Published online Nov 16, 2022. doi: 10.12998/wjcc.v10.i32.11980
Peer-review started: August 30, 2022
First decision: September 9, 2022
Revised: September 23, 2022
Accepted: October 20, 2022
Article in press: October 20, 2022
Published online: November 16, 2022
Processing time: 70 Days and 4.6 Hours
MLL gene rearrangement is a common genetic abnormality of acute myeloid leukemia (AML), which predicts poor prognosis and is important in clinical diagnosis. MLL rearrangement involves many chromosomes, among which, t(4;11) translocation is rare in AML. The present case was t(4;11) AML, acc
An adult male with self-reported symptoms of fatigue, febrility and hyperleukocytosis was diagnosed with AML by morphology and confirmed by imm
t(4;11) AML with hyperdiploid karyotype has not been reported. In this case, t(4;11) was only detected by karyotype analysis and FISH, suggesting their im
Core Tip: t(4;11) translocation is a rare karyotypic abnormality in acute myeloid leukemia (AML). We report for the first time an AML patient with t(4;11) and hyperdiploid karyotype abnormality only detected by karyotype analysis and fluorescence in situ hybridization. This highlights their importance in the diagnosis and prognosis of leukemia. We also describe the phenotype and gene mutation profile of his leukemia cells.
- Citation: Zhang MY, Zhao Y, Zhang JH. t(4;11) translocation in hyperdiploid de novo adult acute myeloid leukemia: A case report. World J Clin Cases 2022; 10(32): 11980-11986
- URL: https://www.wjgnet.com/2307-8960/full/v10/i32/11980.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i32.11980
The MLL gene (also known as KMT2A, mapping at chromosome 11q23) was first identified and reported in 1991, and its rearrangement is a common genetic change in hematopoietic malignancies, such as acute leukemia (AL) and myelodysplastic syndrome[1,2]. The MLL rearrangement (MLL-r) occurs in 2.8%–3.5% of acute myeloid leukemia (AML) cases, indicating poor prognosis[3,4]. Conventional karyotype analysis and molecular genetic technology, fluorescence in situ hybridization (FISH) and reverse-transcription polymerase chain reaction (RT-PCR) are the primary methods to detect MLL-r, used individually or in combination in previous studies[3-10]. Other methods, such as Southern blotting and cDNA panhandle PCR have been used in the exploratory study of MLL-r AL[11-13], and 94 translocation partner genes (TPGs) have been characterized so far[14], indicating the cytogenetic heterogeneity of MLL-r AL. Considering this characteristic, current retrospective research preferred to divide MLL-r into different subgroups based on TPGs[4,15]. The most common MLL-r subgroup is MLL–AF4 [also known as KMT2A–AFF1, or t(4;11)(q21;q23)], which is formed by translocation between MLL and AF4 genes (located on chromosome 4q21), and occurs almost entirely in acute lymphoblastic leukemia (ALL)[16]. By contrast, t(4;11) AML only accounts for 0.8%–1.2% of MLL-r+ AML[9,13,14,16,17]. Limited by the sample size, t(4;11) AML has not been analyzed as a single subgroup, and its characteristics, pathogenesis and therapeutic options have not been established. More information needs to be accumulated about t(4;11) AML.
We here report a case of uncommon t(4;11) AML, review the literature and summarize the diagnostic features of MLL-r AML.
A 52-year-old man was admitted to Shengjing Hospital of China Medical University with complaints of fatigue for 1 mo, febrility for 2 wk and increased leukocytes for 3 d.
The patient exhibited signs of fatigue 1 mo ago and did not receive treatment. After 2 wk, he developed febrility with the highest temperature of 38.0°C, and improved after taking unknown ingredients of traditional Chinese medicine. The patient had no other symptoms or bleeding episodes.
The patient had high blood pressure (160/110 mmHg), and had been taking oral amlodipine (10 mg, qd) and betaproc (100 mg, bid).
The patient denied any medical history, and declared no exposure to chemotherapeutic agents or radioactive elements. No special family history was noted.
Physical examination showed a pale appearance and sternal tenderness. No enlarged lymph nodes or hepatosplenomegaly was noted.
Complete blood count revealed hyperleukocytosis, anemia and thrombocytopenia [white blood cells (WBC) 227.8 × 109/L; neutrophils (N) 25.1 × 109/L; lymphocytes (L) 16.2 × 109/L; monocytes (M) 18.9 × 109/L; red blood cells (RBC) 1.92 × 1012/L; hemoglobin (HGB) 54g/L; platelets (PLT) 27 × 109/L. (reference range: WBC 3.5-9.5 × 109/L; N 1.9-7.2 × 109/L; L 1.1-2.7 × 109/L; M 0.3-0.8 × 109/L; RBC 4.3-5.8 × 1012/L; HGB 130-172g/L; PLT 135-350 × 109/L)]. Creatinine was 199.6 mmol/L (reference range: 59-104 mmol/L) and D-dimer 9196 mg/L (reference range: 0-252 mg/L).
No imaging examination.
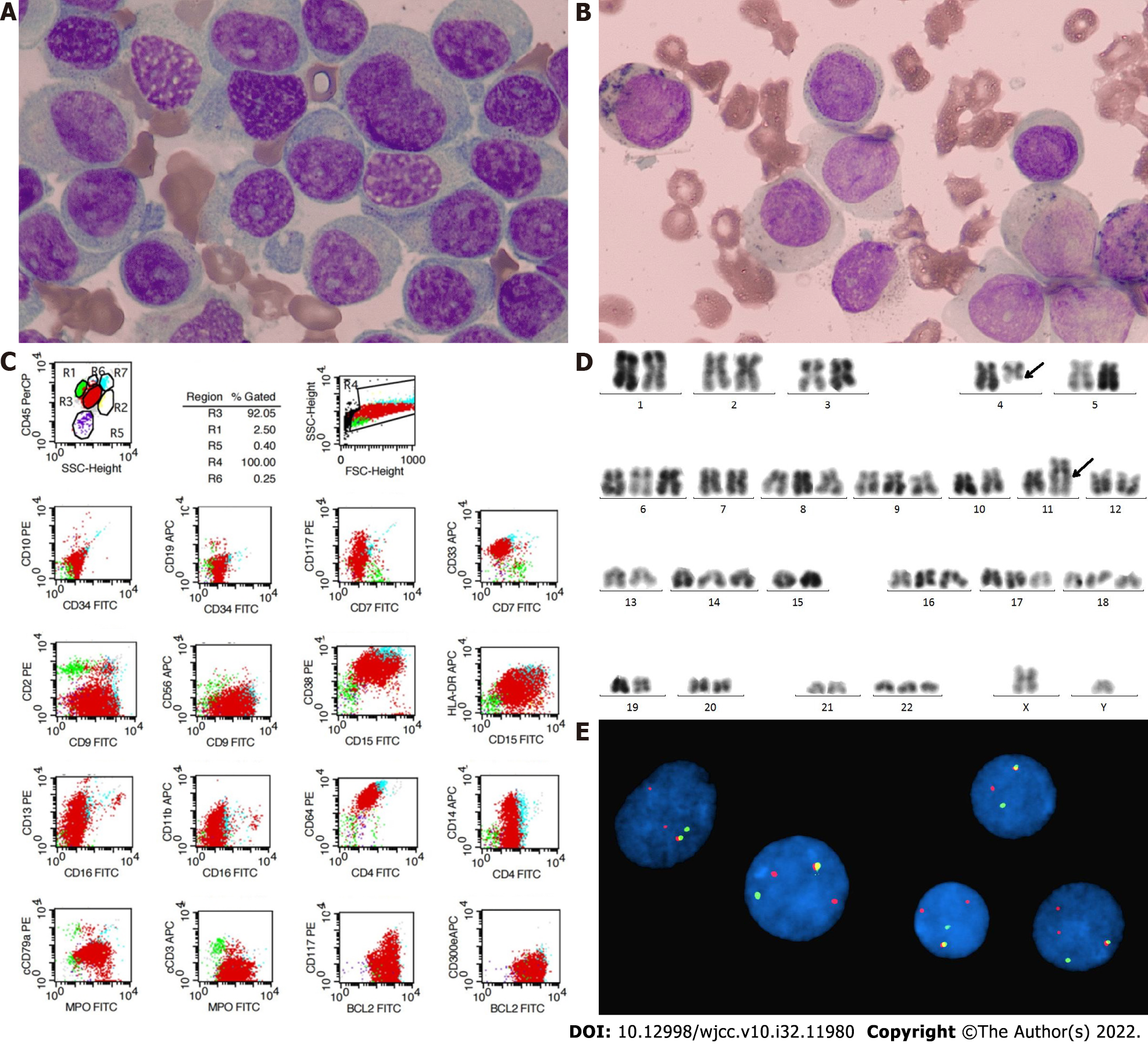
Bone marrow (BM) examination revealed 74.4% of typical premonocytes and 7.6% myelocytes (Figure 1A), and peroxidase staining was weak positive (Figure 1B). Flow cytometry detected 91.87% malignant myeloid cells in BM expressing CD33+, CD9+, CD38+, CD15+, CD64+, cMPO+, BCL2+, HLA-DR+/-, CD13+/-, and CD14+/- (Figure 1C). Cytogenetic test revealed a hyperdiploid karyotype with addition of chromosomes 6, 8, 9, 14, 16, 17, 18, 22 and t(4;11)(q21;q23) balanced translocation on R-banded metaphases (Figure 1D), suggesting MLL rearrangement, which was confirmed by an MLL break-apart FISH probe with 1R1G1Y signal and atypical 2R1G1Y signal (Figure 1E). However, molecular biological analysis showed that none of the common TPGs involved in MLL-r AL (MLL–AF4/ AF6/ AF9/ AF10 /ELL/ ENL /SETP6 /AF17/ AF1q/ AF1p/ AFX) was positive by RT-PCR. Next-generation sequencing found mutations of ASXL1 (exon12: c. 2083C>T), and U2AF1 (exon2: c. 101C>T) and a TET2 mutation (exon3: c. 652G>A) of undetermined significance.
Based on the information above, this case was diagnosed as MLL-r AML with poor prognosis.
After diagnosis was established, the patient began to receive cytoreductive drugs (homoharringtonine and hydroxyurea, 2 mg and 3 g per day, respectively).
The patient died of respiratory and circulatory failure 5 d after the diagnosis.
MLL rearrangement is a common category of genetic abnormalities accounting for 2.8%–3.5% of AML cases, and indicating poor prognosis[3,4]. Among the multiple MLL-r AML subtypes distinguished by TPGs, MLL–AF4, also known as t(4;11)(q21;q23), is rare, especially in adult patients. According to previous reports, t(4;11) only accounts for 0.8%–1.2% of MLL-r AML[9,13,14,17] and 0.05% of all AML[10]. Existing reports on t(4;11) AML differ in age and pathological pattern, covering pediatric, secondary AML and acute megakaryoblastic leukemia[18-20], yet there are no reports of adult de novo AML with t(4;11).
The present case was a newly diagnosed adult case of hyperdiploid AML with t(4;11), and MLL rearrangement was revealed by karyotype and FISH analysis. Confusingly, RT-PCR failed to detect MLL–AF4 fusion gene. We speculate that the MLL gene in this case amplified partially and/or rearranged with at least two TPGs simultaneously, forming an atypical 2R1G1Y positive signal of MLL break-apart probe. Therefore, it was not possible to perform PCR. Due to the sudden death of the patient, deeper verification was not available. Karyotype analysis and molecular genetic methods, including FISH and RT-PCR, are the primary techniques used to detect MLL-r[5,6,11,21]. In clinical practice, however, only a few of the most common fusion genes were included in the RT-PCR panel, which restricts the range of RT-PCR[7,13]. This case demonstrates that combined use of karyotype analysis and FISH may be beneficial for discovery of more MLL-r AMLs[5].
Considering the limited number of cases of t(4;11) AML, we compared the clinical and laboratory features with data of MLL-r AML patients. This case was diagnosed with AML by morphology, the blasts expressed CD33, which matched the majority of MLL-r AML cases reported in the literature[3,9]. MLL-r AML also has common features in karyotype analysis. Vetro showed that additional cytogenetic abnormalities (ACAs) are common in MLL-r AML and 75% of cases have one or two ACAs[22]. However, our case showed eight ACAs besides t(4;11), leading to a hyperdiploid karyotype with chromosome number of 54. To the best of our knowledge, there has been only one adult case of hyperdiploid karyotype with t(4;11) reported in B-ALL[23], and none has been reported in AML. Another characteristic of this case was the mutations of ASXL1 and U2AF1 genes, while most statistical data show that AML with MLL-r is commonly accompanied by mutations of RAS pathway-related genes, such as KRAS and NRAS[7,10,15,22,24]. In terms of prognosis, several studies have shown that all MLL-r AML should be classified into the poor prognosis group regardless of TPGs[3,4,13], and the WBC count at diagnosis, achieving complete remission after the first course of treatment, and transplantation are independent risk factors in multivariate analysis[7,8,9,25]. The effects of immunotherapy and inhibitors targeting MLL-r acute leukemia need to be further explored[16,26-29]. We could not observe any therapeutic effects because the patient died soon after diagnosis.
t(4;11) AML is a rare subgroup of MLL-r AML, and combination with hyperdiploid karyotype has rarely been reported. In this case, t(4;11) was only detected by conventional karyotype analysis and FISH, suggesting the importance of these tests in detection of MLL-r patients. Special genetic information of this case is provided in our report. More data need to be collected for more in-depth studies on t(4;11) AML.
The authors wish to acknowledge Dr. Xuan Liu, Dr. Xiao-Hui Wang, Dr. Fang Chen, and Dr. Shuang Fu, Hematology Laboratory of Shengjing Hospital, for their help in collecting clinical data and advice on manuscript writing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Al-Ani RM, Iraq; Musoni L, Morocco S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa R 3rd, Patel Y, Harden A, Rubinelli P, Smith SD, LeBeau MM, Rowley JD. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991;88:10735-10739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 452] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 2. | Ibrahim S, Estey EH, Pierce S, Glassman A, Keating M, O'Brien S, Kantarjian HM, Albitar M. 11q23 abnormalities in patients with acute myelogenous leukemia and myelodysplastic syndrome as detected by molecular and cytogenetic analyses. Am J Clin Pathol. 2000;114:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 3. | Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003;102:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 259] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 4. | Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1513] [Article Influence: 100.9] [Reference Citation Analysis (1)] |
| 5. | Cox MC, Panetta P, Venditti A, Del Poeta G, Maurillo L, Tamburini A, Del Principe MI, Amadori S. Fluorescence in situ hybridization and conventional cytogenetics for the diagnosis of 11q23+/MLL+ translocation in leukaemia. Br J Haematol. 2003;121:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 6. | Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, Beverloo HB, Chang M, Creutzig U, Dworzak MN, Forestier E, Gibson B, Hasle H, Harrison CJ, Heerema NA, Kaspers GJ, Leszl A, Litvinko N, Nigro LL, Morimoto A, Perot C, Pieters R, Reinhardt D, Rubnitz JE, Smith FO, Stary J, Stasevich I, Strehl S, Taga T, Tomizawa D, Webb D, Zemanova Z, Zwaan CM, van den Heuvel-Eibrink MM. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 7. | Gong XY, Wang Y, Liu BC, Wei H, Li CW, Li QH, Zhao JW, Zhou CL, Lin D, Liu KQ, Wei SN, Gong BF, Zhang GJ, Liu YT, Zhao XL, Li Y, Gu RX, Qiu SW, Mi YC, Wang JX. [Characteristics and prognosis in adult acute myeloid leukemia patients with MLL gene rearrangements]. Zhonghua Xue Ye Xue Za Zhi. 2018;39:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 8. | Menghrajani K, Zhang Y, Famulare C, Devlin SM, Tallman MS. Acute myeloid leukemia with 11q23 rearrangements: A study of therapy-related disease and therapeutic outcomes. Leuk Res. 2020;98:106453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 9. | Issa GC, Zarka J, Sasaki K, Qiao W, Pak D, Ning J, Short NJ, Haddad F, Tang Z, Patel KP, Cuglievan B, Daver N, DiNardo CD, Jabbour E, Kadia T, Borthakur G, Garcia-Manero G, Konopleva M, Andreeff M, Kantarjian HM, Ravandi F. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J. 2021;11:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 10. | Yan Q, Lin YN, Huang XQ, Qian LZ, Ma JT, Zhang H, Chen L, Chen XJ, Mi YC, Ru K. [Analysis of fusion gene expression in acute myeloid leukemia]. Zhonghua Xue Ye Xue Za Zhi. 2021;42:480-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 11. | von Bergh A, Emanuel B, van Zelderen-Bhola S, Smetsers T, van Soest R, Stul M, Vranckx H, Schuuring E, Hagemeijer A, Kluin P. A DNA probe combination for improved detection of MLL/11q23 breakpoints by double-color interphase-FISH in acute leukemias. Genes Chromosomes Cancer. 2000;28:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | De Braekeleer M, Morel F, Le Bris MJ, Herry A, Douet-Guilbert N. The MLL gene and translocations involving chromosomal band 11q23 in acute leukemia. Anticancer Res. 2005;25:1931-1944. [PubMed] |
| 13. | Shih LY, Liang DC, Fu JF, Wu JH, Wang PN, Lin TL, Dunn P, Kuo MC, Tang TC, Lin TH, Lai CL. Characterization of fusion partner genes in 114 patients with de novo acute myeloid leukemia and MLL rearrangement. Leukemia. 2006;20:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 14. | Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, Sutton R, Venn NC, Emerenciano M, Pombo-de-Oliveira MS, Barbieri Blunck C, Almeida Lopes B, Zuna J, Trka J, Ballerini P, Lapillonne H, De Braekeleer M, Cazzaniga G, Corral Abascal L, van der Velden VHJ, Delabesse E, Park TS, Oh SH, Silva MLM, Lund-Aho T, Juvonen V, Moore AS, Heidenreich O, Vormoor J, Zerkalenkova E, Olshanskaya Y, Bueno C, Menendez P, Teigler-Schlegel A, Zur Stadt U, Lentes J, Göhring G, Kustanovich A, Aleinikova O, Schäfer BW, Kubetzko S, Madsen HO, Gruhn B, Duarte X, Gameiro P, Lippert E, Bidet A, Cayuela JM, Clappier E, Alonso CN, Zwaan CM, van den Heuvel-Eibrink MM, Izraeli S, Trakhtenbrot L, Archer P, Hancock J, Möricke A, Alten J, Schrappe M, Stanulla M, Strehl S, Attarbaschi A, Dworzak M, Haas OA, Panzer-Grümayer R, Sedék L, Szczepański T, Caye A, Suarez L, Cavé H, Marschalek R. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 507] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 15. | Bill M, Mrózek K, Kohlschmidt J, Eisfeld AK, Walker CJ, Nicolet D, Papaioannou D, Blachly JS, Orwick S, Carroll AJ, Kolitz JE, Powell BL, Stone RM, de la Chapelle A, Byrd JC, Bloomfield CD. Mutational landscape and clinical outcome of patients with de novo acute myeloid leukemia and rearrangements involving 11q23/KMT2A. Proc Natl Acad Sci U S A. 2020;117:26340-26346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 16. | Britten O, Ragusa D, Tosi S, Kamel YM. MLL-Rearranged Acute Leukemia with t(4;11)(q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy? Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 17. | Meyer C, Hofmann J, Burmeister T, Gröger D, Park TS, Emerenciano M, Pombo de Oliveira M, Renneville A, Villarese P, Macintyre E, Cavé H, Clappier E, Mass-Malo K, Zuna J, Trka J, De Braekeleer E, De Braekeleer M, Oh SH, Tsaur G, Fechina L, van der Velden VH, van Dongen JJ, Delabesse E, Binato R, Silva ML, Kustanovich A, Aleinikova O, Harris MH, Lund-Aho T, Juvonen V, Heidenreich O, Vormoor J, Choi WW, Jarosova M, Kolenova A, Bueno C, Menendez P, Wehner S, Eckert C, Talmant P, Tondeur S, Lippert E, Launay E, Henry C, Ballerini P, Lapillone H, Callanan MB, Cayuela JM, Herbaux C, Cazzaniga G, Kakadiya PM, Bohlander S, Ahlmann M, Choi JR, Gameiro P, Lee DS, Krauter J, Cornillet-Lefebvre P, Te Kronnie G, Schäfer BW, Kubetzko S, Alonso CN, zur Stadt U, Sutton R, Venn NC, Izraeli S, Trakhtenbrot L, Madsen HO, Archer P, Hancock J, Cerveira N, Teixeira MR, Lo Nigro L, Möricke A, Stanulla M, Schrappe M, Sedék L, Szczepański T, Zwaan CM, Coenen EA, van den Heuvel-Eibrink MM, Strehl S, Dworzak M, Panzer-Grümayer R, Dingermann T, Klingebiel T, Marschalek R. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27:2165-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 343] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 18. | He RR, Nayer Z, Hogan M, Cuevo RS, Woodward K, Heyer D, Curtis CA, Peterson JF. Immunotherapy- (Blinatumomab-) Related Lineage Switch of KMT2A/AFF1 Rearranged B-Lymphoblastic Leukemia into Acute Myeloid Leukemia/Myeloid Sarcoma and Subsequently into B/Myeloid Mixed Phenotype Acute Leukemia. Case Rep Hematol. 2019;2019:7394619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 19. | Blütters-Sawatzki R, Borkhardt A, Grathwohl J, Repp R, Rheinisch-Becker I, Bohle RM, Lampert F. Secondary acute myeloid leukemia with translocation (4;11) and MLL/AF4 rearrangement in a 15-year-old boy treated for common acute lymphoblastic leukemia 11 years earlier. Ann Hematol. 1995;70:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 20. | Takita J, Motomura A, Koh K, Ida K, Taki T, Hayashi Y, Igarashi T. Acute megakaryoblastic leukemia in a child with the MLL-AF4 fusion gene. Eur J Haematol. 2009;83:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 21. | Liu P, Zhang R, Ge Z, Lin ZK, Liu J, Qian SX, Zhang SJ, Lu H, Wu HX, Qiu HX, Liu P, Xu W, Chen LJ, Lu C, Lu BB, Qiao C, Qiu HR, Zhu GR, Zhang JF, Wu YJ, Li JY. [Detection and clinical features of MLL gene rearrangement in adult patients with acute leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012;20:1110-1116. [PubMed] [DOI] [Full Text] |
| 22. | Vetro C, Haferlach T, Meggendorfer M, Stengel A, Jeromin S, Kern W, Haferlach C. Cytogenetic and molecular genetic characterization of KMT2A-PTD positive acute myeloid leukemia in comparison to KMT2A-Rearranged acute myeloid leukemia. Cancer Genet. 2020;240:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 23. | Zotova OV, Lukianova AS, Valchuk MO, Karol YS, Shalay OO, Novak VL, Loginsky VE. 11q23/MLL rearrangements in adult acute leukemia. Exp Oncol. 2021;43:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 24. | Grossmann V, Schnittger S, Poetzinger F, Kohlmann A, Stiel A, Eder C, Fasan A, Kern W, Haferlach T, Haferlach C. High incidence of RAS signalling pathway mutations in MLL-rearranged acute myeloid leukemia. Leukemia. 2013;27:1933-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 25. | Wang T, Zhao Y, Zhang QQ, Xu LR. [Clinical Characteristics and Prognostic Influencing Factors of Adult AML Patients with MLL Rearrangement]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 26. | Libura J, Slater DJ, Felix CA, Richardson C. Therapy-related acute myeloid leukemia-like MLL rearrangements are induced by etoposide in primary human CD34+ cells and remain stable after clonal expansion. Blood. 2005;105:2124-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 27. | Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, Ross KN, Perner F, Olsen SN, Pritchard T, McDermott L, Jones CD, Jing D, Braytee A, Chacon D, Earley E, McKeever BM, Claremon D, Gifford AJ, Lee HJ, Teicher BA, Pimanda JE, Beck D, Perry JA, Smith MA, McGeehan GM, Lock RB, Armstrong SA. A Menin-MLL Inhibitor Induces Specific Chromatin Changes and Eradicates Disease in Models of MLL-Rearranged Leukemia. Cancer Cell. 2019;36:660-673.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 287] [Article Influence: 47.8] [Reference Citation Analysis (1)] |
| 28. | Issa GC, Ravandi F, DiNardo CD, Jabbour E, Kantarjian HM, Andreeff M. Therapeutic implications of menin inhibition in acute leukemias. Leukemia. 2021;35:2482-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 29. | Tsakaneli A, Williams O. Drug Repurposing for Targeting Acute Leukemia With KMT2A (MLL)-Gene Rearrangements. Front Pharmacol. 2021;12:741413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (1)] |