Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11993
Peer-review started: August 24, 2022
First decision: September 23, 2022
Revised: September 27, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 16, 2022
Processing time: 75 Days and 18.4 Hours
Polycythemia vera (PV), often attributed to the JAK2 V617F mutation, is characterized by enhanced red blood cell counts in the peripheral blood. PV-associated renal disease is clinically rare; to date, there have been reports of other chronic kidney diseases related to PV, but no reports on PV-associated minimal change disease.
A 37-year-old man presented with proteinuria and high red blood cell count on January 4, 2021. The patient underwent bone marrow and renal biopsies, then was subsequently diagnosed with PV and minimal change in disease. Hydroxyurea was administered and proteinuria remission was achieved. The patient’s last visit was on April 14, 2022.
We inferred that there may be a causal relationship between PV and minimal change disease.
Core Tip: Polycythemia vera (PV) is a myeloproliferative neoplasm that can influence the immune system and affect organs such as the kidneys. This is the first report of PV-associated minimal change disease. In our case, hydroxyurea administration was effective and proteinuria remission was achieved. Therefore, we infer there may be a causal relationship between PV and minimal change disease.
- Citation: Xu L, Lu LL, Gao JD. Minimal change disease caused by polycythemia vera: A case report. World J Clin Cases 2022; 10(32): 11993-11999
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11993.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11993
Polycythemia vera (PV)[1], a myeloproliferative neoplasm (MPN), is the ultimate phenotype of the JAK2 V1617F mutation, and is characterized by erythrocytosis, leukocytosis, thrombocytosis, splenomegaly, and occasional myelofibrosis. PV-associated renal disease is rare. A literature review[2] referred to cases of PV-associated renal diseases, including immunoglobulin (Ig) A nephropathy, membranoproliferative glomerulonephritis, focal segmental glomerulosclerosis, and rapidly progressive glomerulonephritis. However, to the best of our knowledge, there have been no reports of minimal changes in disease. Here, we report the case of our patient.
A 37-year-old man, a base station engineer, was admitted to the Shuguang Hospital affiliated with the Shanghai University of Traditional Chinese Medicine, Department of Nephrology (Shanghai, China), on January 4, 2021. He complained of proteinuria for approximately one month.
During inpatient care, the patient presented with dizziness, slight weakness, no hematuria, no back pain, good appetite, sound sleep, and normal urine and stool.
The patient claimed no history of past illness.
The patient claimed no personal or family history.
Physical examination revealed no abnormities, except for blood pressure, which was 153/106 mmHg.
On December 15, 2020, the patient underwent physical examination, and the report showed positive urine protein (++), blood routine: white blood cell count, 16.531 × 109/L; hemoglobin level, 229 g/L; red blood cell > 7.8 × 1012/L; and platelet count, 489 × 109/L. Biochemistry test: albumin 37 g/L, total albumin 60 g/L, blood uric acid 490 µmol/L, triglyceride 3.87 mmol/L, and blood creatinine 88 µmol/L.
On December 31, 2020, the 24-hour urine total protein quantitative was 5.08 g/24 h.
On January 7, 2021, routine blood tests showed white blood cells: 21.71 × 109/L; platelets, 431 × 109/L; red blood cells, 7.65 × 1012/L; and hemoglobin, 216.7 g/L.
On January 11 and 15, the patient underwent bone marrow and renal biopsies, respectively, after providing informed consent. A bone marrow biopsy revealed dense bone and a small amount of bone marrow tissue. The ratio of hematopoietic cells to structural fat in the area available for observation was 85%:15%, and the grain-to-red ratio was approximately 4–5:1. Hematopoietic cells in all stages of the red line could be seen; megakaryocytes were 7–17 per high-power field, distributed in clusters, and the cell bodies were relatively large with more nuclear lobes.
On January 11, 2021, a bone marrow biopsy showed that PV was first considered, and further genetic examination was recommended to clarify the type. IHC2021-0042: CD34 (individual +), CD117 (individual +), CD15 (partial +), MPO (partial +), CD56 (-), CD138 (individual +), CD61 (discrete +), CD71 (part +), Kappa: Lambda (not restricted expression), and special stain results: mesh (-), Periodic Acid-Schiff stain (+/-), Prussian blue (-).
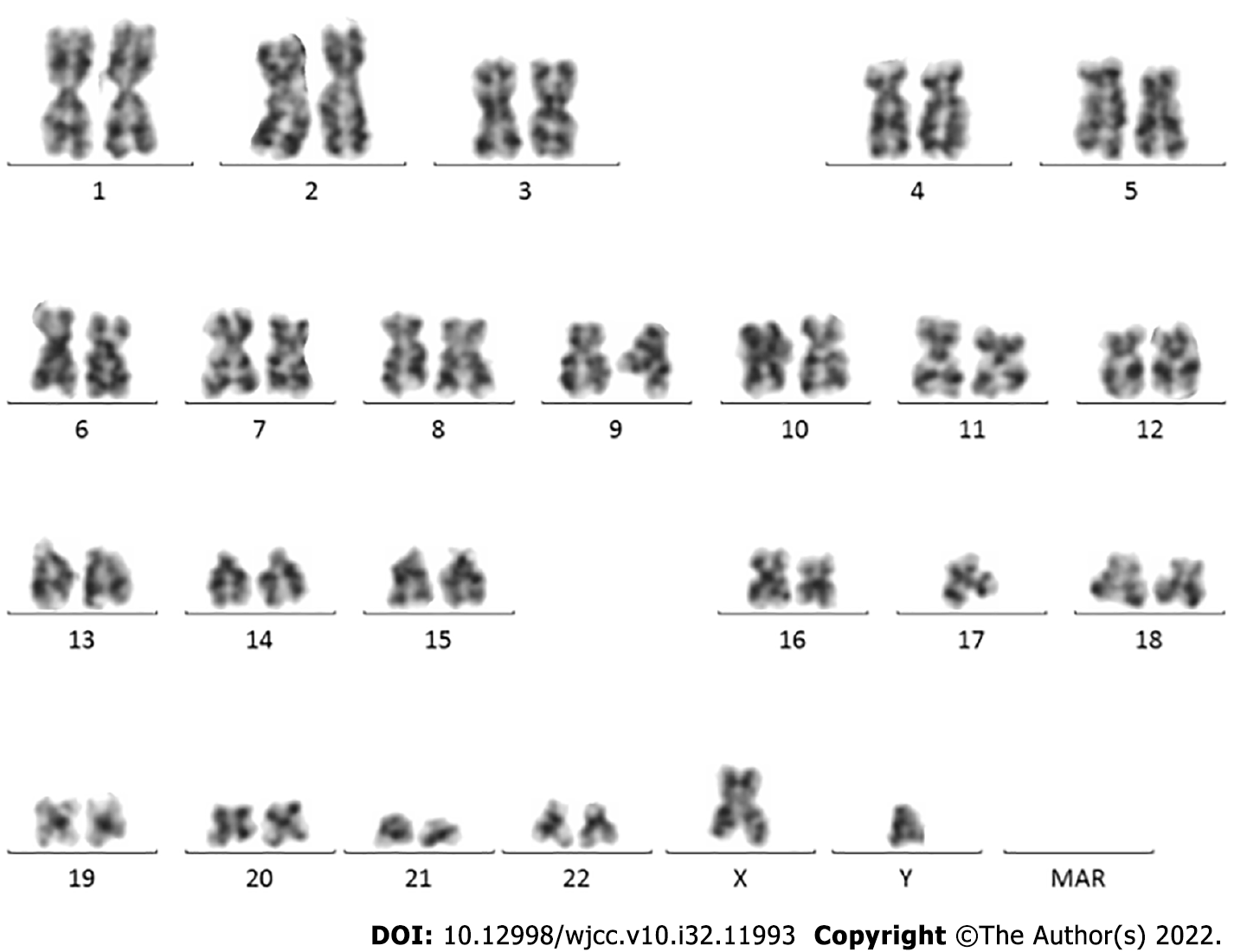
On January 13, 2021, karyotype analysis showed the following karyotype: 46,XY (Figure 1). Gene test: JAK2 V617F (+). The mutation ratio of JAK2 V617F was 86.60%.
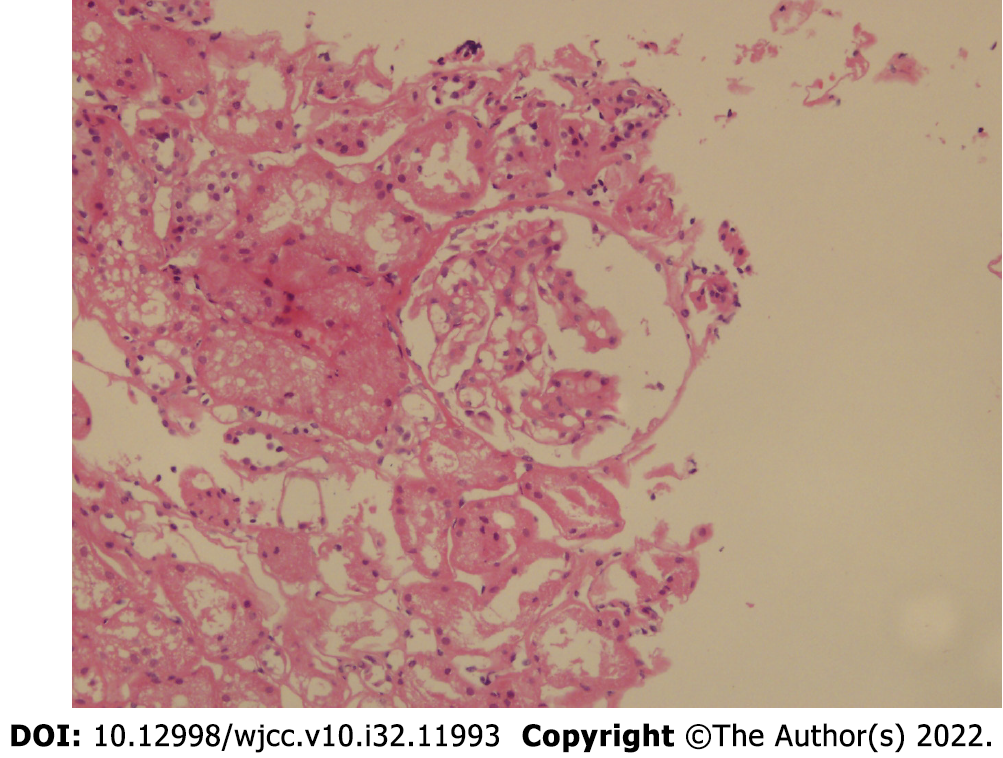
On January 15, 2021, renal biopsy (renal puncture tissue) revealed four glomeruli. The morphology and structure of glomeruli were normal. Some glomeruli showed mild proliferation of mesangial cells and increased matrix. No glomerular sclerosis changes, crescents, or infiltration of inflammatory cells were observed. Protein casts were observed in individual tubules, and some renal tubules showed atrophy and unclear structure. No obvious abnormalities were observed in the renal interstitium or blood vessels. Diagnosis: Minimal change disease (MCD). Fluorescence staining: IgG (-), IgA (-), IgM (-), C3 (-), C4 (-), C1q (-), Fb (+/-). Special staining: PAS (+), silver staining (+), Masson’s trichrome (+), Congo red (-). IHC2021-0045: TGF B1 (-) (Figure 2).
Abdominal ultrasonography showed splenomegaly.
The patient was diagnosed with the following conditions: (1) MCD; (2) PV; and (3) hypertension.
The patient was given hydroxyurea 0.5 g qd p.o. (total duration of administration: 3 mo), olmesartan 20 mg qd p.o. (till last vist) and has been receiving interferon biweekly since cessation of hydroxyurea.
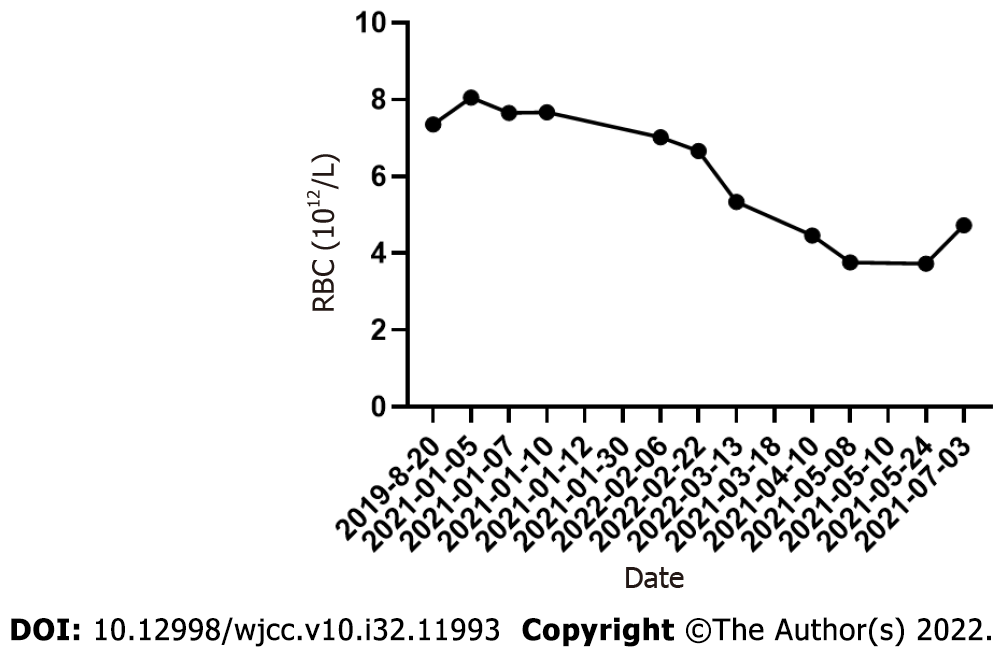
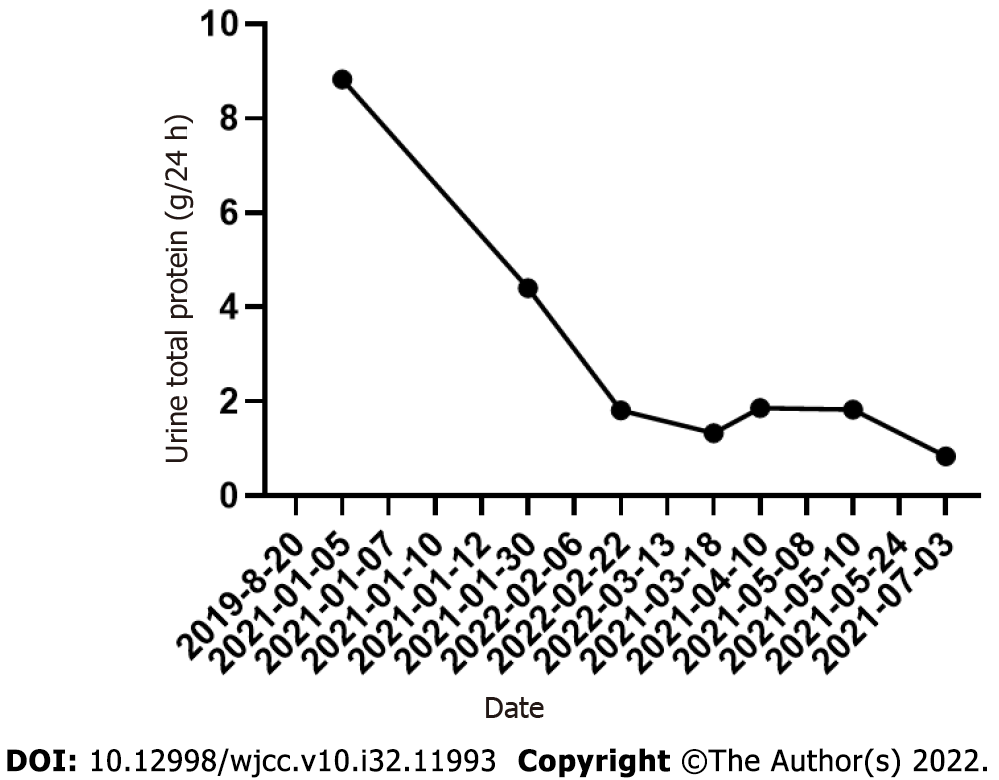
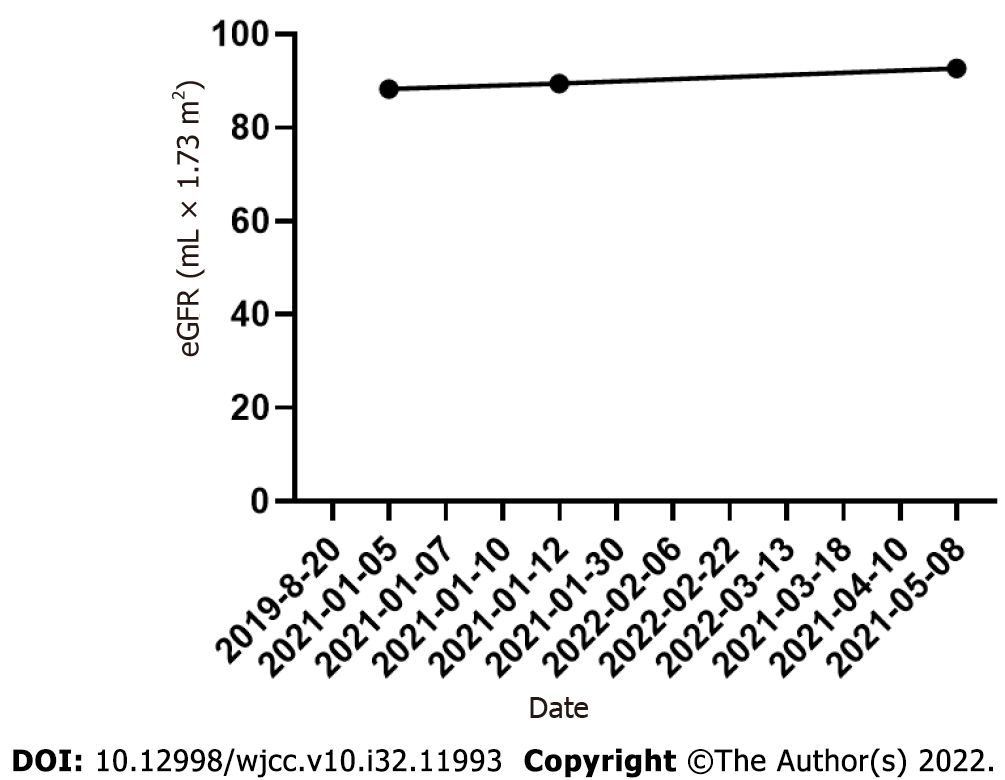
On March 13, 2021, the patient visited the hospital for a second visit. Routine blood test results revealed the following findings: hemoglobin, 167 g/L; red blood cells: 5.34 × 1012/L; white blood cells: 5.5 × 109/L; and platelets, 170 × 109/L. On March 18, 2021, the patient’s 24 h proteinuria was 1.33 g/24 h. The patient is currently in good condition. Other laboratory data for each visit are shown in Table 1 and Figures 3-5.
| Date | WBC (109/L) | HB (g/L) | RBC (1012/L) | PLT (109/L) | Hematocrits | UTP (g/24 h) | eGFR (mL × 1.73 m2) |
| 2019-08-20 | 12.7 | 205 | 7.35 | 474 | 0.636 | - | - |
| 2021-01-05 | 16.57 | 226.1 | 8.05 | 435 | 0.665 | 8.83 | 88.33 |
| 2021-01-07 | 21.71 | 216.7 | 7.65 | 431 | 0.664 | - | - |
| 2021-01-10 | 15.77 | 218 | 7.67 | 500 | 0.659 | - | - |
| 2021-01-12 | - | - | - | - | - | - | 89.47 |
| 2021-01-30 | - | - | - | - | - | 4.4 | - |
| 2022-02-06 | 2.79 | 211 | 7.02 | 168 | 0.63 | - | - |
| 2022-02-22 | 3.97 | 203 | 6.66 | 161 | 0.591 | 1.81 | - |
| 2022-03-13 | 5.51 | 167 | 5.34 | 170 | 0.475 | - | - |
| 2021-03-18 | - | - | - | - | - | 1.33 | - |
| 2021-04-10 | 4 | 141 | 4.46 | 263 | 0.408 | 1.86 | - |
| 2021-05-08 | 4.53 | 125 | 3.76 | 337 | 0.362 | 92.69 | |
| 2021-05-10 | - | - | - | - | - | 1.83 | - |
| 2021-05-24 | 6.3 | 128 | 3.73 | 450 | 0.369 | - | - |
| 2021-07-03 | 6.5 | 156 | 4.73 | 525 | 0.452 | 0.84 | - |
MCD is a primary cause of idiopathic nephrotic syndrome (NS), which manifests as mass proteinuria, resulting in edema and depletion of intravascular volume[3]. MCD occurs much less frequently in adults than in children[3]. The pathological features of MCD include a lack of visible changes on light microscopy and depletion of foot processes on electron microscopy. Podocyte modifications and immune disorders synergize to change the integrity of the glomerular basement membrane (GBM), thereby determining proteinuria[3]. MCD is primarily associated with extra-glomerular diseases, including neoplastic processes and undesirable reactions to drugs.
MCD has been frequently associated with hematologic malignancies such as Hodgkin’s lymphoma[4], and this relationship indicates that MCD is a disease that results from lymphocyte dysfunction. The benefit of immunosuppressants and steroids also implies that the cause of MCD is immune dysregulation. The pathogenesis of PV-associated MCD is likely related to T cell dysfunction. The mechanism is speculated to be a T helper cell class 1 and 2 imbalance in MCD[5,6]. In brief, the main cause of MCD is immune disorders, particularly T cell dysfunction. These T lymphocytes can produce circulating lymphokines which are toxic to GBM, and thus alter their permeability to proteins. In 1974, Shalhoub postulated the existence of a circulating mediator produced by abnormal T-lymphocytes[7]. In our case, T-lymphocyte disorder also induced concurrent MCD with PV. Studies have shown that the immune system is thrown into enormous disruption by myeloproliferative neoplasms (MPNs)[8]. JAK2V617F mutation results in the overexpression of many cytokines and chemokines such as interleukins IL-2, IL-8, as reported in samples from both mice and patients[8,9]. Nephrotic-level proteinuria has been reported in cancer patients treated with IL-2[10]. Evidence indicates that IL-2 is associated with proteinuria[10]. Hermouet et al[11] were the first to report that serum IL-8 Levels were elevated in PV patients. Garin et al[12] speculated that circulating IL-8 may play a role in MCD.
Our patient’s business trip to Chernobyl 8 years ago lasted approximately 4 months. We suspected nuclear contamination, which would have affected the immune system.
Our question in this case was as follows: Was the occurrence of MCD with PV purely coincidental or was it etiologically linked, either by a common pathogenesis or could the MCD be secondary to the PV? We can see that proteinuria remission was positively associated with treatment with hydroxyurea, so it is reasonable to consider a causal relationship.
However, the exact mechanisms underlying the pathogenesis of MCD associated with PV remain unclear. In addition to immune dysregulation, kidney injury might also be attributed to hyperviscosity, abnormal function, and/or excessive neoplastic blood cell activation and growth factor production[13]. Increased blood viscosity and platelet hypersensitivity can induce microcirculatory disturbances, resulting in endothelial damage. Many cytokines and growth factors such as interferon-γ, IL-6, and platelet-derived growth factor, which have documented in patients with MPNs, can directly elicit endothelial damage. Growth factors can be generated locally by marginating platelets and cells of extramedullary hematopoiesis, accompanied by hyperviscosity, which exacerbates endothelial damage[13]. PV can not only result in thrombosis in the main blood vessels[14], but can also induce thrombotic microangiopathy[15]. Glomerular microvasculature is particularly vulnerable to injury due to thr
Nonetheless, two potential mechanisms, microcirculatory disturbances and immune disorders, may explain the development of MCD in PV patients.
Based on our findings, there may be a causal relationship between minimal change in disease and PV. However, this is a clinical inference rather than a critical appraisal of the differences between chance and causal relationships, which would require a study of extensive epidemiological data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Boilard E, Canada; Tanabe K S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Spivak JL. Polycythemia Vera. Curr Treat Options Oncol. 2018;19:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Chen H, Zhang B, Li M, Hu R, Zhou C. Polycythemia vera associated with IgA nephropathy: A case report and literature review. Exp Ther Med. 2015;10:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal Change Disease. Clin J Am Soc Nephrol. 2017;12:332-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 351] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 4. | Jhaveri KD, Shah HH, Patel C, Kadiyala A, Stokes MB, Radhakrishnan J. Glomerular diseases associated with cancer, chemotherapy, and hematopoietic stem cell transplantation. Adv Chronic Kidney Dis. 2014;21:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Souadjian JV, Enriquez P, Silverstein MN, Pépin JM. The spectrum of diseases associated with thymoma. Coincidence or syndrome? Arch Intern Med. 1974;134:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 213] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol. 2016;183:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 592] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Nasillo V, Riva G, Paolini A, Forghieri F, Roncati L, Lusenti B, Maccaferri M, Messerotti A, Pioli V, Gilioli A, Bettelli F, Giusti D, Barozzi P, Lagreca I, Maffei R, Marasca R, Potenza L, Comoli P, Manfredini R, Maiorana A, Tagliafico E, Luppi M, Trenti T. Inflammatory Microenvironment and Specific T Cells in Myeloproliferative Neoplasms: Immunopathogenesis and Novel Immunotherapies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Panteli KE, Hatzimichael EC, Bouranta PK, Katsaraki A, Seferiadis K, Stebbing J, Bourantas KL. Serum interleukin (IL)-1, IL-2, sIL-2Ra, IL-6 and thrombopoietin levels in patients with chronic myeloproliferative diseases. Br J Haematol 2005; 130: 709-715. |
| 10. | Zea AH, Stewart T, Ascani J, Tate DJ, Finkel-Jimenez B, Wilk A, Reiss K, Smoyer WE, Aviles DH. Activation of the IL-2 Receptor in Podocytes: A Potential Mechanism for Podocyte Injury in Idiopathic Nephrotic Syndrome? PLoS One. 2016;11:e0157907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Hermouet S, Godard A, Pineau D, Corre I, Raher S, Lippert E, Jacques Y. Abnormal production of interleukin (IL)-11 and IL-8 in polycythaemia vera. Cytokine. 2002;20:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Garin EH, West L, Zheng W. Effect of interleukin-8 on glomerular sulfated compounds and albuminuria. Pediatr Nephrol. 1997;11:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Büttner-Herold M, Sticht C, Wiech T, Porubsky S. Renal disease associated with myeloproliferative neoplasms and myelodysplastic syndrome/myeloproliferative neoplasms. Histopathology. 2021;78:738-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Vannucchi AM, Guglielmelli P. JAK2 mutation-related disease and thrombosis. Semin Thromb Hemost. 2013;39:496-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1264] [Cited by in RCA: 1127] [Article Influence: 66.3] [Reference Citation Analysis (0)] |