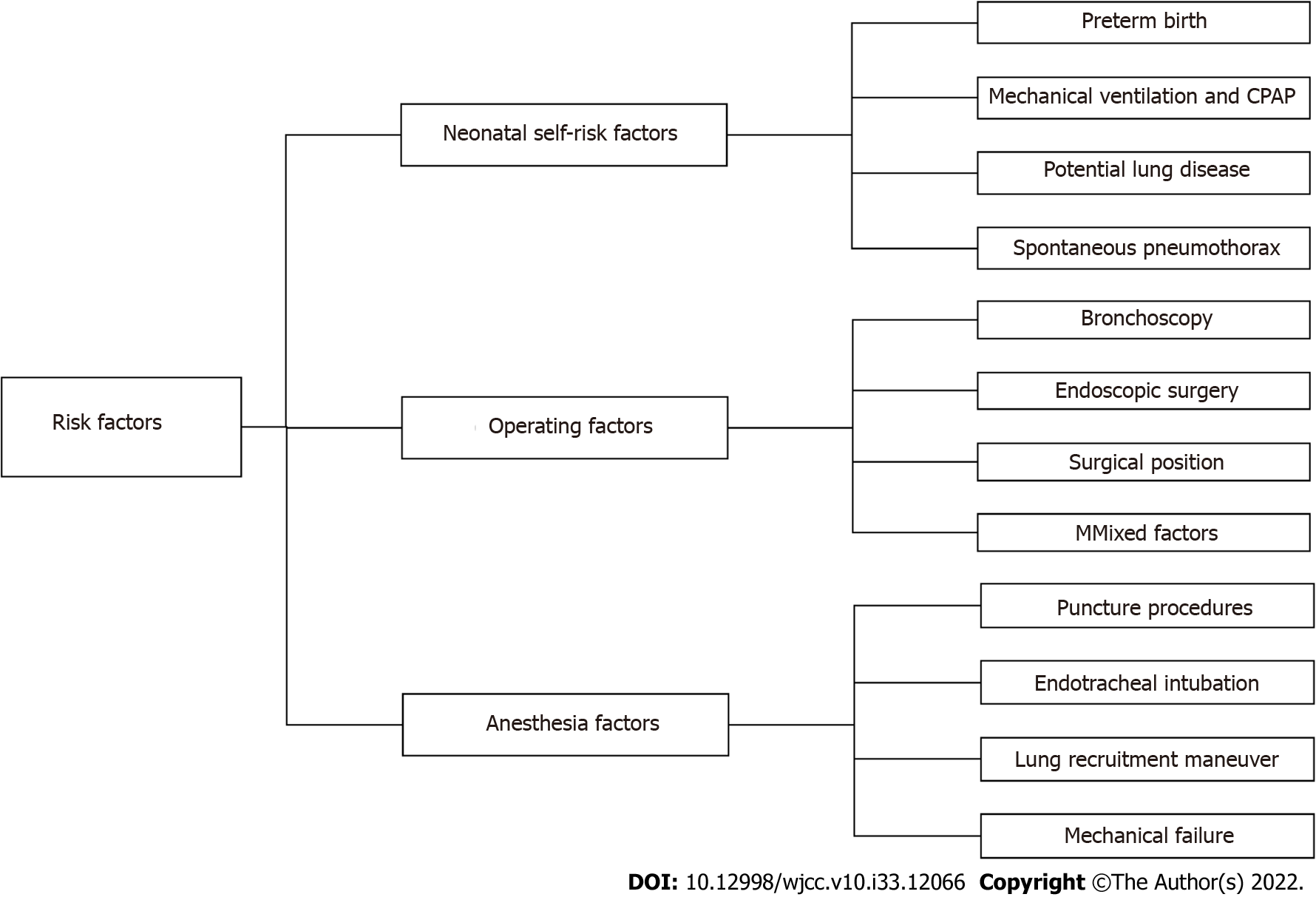
Figure 1 Risk factors for perioperative pneumothorax in neonates.
CPAP: Continuous positive airway pressure.
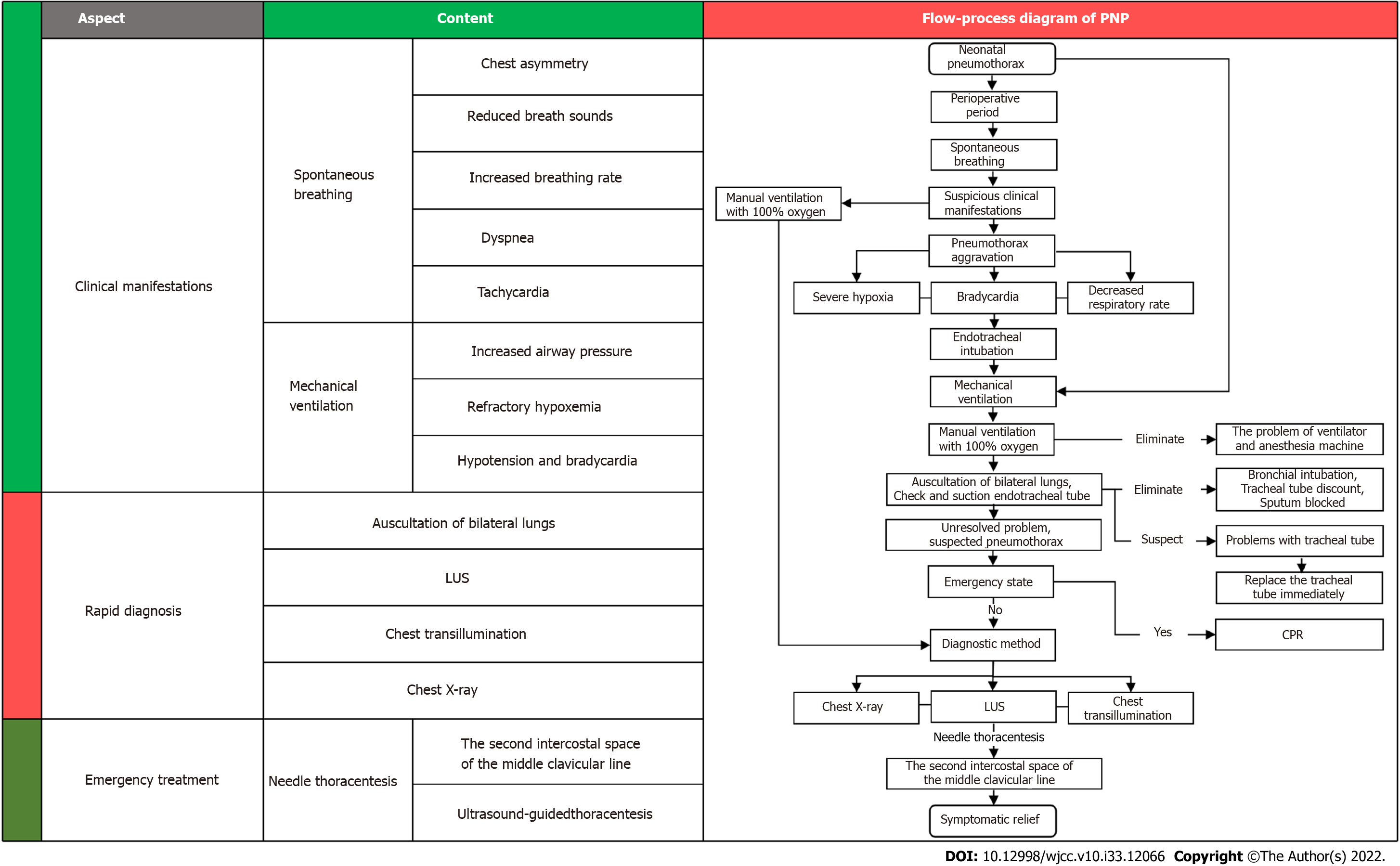
In this review, we summarized the risk factors, clinical manifestations, diagnosis and treatment of PNP and prepared a flow-process diagram for the rapid diagnosis and treatment of patients (Figure 2).
Figure 2 Flow-process diagram for the rapid diagnosis and treatment of perioperative neonatal pneumothorax.
PNP: Perioperative neonatal pneumothorax; LUS: Lung ultrasound; CPR: Cardiopulmonary resuscitation.
RISK FACTORS
Neonatal self-risk factors
Pneumothorax can occur at all ages but more often in the neonatal period. The most important risk factors for NP are prematurity, mechanical ventilation, and continuous positive airway pressure (CPAP). Other risk factors include underlying lung disease and spontaneous pneumothorax[2-5]. Pneumothorax is more likely to occur perioperatively when the newborn has one or multiple risk factors.
Preterm birth: In a multicenter study, researchers reported that the incidence of NP in tertiary hospitals decreased as gestational age increased; by 5.7% in infants aged 28 wk, 1.4% in infants aged 28-37 wk, and 0.8% in infants older than 37 wk[3]. A study showed that for newborns with respiratory distress receiving primary noninvasive respiratory support at 28-36 wk of gestation, prediction in infants of a lower gestational age and with a higher FiO2 require intubation within 72 h of admission[6]. In infants born at < 32 wk of gestation, both bronchopulmonary dysplasia (BPD) and patent ductus arteriosus may require endotracheal intubation for mechanical ventilation. However, intubation is associated with adverse respiratory outcomes, such as pneumothorax and prolonged positive pressure ventilation[7]. Due to the lack of alveolar surfactant in premature infants, alveolar stability decreases, resulting in widespread alveolar collapse and uneven alveolar pressure. Pneumothorax is more likely to occur when airway pressure changes and mechanical ventilation is used[8].
Mechanical ventilation and CPAP: Mechanical ventilation and CPAP therapy are considered to be two of the most important risk factors for NP[2-4]. A few studies have shown the incidence of pneumothorax after mechanical ventilation (25.6%-76%) or CPAP (30%-96%). Some newborns need respiratory support after birth (such as premature infants with patent ductus arteriosus, congenital diaphragmatic hernia, preoperative mechanical ventilation, and CPAP) to improve ventilation, provide adequate oxygenation, and increase tidal volume and airway peak pressure, but this can result in alveolar rupture, and once that occurs, pneumothorax is inevitable[9].
Potential lung disease: When newborns or premature infants have respiratory distress syndrome, meconium aspiration syndrome, neonatal transient shortness of breath, BPD, pneumonia and other potential lung diseases, lack of lung surfactant or sputum obstruction causing uneven alveolar ventilation, some alveoli can rupture due to excessive inflation expansion, thus pneumothorax occurs[4]. In neonates requiring surgery immediately after birth, such as those with esophageal atresia or with esophageal tracheal fistulas, it is easy to aspirate gastric contents into the lungs, thus causing neonatal atelectasis and pneumonia. Prematurity occurs in 30% to 40% of these newborns, and respiratory distress in premature infants may also lead to lung injury, increasing the incidence of PNP[10].
Spontaneous pneumothorax: The incidence of spontaneous pneumothorax in newborns is approximately 1%-2%, and approximately half of all newborns have pneumothorax symptoms, usually diagnosed within a few hours of birth[11,12]. A study by Aly et al[6] showed that the median time to NP diagnosis was 34 h in newborns smaller than 2500 g, but before diagnosis, the newborn may develop tension pneumothorax when undergoing surgery or mechanical ventilation. Park and Lee[13] reported that a newborn with a sudden pneumothorax during esophageal atresia repair was highly suspected of having spontaneous pneumothorax.
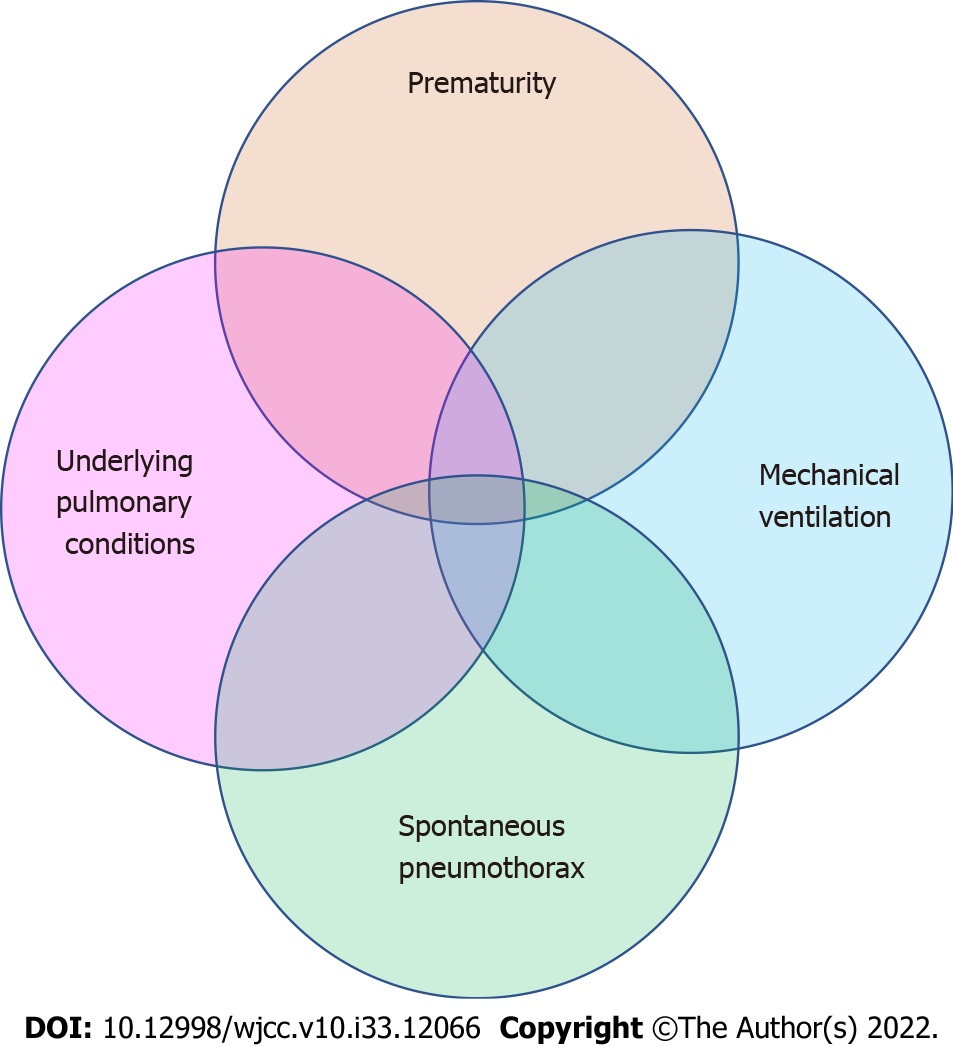
Mixed factors: PNP may be the result of a combination of one or more risk factors (Figure 3). Freed et al[14] reported a 26-wk premature girl who underwent emergency retinal laser photocoagulation surgery in the operating room at 12 wk of age. She had life-threatening complications with ventilation immediately after anesthesia-induced intubation, and the child underwent needle decompression and insertion of a chest drain. Later, it was believed that the child might have had BPD after birth, and she received mechanical ventilation for prolonged periods. The child had poor lung compliance with abdominal distension and required high airway pressure for ventilation, leaving the patient in a high-risk state for pneumothorax, and tension pneumothorax developed during positive pressure ventilation.
Figure 3 Perioperative neonatal pneumothorax may be the result of a combination of one or more risk factors.
Operating factors
During surgical procedures and examinations, neonates experience iatrogenic factors that cause increased airway pressure, rupture of alveoli, or pleural damage that can cause tension pneumothorax.
Bronchoscopy: Previous studies have reported cases of pneumothorax in neonates undergoing rigid bronchoscopy[15,16]. At present, neonatal fiberoptic bronchoscopy (FOB) has replaced rigid bronchoscopy, so the safety of bronchoscopy has been greatly improved. However, serious complications during FOB in neonates have still been reported in the literature, including bronchospasm, severe hypoxia, bradycardia, and tension pneumothorax[17]. The cause of tension pneumothorax in intubated infants may be that the FOB was introduced through the endotracheal tube (ETT) and occupied almost all of the space of the neonatal ETT. While oxygen was being insufflated through the suction channel of the FOB, the gas blown in was more than that exhaled, leading to barotrauma and alveolar rupture[18]. For non-intubated infants, it seems safe to suggest careful control of oxygen flow into the infant's trachea via the FOB. However, some cases of pneumothorax still occurred, and the most likely analysis was that the FOB was pushed into the lobar bronchus to form a seal[19]. Therefore, if a coarser bronchoscope is used in newborns, severe airway obstruction may occur, and the airway pressure at the distal end of the blockage may continuously increase, causing tension pneumothorax.
Endoscopic surgery: The application of endoscopic surgery in newborns can provide a minimally invasive, aesthetic incision that reduces trauma and facilitates postoperative musculoskeletal recovery, especially during thoracic surgery[20]. However, endoscopic surgery requires greater proficiency and anesthesia management, which may prolong the operation time and increase the incidence of surgical complications. Carbon dioxide artificial pneumothorax and single-lung ventilation (SLV) are often used in thoracoscopic surgery to improve the surgical conditions of these procedures[21]. The use of artificial pneumothorax and SLV in neonatal thoracoscopic surgery often causes hypercapnia and spikes in airway pressure, increasing the incidence of barotrauma and pneumothorax, while in some laparoscopic surgeries adjacent to the parietal pleura, the procedure may tear the pleura, leading to the formation of iatrogenic pneumothorax[22].
Surgical position: During lateral recumbent surgery in newborns, the mediastinum compresses the supine lung, and the activity of the diaphragmatic muscles on the recumbent side is more limited than that of the contralateral diaphragm, so neonatal alveoli rupture and pneumothorax occurs when the supine lung is ventilated at a higher airway pressure. Sharma et al[23] reported a case of sudden pneumothorax in a neonatal left recumbent position during tracheoesophageal fistula (TEF) repair, with a high suspicion that the pneumothorax was caused by increased lung compliance and ventilation under high airway pressure after fistula ligation.
Anesthesia factors
When anesthesiologists perform invasive procedures on newborns during the perioperative period, such as deep vein puncture catheterization, peripheral nerve block, endotracheal intubation, and anesthesia circuit failure, they often result in injury and pneumothorax.
Puncture procedures: The significant anatomical differences between newborns and older children or adults are short neck length, small neck space, loose skin and subcutaneous connective tissue, and inconspicuous anatomical markers. Punctures that include upper extremity block, subclavian or intrajugular venous puncture catheterization, are less successful and have complications[24]. Goli et al[25] reported a 32-wk premature infant who underwent peripherally inserted central catheter (PICC) catheterization at 4 d of birth. After the PICC was placed for 2 h, the baby’s oxygen saturation decreased to 45%, and the baby developed tachycardia and shortness of breath. The baby received a chest X-ray again, and the presence of a completely white right chest indicated pneumothorax and collapse of the right lung.
Endotracheal intubation: Pneumothorax caused by endotracheal intubation has the following conditions: (1) The ETT is mistakenly inserted into the bronchus during endotracheal intubation and is not found in time, and mechanical ventilation is performed according to the originally set respiratory parameters. Studies reported by Niwas et al[26] show that NP occurs predominantly on the right side (72.5%), with 67% of these cases associated with endotracheal intubation of the right main bronchi; (2) When SLV is performed, intentional endotracheal-bronchial intubation makes ventilation more effective but causes higher pressure ventilation, which may lead to pneumothorax and lung damage; and (3) When using endotracheal intubation aids such as a bougie, the tracheal wall is damaged. The bougie, also known as the ETT introducer, is often used to expose a difficult airway for laryngoscopic intubation or to assist in the replacement of endotracheal intubation[27]. However, there have been several reports of pneumothorax in neonates who were intubated with the use of the bougie. Kumar and Walker[28] reported cases of perforation of the airway caused by the bougie. Glaisyer and Way[29] reported 3 cases of pneumothorax during surgery in neonates, and no specific cause was determined, but 2 cases were reintubated using the bougie. In two patients with pneumothorax reported by Parekh et al[1], it was suspected that pneumothorax was caused by trauma to the tracheal wall that occurred during bougie-guided endotracheal intubation. In the study by Sakhuja et al[30], the examination before neonatal tracheal intubation showed loss of right breath sounds, and chest photography confirmed pneumothorax immediately after intubation. In the analysis, the suspected causes were that the insertion of the bougie into the airway during the replacement of the intubation was too deep, which may have caused trauma and air leakage. Therefore, the use of the bougie is an unusual choice in neonates. It should be used with extreme caution, paying attention to the depth of insertion and associated complications.
Lung recruitment maneuver (LRM): Lung collapse always occurs during mechanical ventilation, usually after induction of general anesthesia[31]. Compared with adults, the residual capacity of neonatal function is lower than that of closed capacity, and its anatomical and physiological characteristics make it more susceptible to alveolar collapse[32]. Therefore, anesthesiologists often perform LRMs and positive end-expiratory pressure (PEEP) during surgery to prevent induction and mechanical ventilation-related atelectasis[33]. However, they are not risk-free. Pneumothorax and barotrauma are major clinical problems, especially in newborns and infants, as their chest compliance is poor, which makes them particularly sensitive to increased airway pressure and prone to pneumothorax. When safe pressure is exceeded, barotrauma may result in trauma and is accompanied by hemodynamic instability. Recent studies have hoped to use the automatic program of LRM software for related operations, as FLOW-i4.3 Anesthesia System (FLOW-i4.3 Anesthesia System®) automatic step-back relapse operation software is safe and effective for healthy newborn models. No adverse respiratory or hemodynamic events were observed during the implementation of the LRM in the pressure-controlled ventilation mode using a gradually increased PEEP (30/15 cmH2O) approach[34].
Mechanical failure: During the use of the anesthesia machine, the one-way valve can malfunction, and the anesthesia circuit may be kinked, resulting in only gas in (not out), and the pressure in the lungs continues to rise, resulting in barotrauma. In a report by Sabar et al[35], pneumothorax occurred during FOB performed prior to tracheoesophageal fistula repair, which was likely due to a kink in the Jackson-Rees breathing circuit that prevented gas from entering but not exiting.
CLINICAL MANIFESTATIONS
Autonomous breathing neonates
Most NP manifest with asymmetric thoracic fluctuations, weakened breathing sounds, and increased breathing rates. If these manifestations are not detected, patients continue to develop shortness of breath, respiratory distress, hypoxemia, tachycardia, and even cardiac arrest[22]. A study on early neonatal signs with pneumothorax concluded that a sudden increase in respiratory rate helped to reliably identify early signs of impending NP with a sensitivity and specificity of 77% and 90%, respectively[36].
Mechanically ventilated neonates
NP manifests with increased airway pressure, increased end expiratory carbon dioxide, and decreased pulse oxygen saturation. However, during neonatal anesthesia and surgery, changes in ventilation parameters, oxygen saturation and decline are common for many reasons, such as increased airway pressure, hypercapnia, and hypoxemia, which are often similar to pneumothorax. This leads anesthesiologists and surgeons to miss the occurrence of pneumothorax, and anesthesiologists may turn to artificial ventilation, leading to increased peak airway pressure. The strong positive pressure expansion further intensifies the degree of pneumothorax and hypoxemia so that patients even develop tension pneumothorax, a slow heartbeat, and cardiac arrest[37,38].
RAPID DIAGNOSIS
The importance of early diagnosis of pneumothorax is very clear, but it is often difficult to diagnose, especially in the operating room. The infants are covered with sterile sheets and are relatively inaccessible, and there are many other tasks that the anesthesiologist must pay attention to. Therefore, pneumothorax is almost impossible to diagnose within the first minutes of oxygenation deterioration and hemodynamic failure, while the sterile sheets are still on. The diagnosis of pneumothorax is generally based on clinical presentation, lung auscultation and associated respiratory function tests.
Clinical manifestations and respiratory sounds on auscultation
In diagnosing pneumothorax according to the clinical manifestations, other causes similar to pneumothorax manifestations should be excluded. Respiratory sounds on auscultation weaken or disappear on the pneumothorax side, especially in cases of left pneumothorax, since a lack of respiratory sounds may be mistaken for unintentional right bronchial intubation, and a high airway pressure is also present under these circumstances[39].
Chest X-ray and computed tomography
In adults, the most commonly used auxiliary examination for the diagnosis of pneumothorax is still chest X-ray. In the study by Cizmeci et al[40], positive chest X-ray signs were found to be only 46.6% sensitive and 93.3% specific for accurately identifying pneumothorax in neonatal patients placed in the supine position, and 46% did not have occult pneumothorax before clinical diagnosis. Among the studied X-ray positive signs, deep groove and medial stripe signs were suggestive of occult pneumothorax. The report by Alrajhi et al[41] also revealed that chest X-ray reliability is limited in younger preterm and low birth weight infants because pneumothorax may not appear with classic positive signs, and the missed diagnosis rate is up to 30%, resulting in delayed diagnosis. A computed tomography (CT) scan is the gold standard for pneumothorax imaging diagnosis[42]. NP on CT images displays an absence of lung texture in the pleural cavity and sometimes the compressed edge of the affected lung. CT can accurately display the small amount of gas in the pneumothorax and mediastinum, which is very beneficial for clarifying the volume and location of gas accumulation[43]. Simple pneumothorax in mechanically ventilated newborns runs the risk of developing into tension pneumothorax, which may rapidly become life-threatening. Chest X-ray and CT imaging are not easy to obtain in the perioperative period. Therefore, they are not suitable for rapidly diagnosing PNP.
Transillumination
Chest transillumination technology is easier to use in the operating room than chest X-ray equipment, and allows for a rapid diagnosis[44]. A high-strength transmittance portable fiber cold light source is usually used to prevent thermal damage. As the ambient light darkens, the probe is positioned directly above the newborn's nipple while they are in the supine position. The pneumothorax presents as a translucent area within the thorax with an opaque lung parenchyma. A large pneumothorax was reported in a patient at 32 wk gestation with hemodynamic deterioration, and chest ultrasound was not very helpful in diagnosing NP. However, chest transillumination was positive, and an anti-medial NP was found. This study suggests that chest illumination may be more rapid for diagnosing primary NP. Transillumination is the most beneficial method for detecting sudden life-threatening pneumothorax (which requires immediate treatment) because it can be done rapidly, and it is an effective tool for PNP[45]. Notably, strong light radiation causes thermal damage to the skin and photochemical damage to the retina[46]. Attention should be given to protecting the eyes of newborns, and long-term exposure to light radiation should be avoided[47].
Lung ultrasound
Recent studies have shown that lung ultrasound (LUS) is a safe, rapid and effective means to diagnose pneumothorax, especially in the operating room[48]. Pneumothorax can be quickly identified using LUS, even when applied in newborns[49]. In neonates with pneumothorax, LUS also has a higher sensitivity and specificity than chest X-ray. In a meta-analysis[50], the overall specificity and sensitivity of LUS for identifying NP were 98% and 99%, respectively. The most commonly used positive signs for pneumothorax when using LUS are lung sliding and the disappearance of the B-line, which has good sensitivity (100%) for the diagnosis of NP[51]. It has been reported that there are also reliable indicators of pneumothorax in the presence of pleural and A-lines, and their sensitivity and specificity for diagnosing pneumothorax are 100%[52]. The lung point sign is also a positive sign for pneumothorax, which is a transitional phase between the normal (lung sliding) and abnormal (no lung sliding) lung signs and can help providers determine the severity of pneumothorax[53]. Its presence indicates that the pneumothorax is mild to moderate; otherwise, the pneumothorax will be severe[54]. Of course, some positive signs can help exclude pneumothorax. When lung pulse is present (suggesting that the visceral pleura and parietal pleura are in the same location), pneumothorax can be excluded at the examination site. Lung pulse is present when there is atelectasis, but the lung sliding sign is not. Lung pulse and lung sliding signs also occur with endotracheal tube bronchial intubation. For example, during right bronchial intubation, lung sliding signs can appear on the right side, while the left lung collapses with lung pulse without lung sliding signs[55]. Although LUS is influenced by operator experience, it also has high reliability for NP diagnosis, even when used by beginner operators (but prolonged diagnostic time can occur)[52]. Therefore, for experienced operators, portable chest ultrasound diagnosis of pneumothorax is convenient and fast and is suitable for the diagnosis of PNP. In the case of obvious pneumothorax, it is noteworthy that LUS cannot replace clinical judgment and should be actively managed.
EMERGENCY TREATMENT
Changes in ventilation parameters and decreases in oxygen saturation are more common during neonatal anesthesia and surgery, and other common causes must be urgently ruled out before pneumothorax can be diagnosed. Hand ventilation of NP with a T tube and 100% oxygen can exclude circuit problems with the ventilator or anesthesia machine and help to gain understanding regarding the compliance of the lungs. Small endotracheal tubes are prone to bronchial intubation and kinking, and their prolapse is also easily blocked by secretions. They should be carefully and strictly inspected. Endotracheal suction can quickly and safely rule out the above reasons for blockage. If there is still doubt about the tracheal tube, the endotracheal tube should be replaced. In most cases, the problem is resolved quickly. However, when these conditions are not resolved, pneumothorax, although rare, must be considered. Depending on the clinical characteristics of NP, a treatment process is prepared for the perioperative period.
In neonates with tension pneumothorax[32], needle thoracentesis is usually performed with a 22-G intravenous indwelling needle in the midclavicular line of the second intercostal space or the midaxillary line of the fifth intercostal space. Medical evidence shows that compared to traditional thoracic puncture and drainage treatment, puncture and drainage with an intravenous indwelling needle have the same effect and allow for a shorter operation time[56].
Recent research has shown that bedside LUS used in neonates can both improve the accuracy of pneumothorax diagnosis and the effectiveness of thoracic paracentesis. In the four NP patients reported by Gregorio-Hernández et al[57], two patients had anterior thoracentesis before ultrasound, but their pneumothorax problems were not resolved, putting them at an additional risk. A recent consensus by international experts on the neonatal diagnosis of pneumothorax ultrasound and ultrasound-guided thoracentesis is that ultrasound can monitor the development of pneumothorax as well as healing after thoracic drainage[58]. The key steps in the successful implementation of thoracentesis are accurate diagnosis of the presence of pneumothorax and accurate location of puncture points. For formally trained and skilled doctors, this task is easily accomplished with ultrasound, which determines the precise location of the lung points, reduces radiation, and increases the safety of neonatal thoracocentesis[59].